The effect of common bacterial growth media on zinc oxide thin films: identification of reaction products and implications for the toxicology of ZnO
Sanly Liu†
,
Elizabeth Killen†,
May Lim*,
Cindy Gunawan and
Rose Amal
ARC Centre of Excellence for Functional Nanomaterials, School of Chemical Engineering, The University of New South Wales, Sydney, NSW 2052, Australia. E-mail: m.lim@unsw.edu.au
First published on 4th December 2013
Abstract
This study provides a thorough investigation on the effects of the commonly-employed microbial growth medium, namely the peptide-containing luria-bertani broth, tryptic soy broth, the glucose-containing M9 Minimal Salts Media as well as phosphate-buffered saline solution on the dissolution and microstructural transformation of zinc oxide thin film. Morphology and chemical composition of the ZnO film after incubation in the media was thoroughly characterised. In addition, the amount and rate of soluble zinc released by the ZnO thin films was quantified. Exposure of ZnO thin film in the different growth media saw formation of new zinc compounds, resulting from various chemical reactions of zinc with the medium components. Deposition of the new zinc compounds on top of the thin film caused morphological transformation of the film. Zinc leaching was observed in all of the tested media, with significantly higher extent of dissolution observed in peptide-containing organic media, such as luria bertani and tryptic soy broth. Complex organic components, such as amino acids and peptides form complexes with zinc oxide coatings, resulting in complexation-mediated leaching of zinc. Soluble zinc re-precipitates with components in the media, and therefore substantially reduced the amount of dissolved zinc. The results suggest strong influence of solution chemistry on ZnO speciation in a test medium, which have important implications for the mechanistic interpretation of ZnO toxicity.
Introduction
Metal oxides are currently under investigation as functionalised materials for industrial, medical and chemical applications due to their antibacterial effects.1,2 Of the metal oxides, zinc oxide has been shown to have an equal or greater antibacterial effect to aluminium, titanium, and tungsten oxides.3–5 Due to the increased antibacterial effect with decreasing particle size,6,7 nanoparticulate zinc oxide has attracted attention, demonstrating biocidal effects in nanoparticulate form8,9 despite having no reported adverse health effects to humans, including no evidence of carcinogenicity, genotoxicity, and reproduction toxicity.10 Surfaces functionalised with zinc oxide nanoparticles are currently being investigated for their potential to reduce biofouling on metal surfaces,6 reduce infections due to bacterial contamination of medical implants,11 and as oxidative photocatalysts in waste-water treatment.12The antibacterial mechanism of zinc oxide in aqueous conditions is debated, with the most widely contested and substantiated theories being cell damage due to the generation of reactive oxygen species through the formation of electron–hole pairs,13–17 mechanical injury of the target organisms due to nanoparticle internalisation18 and toxicity due to zinc ions leached from zinc oxide nanoparticles.19 The lack of consensus on the mechanism of antibacterial action may be due to all three mechanisms contributing to toxicity, with the dominant effect observed being dependent on testing environment. Due to the lack of consensus, tests conducted on zinc oxide materials are hence often dually aimed at the demonstration of antibacterial qualities and inference of the likely mechanism of toxicity.
Tests of zinc oxide's antibacterial efficacy are usually conducted on a laboratory scale in a controlled environment using cultures of bacteria, commonly E. coli,13,20–24 Staphylococcus aureus,8,25–27 or Pseudomonas aeruginosa27,28 in growth medium, to allow for results to be obtained quickly and reduce variables. These growth media commonly contain inorganic phosphates, sodium, potassium, and chloride ions, as well as complex organic components including tryptone, yeast extract, soya peptone, and glucose. Zinc oxide has been demonstrated to form precipitates with phosphates,29,30 complexes with chloride,31 hydroxide ions,32 and organic compounds such as citrate33 and amino acids.14 Despite the potential for any number of these components to react with the zinc oxide nanomaterials, this is rarely accounted for during tests of the antibacterial efficacy of zinc oxide. As the antibacterial mechanism of ZnO can be highly dependent on water chemistry and particle behavior in the media, a thorough characterisation of the ZnO nanoparticle and its speciation during antibacterial testing is essential for proper interpretation of toxicity data. Li et al.34 recently investigated the influence of medium components on the toxicity of ZnO nanoparticles to Escherichia coli and found that generation of precipitate or zinc complexes dramatically decreased the concentration of free Zn2+ ions, resulting in lower toxicity of nanoparticles to the cell.
In this study, we tested the effect of Luria-Bertani (LB) broth, Tryptic Soy Broth (TSB), Phosphate-Buffered Saline (PBS) solution, and M9 Minimal Salts media on zinc oxide thin films, with an aim to understand the reactions, products, and their implications for the testing of antibacterial effects of zinc oxide thin films. Any change in the morphology and chemical composition of the ZnO thin film after incubation in the media above was investigated. The amount and rate of dissolved zinc released by ZnO thin films in the different media was also quantified.
Experimental procedures
Preparation of zinc oxide thin films
Round silica slips of 18 mm diameter and 0.16 mm thickness were cleaned via immersion in an ultrasonic bath for 10 minutes each in dilute nitric acid (HNO3), acetone ((CH3)2CO), and twice in ethanol (CH3CH2OH). The slides were then dried overnight at 110 °C before being used for coating. Zinc acetate dihydrate (Zn(CH3COO)2·2H2O, Analytical, Univar) was added to ethanol (CH3CH2OH, Absolute, Sigma Aldrich) with magnetic stirring at 500 rpm to make a 1.5 M solution. Monoethanolamine (MEA) (≥99.0%, Sigma Aldrich) was then added dropwise and the solution was stirred for two hours at 60 °C. The molar ratio of MEA to zinc acetate was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The resulting sol was filtered through a 0.2 μm membrane and aged 2 days before coating.
1. The resulting sol was filtered through a 0.2 μm membrane and aged 2 days before coating.
Clean glass substrates were attached with double sided tape to 25 × 25 mm cut glass microscope slides for support. Zinc sol (0.150 mL) was pipetted onto the substrate in the spin coater (Laurel, Model WS-400 BZ-6NPP/Lite). The substrates were spun at 300 rpm for 15 seconds then 5000 rpm for 30 seconds, and placed on a hotplate to evaporate residual solvent. The coating procedure was repeated a total of five times on each substrate. The spin-coated glass slides were placed in glass Petri dishes and annealed at 550 °C for 3 hours with a heating rate of 7 °C min−1. These samples were considered sterile and kept in sterile conditions until testing. Quantitative analysis of the amount of zinc oxide coated on each glass substrate was performed by digesting the sample in nitric acid, followed by ICP-OES (Perkin Elmer Optima 7300). Approximately 2.5 ± 0.3 mg of ZnO was coated on each glass substrate.
Characterisation techniques
The morphologies and chemical composition of the thin film were characterised using a field-emission scanning electron microscope (FE-SEM) equipped with an energy dispersive X-ray detector. Samples were coated using a Chromium Sputter Coater (Emitech K575x) for 30 seconds. The slides were then imaged using an FEI Nova NanoSEM 230. The spot size was varied between 2.0 and 3.0 and the high voltage between 3 and 10 kV, dependent on sample charging. Elemental composition of the samples was analysed using energy-dispersive spectroscopy (EDS) and the data was analysed using Esprit EDS software. Before EDS analysis, the samples were coated with carbon.The crystallinity of the samples was characterised with X-ray diffraction. Samples were mounted on a glass slide and imaged with Philips X'pert Panalytical MRD thin film system with a 1/16° divergence slit and 10 mm mask at 45 kV and 40 mA. Patterns were recorded in the range of 2θ = 5° to 75° with a step time of 50 seconds. Data were analysed with X'pert HighScore Plus software. The average crystallite size was calculated using the Scherrer equation.
Dissolution of zinc oxide coating in different media
Coatings were placed in each well of sterile 12 well plates (Iwaki). Media tested were Tryptic Soy Broth (TSB; Oxoid, 5 g L−1 NaCl, 2.5 g L−1 K2HPO4, 2.5 g L−1 glucose, 3 g L−1 soya peptone, 17 g L−1 casein peptone), Phosphate-Buffered Saline solution (PBS; 137 mM NaCl, 10 mM Na2HPO4, 2.0 mM KH2PO4 and 2.7 mM KCl, (all reagents Univar, Analytical) (pH 7.2)), Luria-Bertani broth (LB; 10 g L−1 Tryptone (Oxoid), 5 g L−1 Yeast extract (Oxoid), 10 g L−1 NaCl (Univar, Australia)), M9 minimal media (M9; 48 mM Na2HPO4, 22 mM KH2PO4, 8.6 mM NaCl, 19 mM NH4Cl, 2 mM MgSO4·7H2O, 100 μM CaCl2 and 20 mM glucose (all reagents Univar, Analytical)). A 2 mL volume of the media being tested was aliquotted into each well, with each media tested in triplicate.Plates were incubated at 37 °C, shaken at 60 rpm for periods of 1 day and 1 week. After the incubation period, the supernatant was removed and the amount of soluble zinc ions was measured with ICP-OES (Perkin Elmer OPTIMA 7300). The background level of Zn in each of the media was also measured, and the reported result has been corrected to take this into account. Phosphorus content in the media was also measured with ICP-OES (Perkin Elmer OPTIMA 7300). No microbial growth was visible over the course of the experiment. Coatings were washed twice with 1 mL of Milli-Q water, shaken at 60 rpm for 10 minutes each at 37 °C, and allowed to air dry. Samples were then characterised using SEM and XRD.
For the leaching kinetics study, plates with coatings and media tested were incubated for periods of 0.5, 1, 1.5, 6 and 24 hours, shaken at 60 rpm and 37 °C. After incubation, the supernatant was removed and analysed with ICP-OES analysis.
Results and discussion
Characterisation of as-synthesised zinc oxide thin films
Sol–gel spin coating synthesis produced consistent films of spherical particles and an average particle size of dSEM = 121 ± 33 nm, as shown below in Fig. 1(a) and (b). The film was shown to be crack free, and composed of a network of dense ZnO aggregates.The XRD pattern of the as-prepared ZnO thin film is presented in the topmost spectrum of Fig. 2. All the diffraction peaks shown were indexed to the hexagonal phase of ZnO (JCPDS 36-1451) with no detection of impurities, which indicates that highly crystalline ZnO products were obtained under the synthesis conditions. XRD analysis shows major peaks at 31.8°, 34.4°, and 36.2°, corresponding to the (1 0 0), (0 0 2), and (1 0 1) directions of crystal growth in ZnO. The peaks were of roughly equivalent height which suggests no preferential crystal growth direction in the hexagonal wurtzite structure.
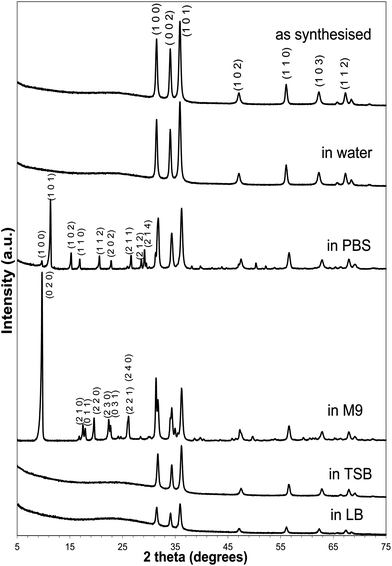 | ||
| Fig. 2 XRD patterns of zinc oxide thin films (from top to bottom): as synthesised, and after 1 week incubation in Milli-Q water, PBS, M9 media, TSB, and LB. | ||
Changes in morphology and chemical composition of ZnO thin film after 1 week incubation in the different media
The thin films were tested for their reactivity in Milli-Q water, Phosphate-Buffered Saline solution (PBS), M9 Minimal Salts Media (M9), Tryptic Soy Broth (TSB), and Luria-Bertani broth (LB) for 1 week. Post-exposure crystalline phases were identified using XRD, with morphology investigated using SEM and elemental composition analysed using energy-dispersive spectroscopy (EDS). Fig. 1, 2 and 3 show a collation of results obtained from tests of zinc oxide thin films after 1 week incubation in the different media.After 1 week incubation in Milli-Q water, zinc oxide coatings displayed no-observable structural change (SEM analysis, Fig. 1(c)) with undetected changes in chemical composition (XRD analysis, Fig. 2) compared to that of the as-prepared ZnO coating. However, according to the literature, the following reactions most likely occur:35,36
1. Hydrolysis of ZnO in water to form Zn(OH)2 on the surface of the coating (eqn (1)). Zn(OH)2 is postulated to have comparable physical morphology as that of ZnO, which is not contradictory to the non-observable structural change of the coating. The absence of crystalline Zn(OH)2 peaks in the XRD spectrum indicates formation of amorphous Zn(OH)2 or alternatively, the quantity of Zn(OH)2 formed was below the detection limit of the XRD.
| ZnO(s) + H2O ↔ Zn(OH)2(s) | (1) |
2. Release of Zn2+ species due to dissociation of Zn(OH)2 (eqn (2)), as detected by ICP-OES (Fig. 4).
| Zn(OH)2(s) ↔ Zn2+(aq) + 2OH−(aq) | (2) |
 | ||
| Fig. 4 The concentration of zinc in supernatant over time for media with and without complex organic components. | ||
Fig. 1(d) shows the SEM image of the ZnO thin film after 1 week incubation in phosphate buffered saline solution. The thin film morphology has been significantly altered from uniform spherical structure to hexagonal bipyramids of non-uniform sizes and smooth facets. EDS analysis revealed detection of Zn, P, O, Na, and minor quantity of K on the surface of the thin film. In agreement, the XRD spectrum showed formation of new peaks at 2θ values of 9.75, 11.39, 15.29, 16.93, 20.66, 22.89, 26.67, 28.61, and 29.20°, which can be matched to the (100), (101), (102), (110), (112), (202), (211), (212), and (114) crystal planes of sodium zinc phosphate hydrate (NaZnPO4·H2O), respectively (JCPDF 01-089-6669). The results indicate that the newly-formed hexagonal bipyramid structure is a crystalline sodium zinc phosphate hydrate. Here, the formation of sodium zinc phosphate hydrate is also confirmed by the observed reduction in aqueous phosphate concentration (Fig. 5). Upon incubation of the ZnO thin film in PBS solution, aqueous phosphate ions could complex with the released Zn2+ (see eqn (2)); forming zinc–phosphate complexes.37 Reaction of zinc–phosphate complexes with sodium ions in PBS solution was proposed to result in the precipitation of a sodium salt of the phosphato–hydroxo–zincate ion, which would reduce the concentration of dissolved zinc in the media. The diffraction pattern did not contain unidentified peaks, indicating the absence of unknown phases. In addition, since the XRD spectrum still displayed the diffraction peaks of the hexagonal wurtzite structure of ZnO even after 1 week incubation in PBS, it could be inferred that ZnO was still present in the coating and sodium zinc phosphate hydrate was formed on top of the ZnO thin film. The intensity of the peaks related to ZnO appeared to decrease following incubation in PBS solution, when compared to the as-synthesised samples or samples that were incubated in Milli-Q water.
Li et al.34 reported that overnight incubation of ZnO nanoparticles (NPs) in PBS solution resulted in microstructural transformation of ZnO to form zinc phosphate (Zn3(PO4)2). In the current work, the incubation period was much longer (1 week), which promotes the precipitation of sodium zinc phosphate hydrate. Another similar work by Lv et al.30 reported the formation of Zn3(PO4)2·4H2O following incubation of ZnO NPs in 10 mM NaNO3 solution spiked with varying concentrations of K2HPO4 from 3 hours to up to 15 days. In their work, aqueous phosphate ions was thought to adsorb on the hydroxylated ZnO surface (Zn(OH)2) via exchange with OH−, forming zinc phosphate layer. In contrast to our work, no formation of sodium zinc phosphate hydrate was reported in the study of Lv et al.,30 which could be due to the much lower sodium concentration used in their study.
Interestingly, different morphological transformation of the ZnO thin film was observed following one week incubation in M9 minimal medium with the surface of the film covered with clusters of slab-like crystals (Fig. 1(e)). XRD pattern of the post-incubated thin film showed appearance of several new peaks at 2θ = 5–30°, which can be closely matched to magnesium zinc phosphate hydrate (H8Mg0.62O12P2Zn2.38, JCPDF 01-089-1562). Indeed, the formation of this compound was further supported by detection of magnesium on the surface of the coating through EDS, along with Zn, P, O, Na, and trace amount of K. The detected magnesium-containing insoluble zinc compound infers reactivity of the released Zn2+ to the magnesium ions present in the culture media.
After 1 week incubation in the peptide-rich TSB, there is no obvious structural transformation of the thin film, apart from an increase in roughness (Fig. 1(f)). From the EDS data, in addition to Zn, P, O, and Na, trace amount of K and Ca were detected, as a result of presence of the Ca-containing casein peptone in TSB. The intensity of the XRD peaks of ZnO were significantly decreased compared to the as-synthesised sample or ZnO sample that was incubated in Milli-Q water, and no extra peak apart from ZnO was detected. Taken together, it appears that there was adsorption of Na+, K+, Ca2+ as well as phosphate ions from the medium onto the hydroxylated surface of ZnO, without the formation of new zinc compounds. The decrease in the intensity of the ZnO diffraction peaks is most likely due to leaching of zinc into the culture medium, as further shown in Fig. 4.
Similar to the TSB, incubation of the thin film for 1 week in the peptide-rich LB medium resulted in decrease of the intensity of the ZnO diffraction peaks due to zinc leaching (Fig. 4), with no detection of extra peaks. From the SEM image (Fig. 1(g)), there was no-observable change in the morphology of the thin film. EDS data showed that besides Zn, P, O, and Na, trace amount of Ca was detected, which indicates the adsorption of phosphate, Na+ and Ca2+ on the surface of the thin film.
Kinetics of leaching/dissolution of zinc oxide coating in the different media
Fig. 4 shows the detected dissolved zinc species corresponding to ZnO thin film incubated in the different media over 24 h period. Incubation of the thin film in Milli-Q water for 0.5 h saw detection of only 2.4 mg L−1 soluble zinc (∼0.2% of total zinc content of the coating). Under the neutral pH condition, the predominant soluble zinc species is free Zn2+, which is consistent with the described two-step reactions for the release of Zn2+ from the thin film in water (eqn (1) and (2)).34 After 1 h, the dissolved zinc concentration increased to 5 mg L−1 (0.4% of total zinc content of the coating), and then decreased over time to less than 1 mg L−1 at 24 h. The latter decrease in the dissolved zinc concentration is most likely due to the reprecipitation of Zn2+ as zinc hydroxide or adsorption of Zn2+ on the surface of the coating.38 In comparison to leaching in Milli-Q water, a lower soluble zinc concentration was observed for the incubation of ZnO thin film in PBS. Over 24 h period, decrease of soluble zinc from 0.7 to 0.3 mg L−1 was observed. The detected low level of soluble zinc in PBS corroborates with the observed formation of sodium zinc phosphate hydrate solids covering the thin film (Fig. 1(d) and 2), which as previously mentioned, was most likely due to the reprecipitation of Zn2+ ions with phosphate. Indeed, we also detected corresponding reduction in the phosphate concentration, as shown in Fig. 5. In addition, phosphate ions may also adsorb on the surface of the thin film to inhibit zinc dissolution.34Incubation of the ZnO thin film coating in M9 minimal medium saw detection of 2.1 mg L−1 soluble zinc (∼0.2% of total zinc content of the coating) after 0.5 h, then decreased to 0.9 mg L−1 at 24 h. Along with the detected reduction in the phosphate concentration (Fig. 5), the decrease in soluble zinc concentration is at least in part attributed to the observed formation of magnesium zinc phosphate hydrate solids (Fig. 1(e), Fig. 2). Despite the earlier described Zn2+-phosphate reprecipitation reaction to form the latter, the concentration of soluble zinc in the M9 medium was still higher when compared to thin film sample incubated in PBS. Unlike PBS, M9 medium contains sulfates and glucose. Sulfates may serve as Zn2+ binding ligands to form complex ions of Zn (ZnSO40) and in turn, increase the release of soluble zinc from ZnO coating.39 Organic matters such as glucose may also enhance zinc dissolution by providing chelating agents for Zn2+ ions.39
Presence of complex organic, such as those comprising the TSB and LB media significantly affected the extent of zinc dissolution of the ZnO coating. Over the 24 h period, 10- to 30-fold higher concentrations of soluble zinc were detected in the TSB and LB media, relative to those not containing complex organic (M9 minimal medium, PBS and Milli-Q water). Rapid leaching of zinc in the TSB and LB media was indicated with detection of higher than 30 mg L−1 dissolved zinc after just 0.5 h of incubation. At 24 h, 91 and 100 mg L−1 dissolved zinc were detected in the TSB and LB media respectively, which correspond to 7% and 8% extent of leaching (relative to total zinc content in the coating). The presence of the amino acid-rich tryptone and yeast extract in TSB and LB media has been known to result in complexation-assisted leaching of zinc from ZnO.34,40 A soft Lewis acid, zinc has been known to have high affinity for donor group of the amino acids Cys (–(S−), pKa = 8.18) and His (−(NH+), pKa = 6).41 The majority of the dissolved zinc species in TSB and LB media exist as zinc–peptide complexes.34 Locked in complexes, the soluble zinc has lower tendency to react with phosphate ions present in the media, which is in agreement to the insignificant reduction in the phosphate concentration observed in LB medium (Fig. 5). Recalling the non-observable changes in the morphology and chemical composition of the ZnO coating, it would be reasonable to deduce that the complexation-mediated leaching of zinc hinders the formation of insoluble zinc phosphate compounds in amino acid-rich media, as opposed to those observed in the phosphate-containing inorganic media. It remains unclear however at this stage, the cause for the decrease in phosphate concentration in TSB medium.
Implications and repercussions for future ZnO toxicity studies
This work provides insights on the medium-dependent speciation of zinc oxide nanoparticles (ZnO NPs), which gives rise to the complex and multiple origins of zinc oxide cytotoxicity to microorganisms. Most media for antimicrobial testing of ZnO NPs contains NaCl, phosphate anions and complex organic components, which are essential for cell growth and pH buffering. Presented in the current work, interactions of ZnO NPs (matrices of NPs forming a thin film coating) with the cell culture media involve (1) reactions and/or adsorption of ions from the medium with the hydroxylated surface of ZnO, (2) complexation-mediated leaching of zinc, and (3) reprecipitation of the leached Zn2+ with anions (PO43−) and/or cations (Mg2+, Na+) in the medium. The interactions result in the formation of various soluble and insoluble zinc compounds, depending on the type of suspending medium. Therefore, it is important to comprehensively assess the zinc speciation as well as the resulting changes to the ZnO NPs for a thorough understanding of the observed cytotoxicity effects.As dissolution of ZnO is one of the main contributors to the toxicity of ZnO NPs, it is critical to assess the dissolution of ZnO in the culture media. The result from this study indicated that high variability of ZnO dissolution can be expected, depending on the matrix in which they are mixed. Low ZnO dissolution was observed in aqueous media without complex organic component. Much higher dissolution of ZnO was detected in media with complex organic component (such as LB and TSB), which indicates complexation-assisted leaching of ZnO, particularly through the formation of organic zinc complexes. In non-organic media, such as Milli-Q water and PBS, most of the dissolved zinc exists as free zinc ions or zinc phosphate complexes.
It is vital that dissolution profile of ZnO in the test medium is investigated and understood before performing any antibacterial testing of ZnO nanoparticles. Since ZnO can be almost completely dissolved in the medium instantaneously,34,42 no actual exposure of ZnO to the bacteria occur and therefore Zn2+ ions were the only species contributing to the observed toxicity effect. In addition, the uptake path mechanism of ions in organisms will be different from ZnO particles. Ionic zinc can enter cells via zinc transmembrane receptors and as bound elements to proteins, while nanoparticles require some form of endocytosis to cross the cellular membrane.43
The current work observed formation/reprecipitation of numerous solid zinc species in high phosphate and non-peptide containing media, which even results in the morphological transformation of ZnO solids. Instead of only ZnO, the organisms or cells will ‘see’ and interact with the various soluble and insoluble zinc compounds. In any given culture medium, it is therefore crucial that the ZnO particles are subjected to chemical composition analysis following the antimicrobial exposure so as to confirm or rule out potential occurrence of new zinc compounds. This has implications on the mechanistic elucidation and interpretation of the origins of zinc toxicity. In the light of the current work, it would be logical to claim that the fundamental elucidation of ZnO toxicity in high phosphate and non-peptide containing media has been over-simplified, that is only two components are realised as the source of toxicity, i.e. the leached zinc and the undissolved ZnO solids. The present work provides evidence of formation of new solid zinc species (for examples, sodium zinc phosphate hydrate and magnesium zinc phosphate hydrate as a result of incubation in PBS and M9 minimal media, respectively) with different shape/morphology/size compared to the ZnO particles. The compositionally- and morphologically-different newly-formed zinc compounds are most likely to cause distinct cellular physiological responses and cytotoxicity compared to the ones inflicted by the original ZnO solids although to date, no investigation has been carried out into the antimicrobial action of sodium and magnesium zinc phosphate hydrate. Physical structure and particle morphology can be a determinant factor to the observed cytotoxicity of nanoparticles, since it may significantly affect their interactions with cell membranes and/or their ability to penetrate into cells and organisms. So far, to the best of our knowledge, no data are available in the literature concerning the effect of hexagonal bipyramid structure or slab like morphology on the ZnO toxicity to cells. However, it was found that rod- or wire-shaped ZnO NPs (one-dimensional structures) could be more toxic than spherical NPs.44 Nanomaterials with one-dimensional structures were indicated to enter the cell nucleus.45 Particles with uneven and rough surface morphology and irregular shapes with corners and edges are more active from a chemical and biological perspective, because edge and corner atoms have weaker bonds to bulk atoms, and therefore bind to foreign atoms and molecules more readily. Size of nanoparticles can also influence their antimicrobial properties, due to the surface area available for interaction with bacteria, and dissolution of particles. A clear example is given by Jones et al.26 with nano (8 nm, 50–70 nm) and micron (>1 μm) formulations of ZnO. The nano-sized ZnO displayed higher extent of growth inhibiting effects on S. aureus compared to that of the micron sized particles. Also confirmed by another study, exposure of S. aureus and E. coli to nano ZnO (7 nm) resulted in increased cellular internalisation of the particles and correspondingly, greater extent of cell death as compared to larger particles (260 and 800 nm).8 The zinc reprecipitation to form particles of different shape/morphology/size can complicate the interpretation of cytotoxicity effect of ZnO towards microorganisms.
Upon exposure to peptide-containing organic media (LB and TSB), the non-observable or minimal changes on the morphology and chemical composition of the zinc solids infers valid interpretation of the cytotoxicity origins from the leached soluble zinc and the undissolved ZnO solids. In those media, the leached zinc exists mostly as zinc–peptide complexes, passivating the tendency to form insoluble zinc solids in the presence of phosphate.
In many studies, a simple zinc salt system (e.g. ZnCl2, or ZnSO4) containing equimolar concentration of zinc has been employed to simulate the activity of leached soluble zinc from ZnO NPs. The approach is even used to simulate the activity of the overall presence of ZnO NPs, or in other words the leached zinc and the undissolved ZnO solids altogether. If there were comparable toxicity effects from the ZnO nanoparticle and the zinc salt at the same Zn2+ concentration as the soluble Zn detected from ZnO dissolution, it could be concluded that soluble zinc plays a dominant role in ZnO toxicity. As elucidated in the current study however, presence of zinc ions in growth media without complex organic (peptide), which usually contains phosphate anions, may result in the formation of insoluble zinc species, such as zinc phosphate. The re-precipitation effects are enhanced in culture media without complex organic (peptide), in which the ZnO NPs-derived soluble zinc species are not locked in complexes, with significant fraction existing as free zinc ions.19,34 Such unintended formation of insoluble zinc species will lower not only the NPs-leached Zn2+ concentration, but also the dosed Zn2+ concentration from zinc salt. This will add an additional layer of complexity to the evaluation of the relative toxicity of ZnO nanoparticles compared to that of the zinc salt control. In such cases, the “soluble zinc” system is in fact containing soluble species with lower Zn2+ concentration than initially dosed and zinc containing particulates. Although the initial forms of zinc in the culture medium were well-defined (i.e. Zn2+ and Cl− for ZnCl2, or Zn2+ and SO42− for ZnSO4), the chemical transformations of zinc species must be considered when interpreting bacterial inactivation results.
Conclusions
In conclusion, the work investigates the changes in zinc oxide thin films after incubation in commonly used aqueous media, more specifically in identifying the formation of new compounds as a result of reaction with components in the media and comparing the dissolution of ZnO in the different media. Common bacterial growth media contain many components, which can influence speciation and dissolution of ZnO, and ultimately, the toxicity of ZnO. The dissolution test results revealed that the amount of soluble zinc was controlled by water chemistry. Complex organic components in media, such as amino acids and peptides can form complexes with zinc oxide coatings, resulting in complexation-mediated leaching of ZnO. Zinc oxide reacts with components in media without complex organic, forming precipitates containing phosphates, sodium or magnesium. The formation of precipitates besides ZnO in media without complex organic calls for reconsideration and re-interpretation of past literature on ZnO toxicity using this media. Considering the dramatic difference of the speciation of ZnO in various aqueous media, future nanotoxicity evaluations should pay more attention on the dynamic transformations of ZnO to be able to properly interpret its toxicity mechanisms. Elucidation of origins of toxicity of ZnO will lead to better assessment of its impact to the environment and human exposure.Acknowledgements
This research was supported under Australian Research Council's Linkage Projects funding scheme (LP110100459). The views expressed herein are those of the authors and are not necessarily those of the Australian Research Council. We also acknowledge funding from Water Corporation and in-kind contribution from Australian Water Quality Centre.Notes and references
- A. J. Huh and Y. J. Kwon, J. Controlled Release, 2011, 156, 128–145 CrossRef CAS PubMed.
- Q. Li, S. Mahendra, D. Y. Lyon, L. Brunet, M. V. Liga, D. Li and P. J. J. Alvarez, Water Res., 2008, 42, 4591–4602 CrossRef CAS PubMed.
- W. Jiang, H. Mashayekhi and B. Xing, Environ. Pollut., 2009, 157, 1619–1625 CrossRef CAS PubMed.
- K. Memarzadeh, M. Vargas, J. Huang, J. Fan and R. Allaker, Key Eng. Mater., 2012, 493, 489–494 Search PubMed.
- A. Kumar, A. K. Pandey, S. S. Singh, R. Shanker and A. Dhawan, Chemosphere, 2011, 83, 1124–1132 CrossRef CAS PubMed.
- J. T. Seil and T. J. Webster, Acta Biomater., 2011, 7, 2579–2584 CrossRef CAS PubMed.
- O. Yamamoto, Int. J. Inorg. Mater., 2001, 3, 643–646 CrossRef CAS.
- G. Applerot, A. Lipovsky, R. Dror, N. Perkas, Y. Nitzan, R. Lubart and A. Gedanken, Adv. Funct. Mater., 2009, 19, 842–852 CrossRef CAS.
- R. Brayner, R. Ferrari-Iliou, N. Brivois, S. Djediat, M. Benedetti and F. Fiévet, Nano Lett., 2006, 6, 866–870 CrossRef CAS PubMed.
- A. Moezzi, A. M. McDonagh and M. B. Cortie, Chem. Eng. J., 2012, 185, 1–22 CrossRef PubMed.
- G. Colon, B. C. Ward and T. J. Webster, J. Biomed. Mater. Res., Part A, 2006, 78, 595–604 CrossRef PubMed.
- S. H. S. Chan, T. Yeong Wu, J. C. Juan and C. Y. Teh, J. Chem. Technol. Biotechnol., 2011, 86, 1130–1158 CrossRef CAS.
- C. Karunakaran, V. Rajeswari and P. Gomathisankar, J. Alloys Compd., 2010, 508, 587–591 CrossRef CAS PubMed.
- Y. Li, W. Zhang, J. Niu and Y. Chen, ACS Nano, 2012, 6, 5164–5173 CrossRef CAS PubMed.
- R. Gopikrishnan, K. Zhang, P. Ravichandran, S. Biradar, V. Ramesh, V. Goornavar, R. B. Jeffers, A. Pradhan, J. C. Hall and S. Baluchamy, J. Mater. Sci.: Mater. Med., 2011, 22, 2301–2309 CrossRef CAS PubMed.
- Y. Xie, Y. He, P. L. Irwin, T. Jin and X. Shi, Appl. Environ. Microbiol., 2011, 77, 2325–2331 CrossRef CAS PubMed.
- D. Sharma, S. Sharma, B. Kaith, J. Rajput and M. Kaur, Appl. Surf. Sci., 2011, 257, 9661–9672 CrossRef CAS PubMed.
- R. Wahab, A. Mishra, S.-I. Yun, Y.-S. Kim and H.-S. Shin, Appl. Microbiol. Biotechnol., 2010, 87, 1917–1925 CrossRef CAS PubMed.
- N. M. Franklin, N. J. Rogers, S. C. Apte, G. E. Batley, G. E. Gadd and P. S. Casey, Environ. Sci. Technol., 2007, 41, 8484–8490 CrossRef CAS.
- A. Kumar, A. K. Pandey, S. S. Singh, R. Shanker and A. Dhawan, Free Radical Biol. Med., 2011, 51, 1872–1881 CrossRef CAS PubMed.
- R. Dutta, B. P. Nenavathu, M. K. Gangishetty and A. Reddy, Colloids Surf., B, 2012, 94, 143–150 CrossRef CAS PubMed.
- K. Ghule, A. V. Ghule, B. J. Chen and Y. C. Ling, Green Chem., 2006, 8, 1034–1041 RSC.
- S. D. Gittard, J. R. Perfect, N. A. Monteiro-Riviere, W. Wei, C. Jin and R. J. Narayan, Appl. Surf. Sci., 2009, 255, 5806–5811 CrossRef CAS PubMed.
- M. Gondal, M. Dastageer, A. Khalil, K. Hayat and Z. Yamani, J. Nanopart. Res., 2011, 13, 3423–3430 CrossRef CAS.
- M. A. Ansari, H. M. Khan, A. A. Khan, A. Sultan and A. Azam, World J. Microbiol. Biotechnol., 2012, 1–9 Search PubMed.
- N. Jones, B. Ray, K. T. Ranjit and A. C. Manna, FEMS Microbiol. Lett., 2008, 279, 71–76 CrossRef CAS PubMed.
- P. Sivakumar, S. Balaji, V. Prabhawathi, R. Neelakandan, P. Manoharan and M. Doble, Carbohydr. Polym., 2010, 79, 717–723 CrossRef CAS PubMed.
- C. Jayaseelan, A. A. Rahuman, A. V. Kirthi, S. Marimuthu, T. Santhoshkumar, A. Bagavan, K. Gaurav, L. Karthik and K. Rao, Spectrochim. Acta, Part A, 2012, 90, 78–84 CrossRef CAS PubMed.
- B. Boonchom, R. Baitahe, S. Kongtaweelert and N. Vittayakorn, Ind. Eng. Chem. Res., 2010, 49, 3571–3576 CrossRef CAS.
- J. Lv, S. Zhang, L. Luo, W. Han, J. Zhang, K. Yang and P. Christie, Environ. Sci. Technol., 2012, 46, 7215–7221 CrossRef CAS PubMed.
- A. Goux, T. Pauporté, J. Chivot and D. Lincot, Electrochim. Acta, 2005, 50, 2239–2248 CrossRef CAS PubMed.
- A. Zirino and S. Yamamoto, Limnol. Oceanogr., 1972, 661–671 CrossRef CAS.
- I. A. Mudunkotuwa, T. Rupasinghe, C. M. Wu and V. H. Grassian, Langmuir, 2011, 28, 396–403 CrossRef PubMed.
- M. Li, L. Zhu and D. Lin, Environ. Sci. Technol., 2011, 45, 1977–1983 CrossRef CAS PubMed.
- J. Han, W. Qiu and W. Gao, J. Hazard. Mater., 2010, 178, 115–122 CrossRef CAS PubMed.
- A. Degen and M. Kosec, J. Eur. Ceram. Soc., 2000, 20, 667–673 CrossRef CAS.
- S. Ziemniak, M. Jones and K. Combs, J. Solution Chem., 1992, 21, 1153–1176 CrossRef CAS.
- S. Mustafa, P. Shahida, A. Naeem, B. Dilara and N. Rehana, Langmuir, 2002, 18, 2254–2259 CrossRef CAS.
- H. Ma, P. L. Williams and S. A. Diamond, Environ. Pollut., 2013, 172, 76–85 CrossRef CAS PubMed.
- C. Gunawan, W. Y. Teoh, Ricardo, C. P. Marquis and R. Amal, Part. Part. Syst. Charact., 2013, 30, 375–380 CrossRef CAS.
- S. J. Lippard and J. M. Berg, Principles of Bioinorganic Chemistry, University Science Books, Mill Valley, CA, 1994 Search PubMed.
- R. B. Reed, D. A. Ladner, C. P. Higgins, P. Westerhoff and J. F. Ranville, Environ. Toxicol. Chem., 2012, 31, 93–99 CrossRef CAS PubMed.
- T. W. Turney, M. B. Duriska, V. N. Jayaratne, A. Elbaz, S. J. O'Keefe, A. S. Hastings, T. J. Piva, P. Wright and B. N. Feltis, Chem. Res. Toxicol., 2012, 25, 2057–2066 CrossRef CAS PubMed.
- X. Peng, S. Palma, N. S. Fisher and S. S. Wong, Aquat. Toxicol., 2011, 102, 186–196 CrossRef CAS PubMed.
- H. Yang, C. Liu, D. Yang, H. Zhang and Z. Xi, J. Appl. Toxicol., 2008, 29, 69–78 CrossRef PubMed.
Footnote |
| † S. L. and E. K. contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2014 |



