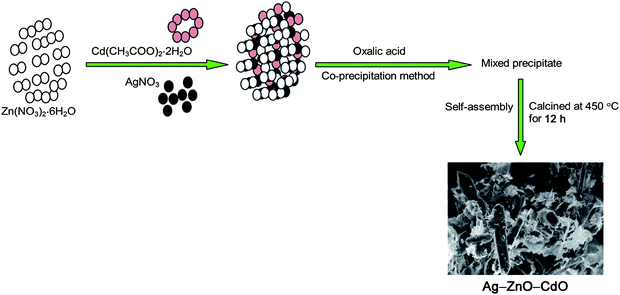
Facile fabrication of highly efficient, reusable heterostructured Ag–ZnO–CdO and its twin applications of dye degradation under natural sunlight and self-cleaning†
Subramanian Balachandrana,
Sarojini Gopinathan Praveenb,
Rengasamy Velmuruganc and
Meenakshisundaram Swaminathan*a
aDepartment of Chemistry, Annamalai University, Annamalainagar – 608 002, India. E-mail: chemres50@gmail.com; Fax: +91-4144-220572; Tel: +91-4144-220572
bDepartment of Physics, N. M Christian College, Marthandam, Kanyakumari – 629165, Tamil Nadu, India
cPresent address, Department of Chemistry, S. K. P. Engineering College, Thiruvannamali, Tamil Nadu, India
First published on 5th December 2013
Abstract
A metal doped coupled semiconductor oxide, Ag–ZnO–CdO was fabricated by a simple co-precipitation method and characterized by X-ray diffraction (XRD), field emission scanning electron microscopy (FE-SEM), elemental mapping, high-resolution scanning electron microscopy (HR-SEM), transmission electron microscopy (TEM), energy dispersive spectroscopy (EDS), X-ray photoelectron spectroscopy (XPS), Brunauer–Emmett–Teller (BET) surface area measurement, UV-Vis diffuse reflectance spectroscopy (DRS) and photoluminescence spectroscopy (PL). XRD and EDS reveal the presence of CdO and metallic Ag in the catalyst. FESEM shows a mixture of hexagonal nanosheets, nanoclusters and nanoparticles with a large number of cavities. HR-SEM and TEM images of the catalyst show that ZnO particles have pentagonal or hexagonal plate-like structure. Cadmium oxide and silver clusters are formed on the clear smooth surface of ZnO. Ag–ZnO–CdO has increased absorption in the UV and Visible region when compared to ZnO. This three component nanojunction system exhibited enhanced photocatalytic activity for the degradation of acid black 1 (AB 1) and acid violet 7 (AV 7) under sunlight far exceeding those of the single and two component systems. Ag–ZnO–CdO was found to be stable and reusable without appreciable loss of catalytic activity up to five runs. This metal doped coupled oxide shows increased hydrophobicity, when compared to CdO or ZnO. Our results provide new insights of the performance of a solar active photocatalyst with self-cleaning properties.
1 Introduction
In the last few decades, semiconductor photocatalysis using TiO2 and ZnO has drawn much attention and the research has been focused on using them in organic synthesis and environmental remediation. The advanced oxidation process (AOP) is a promising green technology for the degradation of toxic contaminants, especially for the removal of organic pollutants with solar energy.1 Recently, the interest of researchers has grown dramatically regarding the application of AOPs using semiconductor photocatalysts, which mainly involved the selective synthesis of efficient solar-light-driven photocatalysts.2–5ZnO is a good semiconductor photocatalyst due its unique optoelectronic properties, environmental stability and low cost.6 Nevertheless, its large band gap (3.2 eV) limits its use under UV light, which constitutes only 5% of the total solar spectrum. The photocatalytic efficiency of a material strongly depends on the photogenerated electron–hole recombination rate and solar energy utilization.7 When day lighting features are properly implemented they can reduce lighting-related energy requirements by 25%. Zinc oxide nanomaterials have received great attention due to its size dependent properties. Two dimensional (2D) nanostructures, such as nanoplates, nano fibres, nanosheets and nanowalls, are suggested to be ideal components for potential applications in numerous fields, such as water purification,8 lasers,9 sensors,10 solar cells,11 and field emission devices.12
Cadmium oxide (CdO) is an n-type semiconductor, attracting tremendous attention due to its interesting properties like a wide direct band gap of 2.27 eV and a narrow indirect band gap of 0.55 eV. CdO is a promising photocatalyst for the optoelectronic applications.13 Coupling of visible active CdO with ZnO can reduce the electron–hole recombination, enhance the solar light absorption and increase the photocatalytic activity of ZnO.
The surface modification of semiconductors with noble metals (Ag, Pt, Au) has attracted significant attention especially in heterogeneous photocatalysis. This can reduce the recombination of the photogenerated electron–hole pairs and lengthen their lifetime through the conduction band electron trapping. Ag is known as electron sinks due to the Schottky barrier at the metal-semiconductor interface.14 In addition, Ag could be a promising mediator for industrial application compared to other noble metals due to its low cost and nontoxicity.
The non-wettability or the water repellant property of a material has a deep influence on our daily life and in the industries manufacturing self cleaning materials, micro-fluid chips and micro-reactors. Hydrophobicity is a property showing non-wettability of a solid surface and it has received great attention due to its practical applications. Water contact angle (WCA) measurements will provide the hydrophobicity of the materials.15 Modified semiconductor oxide coated surfaces show an increased hydrophobicity.
Herein we report the preparation of a coupled, Ag doped photocatalyst, Ag–ZnO–CdO, by facile co-precipitation and thermal decomposition process for the first time. The photocatalytic activity of Ag–ZnO–CdO has been studied using degradation of two azo dyes acid black 1 and acid violet 7 under natural sun light. We believe that our findings can open a new and effective avenue to further improve the photocatalytic activities based on those coupling and loading systems and self-cleaning applications.
2 Experiments
2.1 Reagents
Zinc nitrate hexahydrate, oxalic acid dihydrate, silver nitrate, cadmium acetate, and ethanol were obtained from Himedia chemicals. AB 1 (Colour Chem, Pondicherry, molecular formula C22H14N6Na2O9S2 and molecular weight: 616.57), AV 7 (Colour Chem, Pondicherry, molecular formula C20H16N4Na2O9S2 and molecular weight: 566.48) and ZnO (Merck chemicals, surface area 5 m2 g−1, particle size 4.80 μm) were used as received. A gift sample of TiO2–P25 (80% anatase, 20% rutile with BET surface area 50 m2 g−1 and mean particle size of 30 nm) was supplied by Evonik, Germany. Deionized distilled water was employed throughout experiments.2.2 Synthesis of catalysts
2.3 Characterization
X-ray diffraction (XRD) patterns were recorded with a Siemens D5005 diffractometer using Cu Kα (k = 0.151![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 418 nm) radiation. Maximum peak positions were compared with the standard files to identity the crystalline phase. The surface morphology of the Ag–ZnO–CdO was studied by a field emission scanning electron microscope (FESEM) (Model ULTRA-55). HR-SEM images were taken using FEI Quanta FEG 200 High Resolution Scanning Electron Microscope. EDS analysis was performed on gold coated samples using a JEOL JSM-5610 SEM equipped with EDS. HR-TEM images were taken using the 200 kV Ultra High Resolution Transmission Electron Microscope (JEOL-2010) having high resolution Optical microscope (Leica microscope). X-ray photoelectron spectra (XPS) of the catalysts were recorded in an ESCA-3 Mark II spectrometer (VG Scientific Ltd., England) using Al Kα (1486.6 eV) radiation as the source. The spectra were referenced to the binding energy of C (1s) (285 eV). The specific surface areas of the samples were determined through nitrogen adsorption at 77 K on the basis of BET equation using a micrometrics ASAP 2020 V3.00 H. A Perkin Elmer LS 55 fluorescence spectrometer was employed to record the photoluminescence (PL) spectra of the oxides at room temperature. Diffuse reflectance spectra were recorded using Shimadzu UV-2450. UV absorbance measurements were taken using Hitachi-U-2001 spectrometer.
418 nm) radiation. Maximum peak positions were compared with the standard files to identity the crystalline phase. The surface morphology of the Ag–ZnO–CdO was studied by a field emission scanning electron microscope (FESEM) (Model ULTRA-55). HR-SEM images were taken using FEI Quanta FEG 200 High Resolution Scanning Electron Microscope. EDS analysis was performed on gold coated samples using a JEOL JSM-5610 SEM equipped with EDS. HR-TEM images were taken using the 200 kV Ultra High Resolution Transmission Electron Microscope (JEOL-2010) having high resolution Optical microscope (Leica microscope). X-ray photoelectron spectra (XPS) of the catalysts were recorded in an ESCA-3 Mark II spectrometer (VG Scientific Ltd., England) using Al Kα (1486.6 eV) radiation as the source. The spectra were referenced to the binding energy of C (1s) (285 eV). The specific surface areas of the samples were determined through nitrogen adsorption at 77 K on the basis of BET equation using a micrometrics ASAP 2020 V3.00 H. A Perkin Elmer LS 55 fluorescence spectrometer was employed to record the photoluminescence (PL) spectra of the oxides at room temperature. Diffuse reflectance spectra were recorded using Shimadzu UV-2450. UV absorbance measurements were taken using Hitachi-U-2001 spectrometer.
2.4 Photocatalytic experiments
All photocatalytic experiments were carried out under similar conditions on sunny days of April–May 2012 between 11 am and 2 pm. An open borosilicate glass tube of 50 mL capacity, 40 cm height and 20 mm diameter was used as the reaction vessel. The suspensions were magnetically stirred in the dark for 30 min to attain adsorption–desorption equilibrium between the dye and Ag–ZnO–CdO. Irradiation was carried out in the open-air condition. 50 mL of dye solution with Ag–ZnO–CdO was continuously aerated by a pump to provide oxygen and for the complete mixing of reaction solution. During the illumination time no volatility of the solvent was observed. After dark adsorption the first sample was taken. At specific time intervals 2 mL of the sample was withdrawn and centrifuged to separate the catalyst. 1 mL of the centrifugate was diluted to 10 mL and its absorbance was measured at 319 and 306 nm for AB 1 and AV 7 respectively to monitor the concentration of dyes.Solar light intensity was measured for every 30 min and the average light intensity over the duration of each experiment was calculated. The intensity of solar light was measured using LT Lutron LX-10/A Digital Lux meter and the intensity was 1250 × 100 ± 100 Lux.16
2.5 Contact angle measurements
The water contact angles on the catalyst coated surfaces were measured using a Drop Shape Analyzer (DSA) (Krüss GmbH, Germany). The volume of water droplet was approximately 4 μl and the average of 5 measurements is reported as the water contact angle (WCA) on the substrate. Ag–ZnO–CdO modified silane coatings were successfully fabricated on a glass substrates using spin coating method at room temperature. Ag–ZnO–CdO coated substrates were sintered at 125 °C for 2 h with heating rate of 5 °C min−1 in programmed furnace to ensure densification of the gel network.3 Results and discussion
X-ray diffractograms (Fig. 1a–e) show the typical XRD patterns of the (a) prepared ZnO, (b) Ag–ZnO, (c) CdO, (d) Ag–CdO and (e) Ag–ZnO–CdO (3 wt% of Ag and 3 wt% CdO) samples. Characteristic peaks of ZnO at 31.68, 34.36, 36.18, and 56.56° correspond to (100), (002), (101), and (110) diffraction peaks of wurtzite ZnO (JCPDS file no. 36-1451).17 The XRD pattern of Ag–ZnO–CdO obviously shows additional peaks at 2θ = 38.2 and 64.60° in Ag–ZnO–CdO, which are due to the (111) and (220) planes of silver. This indicates that the ZnO surface is covered with silver particles. Red color can be indexed to face-centered-cubic (fcc) structure of metallic Ag (JCPDS file no. 04-0783). No peaks corresponding to Ag2O are detected.18 Peaks at 2θ = 33.8, 54.7 and 68.1° in Ag–ZnO–CdO can be indexed to (111), (311) and (222) planes of cadmium oxide.19 There is no remarkable shift of other diffraction peaks and no crystalline impurities are observed. The Scherrer equation (eqn (1)) was employed for the precise calculation of the crystallite sizes of Ag–ZnO–CdO.
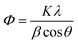 | (1) |
The morphology, size, and crystal structure of the as-synthesized product were determined from the analysis of HR-SEM, FE-SEM and TEM images. The HR-SEM images of Ag–ZnO–CdO at three different magnifications are shown in Fig. S2.† ZnO particles have 3D-pentagonal or hexagonal plate-like structure. Cadmium oxide and silver clusters are formed on the clear smooth surface of ZnO and show aggregations.
To visually study the morphology of CdO, Ag–CdO, Ag–ZnO, Ag–ZnO–CdO, we used field emission scanning electron microscopy (FESEM). Before taking FE-SEM images, Au was covered on the surface of samples. The images of CdO, Ag–CdO, Ag–ZnO, Ag–ZnO–CdO are shown in Fig. 2a–d respectively. Fig 2a shows the plate like structure of CdO. Dispersion of Ag nanoparticles on CdO are seen clearly Fig. 2b. Fig. 2c reveals the homogeneous dispersion of Ag on ZnO with micro cavities. Interestingly we got the different morphology of Ag–ZnO–CdO as seen in Fig. 2d. Ag–ZnO–CdO shows a mixture of hexagonal nanosheets, nanoclusters and nanoparticles with a large number of cavities. These cavities favour the adsorption of dye molecules on the surface of the heteroarchitectured material. The dispersion of nanoclusters on Ag and CdO on hexagonal sheets of ZnO was also shown by HR-SEM images (Fig. S2c and S2d†). Since we have not used any template or surfactant during co-precipitation or the thermal decomposition process, this heterostructure containing nanosheets, nanoclusters and nanoparticles might have been obtained by a self assembly process as shown in Scheme 1.
To confirm the distribution of Ag, Zn, Cd and O in the surface of the catalyst, elemental mapping of FE-SEM was carried out. Fig. 3a exhibits FE-SEM image of Ag–ZnO–CdO, while Fig. 3b–e show the elemental mapping in different colors for Ag (black), Zn (green), O (red) and Cd (blue) respectively. It is evident from the Fig. 3a that Zn and O are higher in density when compared to Ag and Cd and there is a homogenous distribution of Ag, Cd, Zn and O in the catalyst. Thus elemental mapping shows that catalyst is composed of Ag, Zn, Cd and O. This also indicates the purity of the catalyst Ag–ZnO–CdO. The presence of elements Ag, Zn, Cd and O in the catalyst was further confirmed by EDS recorded from the selected area (Fig. S3†).
 | ||
| Fig. 3 Elemental mapping of Ag–ZnO–CdO (a) elemental mapping of FE-SEM, (b) Ag, (c) Zn, (d) O and (e) Cd. | ||
The structural features of Ag–ZnO–CdO photocatalyst can be observed more clearly in the TEM images. Fig. 4a–c shows the TEM images of the Ag–ZnO–CdO sample annealed at 450 °C .The particles posses predominantly three dimensional pentagonal and hexagonal structures with round edges and have no sign of crystal defects. The TEM images of Ag–ZnO–CdO have some small dots and dark black shadow like sticking onto the surface (Fig. 4a–c) and this may be due to the presence of Ag and CdO. The selected area electron distribution pattern (recorded from the silver) (Fig. 4d) shows concentric ring with intermittent bright dots, indicating that the samples are highly crystalline in nature. The presence of diffraction planes of Ag (111) and CdO (200, 220, 311) is clearly seen in SAED pattern. Fig. 4e shows the high-resolution TEM image of the Ag–ZnO–CdO sample with the lattice spacing 0.256 nm, corresponding to the (101) planes of wurtzite ZnO. Similarly the d spacings corresponding to CdO (0.34 nm) and Ag (0.234 nm) are also seen. The results match exactly with XRD pattern discussed earlier.
 | ||
| Fig. 4 TEM images of Ag–ZnO–CdO (a–c) 200 nm, (d) SAED pattern and (e) HR-TEM image (lattice fringes of Ag–ZnO–CdO). | ||
In order to determine the elements and their oxidation state in Ag–ZnO–CdO, XPS study was carried out. The binding energy peaks of Zn, O, Ag and Cd were also analyzed. In Fig. 5a, the O1s profile is asymmetric and can be fitted to two symmetrical peaks (α and β locating at 531.1 and 533.2 eV, respectively), indicating two different kinds of O species in the sample. The peaks α and β should be associated with the lattice oxygen (OL) of ZnO/CdO and chemisorbed oxygen (OH) caused by the surface hydroxyl,20 respectively. Fig. 5b presents the XPS spectra of Zn 2p, and the peak positions of Zn 2p1/2 and Zn 2p3/2 locate at 1045.2 eV and 1022.2 eV respectively. Comparing the peak positions to those in the Handbook of X-Ray Photoelectron Spectroscopy,21 we can conclude that Zn is in the state of Zn2+, but there is a downward shift of 0.2 eV when compared to the standard peak position of Zn 2p3/2 at 1022 eV. The Cd 3d5/2 and Cd 3d3/2 peaks (Fig. 5c) centered at 405.3 and 412.1 eV with a spin–orbit separation of 6.7 eV are assigned to Cd2+ of CdO and it is consistent with the reported values.22 In Fig. 5d, peaks at 368.4 and 374.5 eV are assigned to Ag 3d5/2 and Ag 3d3/2, respectively. These peaks have a splitting of 3d doublet with 6.1 eV indicating the presence of metallic silver.23 No additional peaks were observed above 374 eV, which clearly demonstrated the absence of an oxidized form of silver.
The optical properties of Ag–ZnO–CdO were explored by UV-Vis diffuse reflectance and photoluminescence (PL) spectroscopy. The diffuse reflectance spectra of ZnO and Ag–ZnO–CdO are displayed in Fig. 6. Ag–ZnO–CdO shows a strong absorption in UV and a slightly increased absorption in the visible region of light. This reveals that Ag–ZnO–CdO can be used as an UV and Visible light active semiconductor photocatalytic material. We have recorded DRS spectra of Ag–ZnO with the different concentrations of CdO and they are displayed in Fig. S4.† The increase in the concentration of CdO from 1 to 5 wt% in Ag–ZnO increased the absorption in the visible region of light due to low band gap of CdO (2.27 eV). There is no change in the absorption edge. The photoluminescence (PL) can be used to find out the fate of electron–hole pairs in semiconductor particles.24 Fig. 7 presents the photoluminescence spectra of the prepared ZnO and Ag–ZnO–CdO. Both spectra mainly consist of two emission bands: a band at 416 nm (2.98 eV) and a weak blue-green band at 485 nm (2.56 eV). The strong UV emission at 416 nm corresponds to the e-hole recombination.25–27 The weak blue green emission is due to surface defects in the ZnO powders as in the case of ZnO nanowires reported by Wang and Gao.28 Reduction of PL intensity at 416 nm by Ag–ZnO–CdO when compared to prepared ZnO indicates the suppression of recombination of the photogenerated electron–hole pair.
The pore structure of the Ag–ZnO–CdO composite sample was investigated by nitrogen adsorption–desorption isotherms and the pore size distribution was calculated by BJH method according to the desorption branch. The N2 adsorption–desorption isotherms of the synthesized Ag–ZnO–CdO exhibited a hysteresis loop, typical of type III pattern according to the classification of IUPAC.29 A sharp increase in the adsorption volume of N2 was observed and located in the P/P0 range of 0.8–0.99. This sharp increase can be attributed to the capillary condensation, indicating the good homogeneity of the sample and macro pore size for the P/P0 position of the inflection point is related to the pore size.30 Average pore radius of Ag–ZnO–CdO, shown by pore size distribution curve (inset of Fig. 8) is 25 nm (250 Å). The pore size distribution of the Ag–ZnO–CdO sample thus confirms the macroporous structure. Surface area measurements, made by the BET method, provide the specific surface area of Ag–ZnO–CdO as 26.0 m2 g−1, which is higher than prepared ZnO (11.52 m2 g−1). The single point adsorption total pore volume of pores less than 996.84 Å radius at P/P0 = 0.9902 is 0.192 cm3 g−1. Moreover, it has to be emphasized that this type of isotherm indicates the presence of macroporous structure in Ag–ZnO–CdO.
3.1 Photocatalytic activity
Azo dyes are causing serious environmental pollution and endangering public health due to their resistance to biodegradability and to their easy transformation into genotoxic and carcinogenic amines by different mechanisms. Experiments were carried out under the same conditions to test the photodegradability of acid black 1 (AB 1) by ZnO, ZnO–CdO, Ag–ZnO, and Ag–ZnO–CdO catalysts and the results are presented in Fig. 9. Dye is resistant to self photolysis and for same experiment with Ag–ZnO–CdO in the dark, a small decrease in dye concentration was observed due to the adsorption of dye on the catalyst. AB 1 undergoes almost complete degradation in the presence of Ag–ZnO–CdO and natural sunlight light in 45 min. But, prepared ZnO, ZnO–CdO, Ag–ZnO, Ag–CdO and TiO2–P25 produced 82, 85, 80, 78 and 72% degradations, respectively in 45 min. This shows that Ag–ZnO–CdO is more efficient in AB1 degradation than other photocatalysts. | ||
| Fig. 9 Primary analysis: AB 1 dye concentration = 3 × 10−4 M, catalyst suspended = 3 g L−1, pH = 11, airflow rate = 8.1 mL s−1, Isolar = 1250 × 100 Lux ± 100. | ||
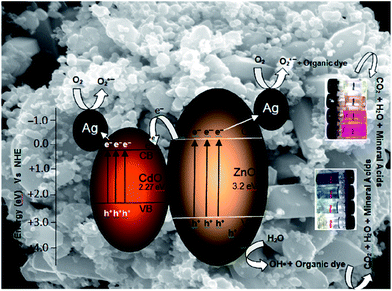
The photocatalytic efficiency of this catalyst in natural sunlight was also tested with AV 7 dye degradation. Photodegradability by ZnO, ZnO–CdO, Ag–ZnO, and Ag–ZnO–CdO catalysts are presented in Fig. 10. When the dye was irradiated without catalyst, there was negligible degradation. Almost complete degradation of AV 7 dye with Ag–ZnO–CdO and solar light was observed in 50 min. Percentages of degradation with prepared ZnO, ZnO–CdO, Ag–ZnO, Ag–CdO and TiO2–P25 are 78, 83, 80, 74 and 72% respectively in 50 min. In AV 7 degradation also, Ag–ZnO–CdO is most efficient. Color changes at different irradiation times shown in bottles indicate the dye degradation (Fig. 9 and 10). UV spectral changes of AB 1 and AV 7 at different irradiation times with Ag–ZnO–CdO catalyst are shown in Fig. S5 and S6.† There is a gradual decrease in intensity without the appearance of new absorption peaks. This reveals that the intermediates formed during degradation do not absorb at analytical wavelength. Photocatalytic activity is influenced by many factors in which specific surface area and the transport properties of photoinduced charge carriers are two key factors. The Ag–ZnO–CdO is fairly active for photocatalytic degradation AB 1 as well as AV 7 dyes, because it has larger BET surface area (26.0 m2 g−1) than prepared ZnO (11.5 m2 g−1) to facilitate more efficient contact of the heterostructured Ag–ZnO–CdO with organic dye contaminants and thus resulting in enhanced photocatalytic activity. A mechanism involving transport properties of photoinduced charge carriers between the band energy levels of Ag–ZnO–CdO is proposed in Scheme 2 and discussed separately. The effects of pH and catalyst loading on photodegradation of dyes were studied with Ag–ZnO–CdO under natural sun light to find out optimum conditions.
 | ||
| Fig. 10 Primary analysis: AV 7 dye concentration = 5 × 10−4 M, catalyst suspended 3 g L−1, pH = 11, airflow rate = 8.1 mL s−1, Isolar = 1250 × 100 Lux ± 100. | ||
3.2 Influence of initial pH
Percentages of dye degradation at different pH from 3 to12 for AB1 and AV 7 are shown in Fig. S7.† It is observed that the degradation rate increases with increase in pH up to 11 and then it decreases. The optimum pH is found to be 11 for both AB 1 and AV 7 dyes. The acid–base property of the metal oxide surface can have considerable implications on their photocatalytic activity. The presence of large quantities of hydroxide ions on the particle surface as well as in the reaction medium at pH 11 favors the formation of hydroxyl radical, which is the principal oxidizing species responsible for the dye degradation process. To find out the reason for the effect of pH on degradation efficiency, zero point charge (ZPC) of the catalyst was determined by potentiometric titration method.31 Zero point charge of Ag–ZnO–CdO was found to be 10.6, which is higher than ZPC of ZnO (9.2) and CdO (10.4). When the pH is above ZPC, the surface charge density of the catalyst becomes negative. This decreases the adsorption of dye molecules, which exist anionic at pH above 11. Hence the degradation efficiency is low at pH 12. To confirm this, an experiment to find out the adsorption of dye in dark at different pH was carried out. The percentages of adsorption, after the attainment of adsorption equilibrium, are 2.6, 5.3, 7.1, 7.8, 10.2 and 8.5 at pH 3, 5, 7, 9, 11 and 12 respectively. Adsorption of dye on Ag–ZnO–CdO is high at pH 11. At higher pH values (above 11), surface of the catalyst is negatively charged and the electrostatic attraction between dye anions and negatively charged catalyst becomes weak resulting in reduced adsorption.32 This leads to the decrease in degradation efficiency at pH 12. This reveals the role of ZPC in pH effect on catalyst efficiency in the photodegradation. Low removal efficiency at acidic pH range may be due to the dissolution of ZnO in Ag–ZnO–CdO.3.3 Effect of photocatalyst dosage
The influence of the photocatalyst dosage on the degradation of AB 1 and AV 7 has been investigated employing different concentrations of Ag–ZnO–CdO. The results are presented in Fig. S8.† In the case of AB 1 dye, the increase in catalyst from 1 to 3 g L−1 increases the dye removal. Further increase in catalyst amount above 3 g L−1 decreases the dye removal efficiency. In the case of AV 7, dye removal increases up to 2 g L−1 and further increase in the amount of the catalyst decreases the removal rate. The enhancement of removal rate is because of (a) the increase in the weight of the catalyst which increases the number of dye molecules adsorbed, (b) the increase in the density of particles in the area of illumination. Hence, under these experimental conditions, 3 g L−1 for AB 1 and 2 g L−1 for AV 7 are found to be optimum for efficient dye degradation. At higher concentration of the catalyst (above 3 g L−1 for AB 1 and 2 g L−1 for AV 7), the decrease in efficiency is due to the light scattering by catalyst particles.163.4 Reusability of the catalyst
The reusability of Ag–ZnO–CdO was tested for the degradation of AB 1 dye under identical reaction conditions. After complete degradation, the catalyst was separated and washed with large amount of deionized water. The recovered catalyst was dried in hot air oven at 100 °C for 90 min and used for second run. Fig. 11 shows the results of AB 1 degradations for five runs. Ag–ZnO–CdO exhibits remarkable photostability as the AB 1 degradation percentages are 100%, 98%, 96.0%, 96% and 96% in the first, second, third, fourth and fifth runs respectively for 45 min. In case of AV 7 degradation with Ag–ZnO–CdO, 100%, 98%, 97%, 95%, and 95% of degradations in the first, second, third, fourth and fifth runs are observed for 50 min, respectively. There is no significant change in the degradation efficiency of Ag–ZnO–CdO for both dyes. In order to find out the morphological change in the catalyst during the degradation, we had taken XRD spectra of fresh and used Ag–ZnO–CdO photocatalysts and they are given in Fig. S9.† It was found that the crystal structure of Ag–ZnO–CdO photocatalyst after 5 cycles did not change during the reaction, indicating the stability of photocatalyst. Hence the catalyst can be reused for continuous treatment of wastewater. Furthermore Ag–ZnO–CdO can be easily separated when compared to other catalysts used. After completion of degradation reaction the solution was tested with Na2S solution and there is no precipitation of Cadmium sulfide. Hence, there is no dissolution of Cd2+ ion and it confirms the Cd2+ in the lattice of ZnO–CdO material. As there is no leaching of Cd2+, this catalyst is non-toxic for the wastewater treatment.3.5 Mechanism of degradation
A mechanism based on the band energy levels of ZnO and CdO is proposed in Scheme 2 for the degradation of dye. Both ZnO and CdO are n-type semiconductors with band gap energies 3.2 eV, 2.27 eV respectively and exhibit a strong absorption threshold both in the UV and visible region. The CB edges of ZnO and CdO are situated at −0.31 and +0.11 eV, respectively. The CB edge of CdO is more positive than that of ZnO, as shown in Scheme 2. Hence CdO can act as a sink for the photogenerated electrons in the coupled oxide. This makes the charge separation more efficient. In addition to this ‘Ag’ metal can trap the electron from ZnO and CdO, inhibiting the recombination of electrons and holes in Ag–ZnO–CdO. This increases the photocatalytic activity of Ag–ZnO–CdO. Holes remained on ZnO valence band and electrons concentrated on CdO conduction band, enabled this type of three component system with strong oxidation and reduction capabilities to generate superoxide (O2˙−), and hydroxyl radicals (˙OH) for the dye degradation. Hydroxyl radical formation by ZnO photocatalysis has been well established.This charge transfer mechanism permits higher degradation of dyes than can be achieved by a typical single and two component systems (ZnO, TiO2–P25, CdO–ZnO and Ag–ZnO) in dye degradation under irradiation by natural sun light. In addition to this Ag metal can also trap the electron from ZnO and CdO, reducing the recombination of electrons and holes in Ag–ZnO–CdO. This may also increase the photocatalytic activity of Ag–ZnO–CdO.
4 Contact angle measurements
Surface wettability or the hydrophobicity of the catalyst is revealed by water contact angle. If a surface has a contact angle with water that is greater than 90°, then the surface is classed as hydrophobic and if the contact angle is less than 90°, the surface is hydrophilic. Water contact angles were measured on glass slides coated with TEOS, TEOS + ZnO and TEOS + Ag–ZnO–CdO to analyze the hydrophobicity of the catalysts. The images of water drops on coated and uncoated glass slides are shown in Fig. 12. The catalyst nano particles were coated on the slides by spin coating method using silica sol. The film surface is hydrolytically stable because Si–C bonds are modified by catalyst particles. Water contact angle (WCA) of 39° on uncoated glass slide (CA1) shows the hydrophilicity and this WCA increases gradually on glass slides coated with TEOS (50.6°) (CA2), TEOS + ZnO (73.3°) (CA3) and TEOS + Ag–ZnO–CdO (101.1°) (CA4). Hydrophobicity increases and reaches a maximum value of 101.1° with TEOS + Ag–ZnO–CdO coated glass slides. This shows that the surface coated with TEOS + Ag–ZnO–CdO has more hydrophobic character. This decrease in surface wettability leads to a self cleaning property of the catalyst.15,33,34 | ||
| Fig. 12 Water contact angle measurements (a) uncoated glass slide (CA1), (b) TEOS coated (CA2), (c) TEOS + ZnO coated glass slide (CA3) and (d) TEOS + Ag–ZnO–CdO coated glass slide (CA4). | ||
5 Conclusions
Ag–ZnO–CdO semiconductor photocatalyst was synthesized by a simple and cost effective co-precipitation method and characterized. XRD and XPS reveal the presence of CdO, Ag and ZnO in the catalyst. FE-SEM shows a mixture of hexagonal nanosheets, nanoclusters and nanoparticles with a large number of cavities. HR-SEM and TEM images show heterostructure of Ag–ZnO–CdO with pentagonal and hexagonal plates. It has increased absorption in the visible and UV region when compared to ZnO. The excellent photocatalytic activity stems from the different conduction band edge positions of CdO and ZnO, which promote electron transfer from one semiconductor to the other. Ag–ZnO–CdO was found to be stable and reusable without appreciable loss of catalytic activity up to five runs. It will be very useful as industrial green catalyst for effective treatment of dye effluents under natural sunlight and as a self cleaning material.Acknowledgements
We are grateful to the Council of Scientific and Industrial Research, New Delhi, for the financial support through research Grant no. 21(0799)/10/EMR-II. One of the authors Mr S. Balachandran is thankful to UGC Networking Resource Centre, University of Hyderabad for providing characterization facility and to Dr Tushar Jana, School of Chemistry, University of Hyderabad for giving the laboratory facility. Authors are also grateful to Dr P. V. Satyam, Institute of Physics, Bhubaneswar for using HR-TEM facility.Notes and references
- M. R. Hoffman, S. T. Martin, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69 CrossRef.
- J. Yu and A. Kudo, Adv. Funct. Mater., 2006, 16, 2163 CrossRef CAS.
- A. Kudo, K. Ueda, H. Kato and I. Mikami, Catal. Lett., 1998, 53, 229 CrossRef CAS.
- S. Kohtani, J. Hiro, N. Yamamoto, A. Kudo, K. Tokumura and R. Nakagaki, Catal. Commun., 2005, 6, 185 CrossRef CAS PubMed.
- L. Zhou, W. Z. Wang, S. W. Liu, L. S. Zhang, H. L. Xu and W. Zhu, J. Mol. Catal. A: Chem., 2006, 252, 120 CrossRef CAS PubMed.
- C. Lizama, J. Freer, J. Baeza and H. D. Mansilla, Catal. Today, 2002, 76, 235 CrossRef CAS.
- X. Pian, B. Lin, Y. L. Chen, J. D. Kuang, K. Z. Zhang and L. M. Fu, J. Phys. Chem. C, 2011, 115, 6531 CAS.
- M. Y. Lu, M. P. Lu, Y. A. Chung, M. J. Chen, Z. L. Wang and L. J. Chen, J. Phys. Chem. C, 2009, 113, 12878 CAS.
- D. M. Bagnall, Y. F. Chen, Z. Zhu, T. Yao, S. Koyama, M. Y. Shen and T. Goto, Appl. Phys. Lett., 1997, 70, 2230 CrossRef CAS PubMed.
- Q. Wan, Q. H. Li, Y. J. Chen, T. H. Wang, X. L. He, J. P. Li and C. L. Lin, Appl. Phys. Lett., 2004, 84, 3654 CrossRef CAS PubMed.
- H. Rensmo, K. Keis, H. Lindstrom, S. Sodergren, A. Solbrand, A. Hagfeldt, S. E. Lindquist, L. N. Wang and M. Muhammed, J. Phys. Chem. B, 1997, 101, 2598 CrossRef CAS.
- Y. K. Tseng, C. J. Huang, H. M. Cheng, I. N. Lin, K. S. Liu and I. C. Chen, Adv. Funct. Mater., 2003, 13, 811 CrossRef CAS.
- Y. M. Wu, M. Y. Xing, J. L. Zhang and F. Chen, Appl. Catal., B, 2010, 97, 182 CrossRef CAS PubMed.
- C. Ren, B. Yang, M. Wu, J. Xu, Z. Fu, Y. lv, T. Guo, Y. Zhao and C. Zhu, J. Hazard. Mater., 2010, 182, 123 CrossRef CAS PubMed.
- K. Jeevajothi, R. Subasri and K. R. C. Soma Raju, Ceram. Int., 2013, 39, 2111 CrossRef CAS PubMed.
- R. Velmurugan and M. Swaminathan, Sol. Energy Mater. Sol. Cells, 2011, 95, 942 CrossRef CAS PubMed.
- D. Yiamsawas, K. Boonpavanitchakul and W. Kangwansupamonkon, Journal of Microscopy Society of Thailand, 2009, 23, 75 Search PubMed.
- D. Lin, H. Wu, R. Zhang and W. Pan, Chem. Mater., 2009, 21, 3479 CrossRef CAS.
- R. Saravanan, H. Shankar, T. Prakash, V. Narayanan and A. Stephen, Mater. Chem. Phys., 2011, 125, 277 CrossRef CAS PubMed.
- J. J. F. Moudler, W. F. Stickle, P. E. Sobol and K. D. Bomben, Handbook of X-ray Photoelectron Spectroscopy, ed. J. Chastain and R. C. King, Jr, Physical Electronics Division, Perkin-Elmer Corp, Eden Prairie, MN, 1995, p. 127 Search PubMed.
- C. D. Wagner, W. M. Riggs, L. E. Davis and J. F. Moulder, Handbook of X-ray Photoelectron Spectroscopy, Perkin Elmer, Eden Prairie, 1979, p. 81 Search PubMed.
- L. Wu, J. C. Yu and X. Z. Fu, J. Mol. Catal. A: Chem., 2006, 244, 25 CrossRef CAS PubMed.
- L. Kuai, B. Geng, X. Chen, Y. Zhao and Y. Luo, Langmuir, 2010, 26, 18723 CrossRef CAS PubMed.
- F. B. Li and X. Z. Li, Appl. Catal., A, 2002, 228, 15 CrossRef CAS.
- V. Stikant and D. R. Clarke, J. Appl. Phys., 1998, 83, 5447 CrossRef PubMed.
- S. C. Lyu, Y. Zhang, H. Ruh, H. Lee, H. Shim, E. Suh and C. J. Lee, J. Chem. Phys. Lett., 2002, 363, 134 CrossRef CAS.
- L. Bergman, X. B. Chen, J. L. Morrison, J. Huso and A. P. Purdy, J. Appl. Phys., 2004, 96, 675 CrossRef CAS PubMed.
- J. Wang and L. Gao, Solid State Commun., 2004, 132, 269 CrossRef CAS PubMed.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquerol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603 CrossRef CAS.
- T. Sreethawong, Y. Yamadab, T. Kobayashi and S. Yoshikawa, J. Mol. Catal. A: Chem., 2005, 241, 23 CrossRef CAS PubMed.
- S. Subramanian, J. S. Noh and J. A. Schwarz, J. Catal., 1988, 114, 433 CrossRef CAS.
- G. A. Parks, Chem. Rev., 1965, 65, 177 CrossRef CAS.
- R. V. Lakshmi, T. Bharathidasan, P. Bera and B. J. Basu, Surf. Coat. Technol., 2012, 206, 3888 CrossRef CAS PubMed.
- K. M. S. Meera, R. M. Sankar, A. Murali, S. N. Jaisankar and A. B. Mandal, Colloids Surf., B, 2012, 90, 204 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra45381b |
| This journal is © The Royal Society of Chemistry 2014 |