Preparation and sensing properties of hierarchical 3D assembled porous ZnO from zinc hydroxide carbonate
Zhidong Lin*a,
Fei Guoa,
Chen Wanga,
Xuehua Wanga,
Ke Wanga and
Yang Qub
aProvincial Key Laboratory of Plasma Chemistry & Advanced Materials, Wuhan Institute of Technology, Wuhan, P. R. China. Tel: +86 27 87195661E-mail: zhidong.lin@126.com
bBiochemical Institute, Department of Applied Chemistry, College of Science, Huazhong Agricultural University, Wuhan, P. R. China
First published on 5th November 2013
Abstract
The precursor hydrozincite was synthesized through hydrothermal reaction and hierarchically assembled 3D porous ZnO was prepared by calcination of the precursor at 400 °C for 15 min. The 3D porous ZnO was composed of micrometer spheres which were assembled from nanoflakes. The sensor based on 3D porous ZnO showed improved ethanol response compared to 2D porous ZnO nanoplates. The highest sensitivity of 77.5, 9.8 and 49.7, respectively, for the sensor based on 3D porous ZnO-2 were obtained for 100 ppm acetone, benzene and ethanol at the operation temperature of 420 °C, 420 °C and 340 °C, respectively. The sensor also show selectivity to acetone at the operation temperature of 420 °C. The highest sensitivity of the sensor based on ZnO-3 was of 59.9 to 100 ppm ethanol, the response increased linearly with the concentration of ethanol in the range of 10–1000 ppm. All the response and recovery times of the sensors were less than 20 s. The good sensing performance of the 3D porous ZnO sensor indicated that the hierarchically assembled 3D porous ZnO could be a promising candidate for highly sensitive gas sensors.
1. Introduction
Zinc oxide (ZnO), as one of the most important wide-band gap semiconductors, has been widely used for its non-toxicity and low-cost, which has a direct wide band gap (3.37 eV) and a strong exciton binding energy (60 meV) at room temperature. For its special electrical, optical and catalytic properties, ZnO provides extensive possible applications in sensors, piezoelectric and photoelectric devices, solar cells, light emitting diodes and catalysis.1–3 Nano-scale porous ZnO with high surface area can enhance physicochemical and electrochemical properties of certain applications such as gas sensors,4,5 photocatalysts,6,7 lithium-ion batteries and dye-sensitized solar cell photoelectrodes.8 Various ZnO morphologies, such as 1D rods, wires, tubes, needles, 2D belts and sheets, 3D brushes, flowers,9–14 and hollow spherical architectures,15–17 have been prepared through various routes including metal catalyzed growth, chemical vapor deposition (CVD), precipitation, sol–gel, microemulsion, hydrothermal and so on.In recent years, three dimensionally (3D) assembled micro/nano porous structures with high porosity and surface area, have attracted enormous attention due to their unique properties. The hierarchical 3D assembled porous structures are of key importance to some specific applications like sensors,18–20 lithium-ion batteries,21 photo-catalysis and solar cells22–24 for their enhanced properties originating from their high porosity and surface area which promotes molecular transport through the pores. Compared with other nanostructures, hierarchically porous materials are promising candidates because their special structures can usually provide a large surface-to-volume ratio that can greatly facilitates gas diffusion and mass transport in sensor materials, thus improving the sensitivity and response time of the gas sensor.25,26
Hierarchical porous ZnO micro/nano-structures were prepared by solution based chemical methods in combination with a calcination step.27–29 Different synthetic approaches have been developed for 3D assembled porous ZnO structures through a zinc carbonate or hydrozincite intermediate by heating the solution containing a soluble zinc precursor, urea/ammonium carbonate and a different surfactant.17–21 While the zinc carbonate or hydrozincite intermediates form porous ZnO during the calcination, a large amount of CO2 and H2O are generated which creates a reasonable amount of pores in the structure. Therefore, it is of great significance to develop a simple, economical and time efficient method for 3D assembled ZnO porous structures.30
In this paper, we report a simple hydrothermal treatment method combining with a calcination process for the synthesis of hierarchically assembled porous ZnO structures. The precursor hydrozincite [Zn5(CO3)2(OH)6] 3D spheres assembled from nanoflakes were prepared from zinc chloride and hexamethylenetetramine without any surfactant. An identical morphology of ZnO is obtained by annealing the precursor at 400 °C for 15 min in air atmosphere. The gas sensing properties of 3D spherical porous ZnO were studied. The results show that hierarchically 3D porous ZnO spheres would be a promising material for preparing devices with good gas sensing properties. We have also synthesized ZnO with different morphologies by changing the hydrothermal treatment time. The fabrication of hierarchical porous ZnO micro/nano-structures enables greater control of the local chemical environment, with potential applications in gas sensors, sorbents, carriers, and other fields.
2. Experimental
2.1. Materials synthesis
The chemical reagents including zinc chloride and hexamethylenetetramine were of analytical grade and used without further purification. All the chemical reagents were purchased from Shanghai Chemical Reagent Co. Ltd.In a typical synthesis, 30 mL of 0.2 M zinc chloride solution was added into 30 mL of 0.2 M hexamethylenetetramine solution. The mixture was transferred into a Teflon-lined stainless steel autoclave with a capacity of 100 mL. The autoclave was sealed and kept in an oven at a constant temperature of 180 °C for 6 h. Subsequently, the autoclave was taken out and allowed to cool to room temperature naturally. The precipitate was collected by centrifugation and washed several times with deionized water, and dried at 80 °C for 12 h in air. The precursor hydrozincite was obtained. Then, the hydrozincite powders were annealed in a furnace at 400 °C for 15 min in air to form hierarchical porous ZnO micro/nano-structures. To study the effect of hydrothermal reaction time, we obtained three samples with different times. The precursor-1, precursor-2 and precursor-3 were prepared by 2 h, 6 h and 16 h hydrothermal time, respectively. The corresponding annealed samples were named as ZnO-1, ZnO-2, ZnO-3 respectively. The phase composition and morphology of the material particles were investigated by X-ray diffraction (XRD) and scanning electron microscopy (SEM), respectively.
The gas sensor is fabricated as a side heated structure, the paste prepared from hierarchical porous ZnO ethanol solution was coated onto an Al2O3 tube (4 mm in length, 1.2 mm in external diameter and 0.8 mm in internal diameter) on which Au electrodes and Pt wires had been fixed at both ends. Finally, a heater of Ni–Cr wire with resistance of about 35 Ω was inserted into the Al2O3 tube to control the operating temperature within the range of 80–500 °C. The gas sensors were aged at 350 °C for 24 hours in order to improve stability and repeatability.
2.2. Characterization
XRD data were collected in the 2θ region of 6–80° with a scan rate of 4° min−1 on a Bruker D8 Advance diffractometer with theta–theta geometry and Cu Kα1 radiation (λ = 0.154056 nm). N2 sorption isotherms were measured with a NOVA 2000e at 77 K. Before the measurements, all samples were degassed at 120 °C under vacuum for 12 h. The pore volumes and pore size distributions were derived from the adsorption branches of the isotherms using the Barrett–Joyner–Halenda (BJH) model. The specific surface areas Sp (m2 g−1) were estimated via the BET method. Scanning electron micrographs were obtained using an emission SEM JEOL JSM-6460LV fitted with an energy dispersive spectrometer (EDS) for elemental analysis.Gas sensing properties were measured under room conditions (humid range 30–60%) using a static test system made by Henan Hanwei Electronics Co. Ltd which included a test chamber (about 18 L in volume) and a data acquisition–processing system. The operating temperature of a gas sensor was adjusted through varying the heating voltage, because each heating voltage has its corresponding operating temperature. Sensor response in this paper is defined as S = Ra/Rg, in which Ra and Rg are the resistance of the sensor in air and in tested gas, respectively. The response and recovery time were expressed as the time taken for the sensor output to reach 90% of its saturation after applying or switching off the gas in a step function.
3. Results and discussion
3.1. Structure and morphology
To determine the chemical composition of the prepared powders, we first presented XRD patterns of the samples prepared by hydrothermal reaction for 2 h, 6 h and 16 h as shown in Fig. 1(a) and (b). The obtained diffraction patterns of the sample prepared from 6 h and 16 h hydrothermal reaction can be indexed as monoclinic hydrozincite [Zn5(CO3)2(OH)6] (JCPDS 19-1458), in good agreement with the previously reported hydrozincite.31 The absence of any other additional peaks confirms the formation of the pure hydrozincite phase. The patterns of the sample prepared from 2 h was different, and can be indexed as zinc carbonate hydroxide hydrate [Zn4CO3(OH)6·H2O] (JCPDS 11-0287), and without other peaks. The hierarchically assembled 3D porous ZnO nanostructures were obtained by the calcination of hydrozincite. The XRD patterns of the products, ZnO-1, ZnO-2 and ZnO-3 obtained after the calcination of the synthesized [Zn4CO3(OH)6·H2O] or hydrozincites at 400 °C for 15 min (Fig. 1c), exhibit well resolved X-ray diffraction peaks , indexed to (100), (002), (101), (102), (110), (103), (200), (112), (201), (004) and (202) planes of the hexagonal structure of ZnO (JCPDS 36-1451). In the XRD pattern, no peak for precursor was identified, confirming that the phase of the precursor was fully decomposed to ZnO. The average crystallite sizes of the precursors and ZnO samples after sintering calculated from X-ray line broadening using Scherrer's equation are listed in Table 1. Owing to the different hydrothermal reaction times, the precursors have different crystallites and the FTIR experiments again confirmed the formation of intermediate hydrozincite and Zn4CO3(OH)6·H2O. | ||
| Fig. 1 The XRD patterns of the precursors (a), (b) and ZnO samples after calcination (c). The standard diffraction patterns are shown at the bottom as references. | ||
| Reaction time (h) | Precursor type | Precursor crystallite size (nm) | SBET of precursor (m2 g−1) | ZnO crystallite size (nm) | SBET of ZnO (m2 g−1) | Morphology of ZnO |
|---|---|---|---|---|---|---|
| 2 | Zinc carbonate hydroxide hydrate [Zn4CO3(OH)6·H2O] | 16.8 | 19.6 | 16.6 | 38.4 | Particle |
| 6 | Hydrozincite [Zn5(CO3)2(OH)6] | 16.5 | 25.3 | 16.9 | 35.8 | 3D nanoflake |
| 16 | Hydrozincite [Zn5(CO3)2(OH)6] | 22.2 | 18.6 | 18.3 | 29.3 | 3D nanoflake |
The morphology of the precursors and calcined ZnO were determined by SEM. The Zn4CO3(OH)6·H2O obtained by hydrothermal reaction for 2 h formed nanoflakes less than 200 nm in size, shown in Fig. 2a, the hexagonal ZnO particles formed after sintering 15 min at 400 °C (shown in Fig. 2b). Fig. 2(c) and (e) display the SEM images of the Zn5(CO3)2(OH)6 precursors prepared by hydrothermal reaction for 6 h and 16 h, which show that the products are composed of flake shapes. Upon calcination the hydrozincite decomposed to ZnO, the morphologies of the resultant ZnO were retained, shown as Fig. 2(d) and (f). The SEM image of ZnO-3 (Fig. 2f) confirms that the presence of a porous flake structure that is almost identical to the parental hydrozincite. However, the smooth surface of the flakes transformed to a porous structure after calcination and the pores were generated in the flakes due to the evolution of CO2 and H2O.28
The low-magnification SEM images (Fig. 2(d) and (f)) show that the morphology of the ZnO was 3D spheres assembled by 2D flakes. Closer inspection reveals that finer nanoflakes are assembled within the same layer towards 2D ZnO flakes. The thickness of the individual flakes is about 20 nm, corresponding to the average crystallite size.
The following are the reactions involved during the synthesis of Zn4CO3(OH)6·H2O, hydrozincite and ZnO microspheres:30,32–34
 | (1) |
| NH3 + H2O → NH4+ + OH− | (2) |
 | (3) |
| 4Zn2+ + CO2 + 6OH− + 2H2O → Zn4CO3(OH)6·H2O + 2H+ | (4) |
| 5Zn2+ + 2CO2 + 6OH− + 2H2O → Zn5(CO3)2(OH)6 + 4H+ | (5) |
| Zn4CO3(OH)6·H2O → 4ZnO + CO2 + 4H2O | (6) |
| Zn5(CO3)2(OH)6 → 5ZnO + 2CO2 + 3H2O | (7) |
As indicated by reactions 1 and 3, firstly, hexamethylenetetramine is decomposed to ammonia and formaldehyde at low temperature, about 70 °C (eqn (1)), and when hydrothermal temperature exceeds 150 °C, hexamine should be decomposed as eqn (3) to form carbonate. The dissociation of hexamethylenetetramine generates hydroxide and carbonate, which react with zinc ions to form the precursor Zn4CO3(OH)6·H2O or hydrozincite (eqn (4) and (5)). Finally, after calcination in air at 400 °C (eqn (6) and (7)) for 15 min, porous ZnO is obtained. From the XRD and SEM results, it is evident that the formation of assembled hydrozincite flakes needs more than 6 h hydrothermal reaction. The reason is unclear at present.
The precursors were analyzed by FTIR spectroscopy. Fig. 3 shows FTIR spectra of precursors obtained by different hydrothermal times in the range of 400–4000 cm−1 at room temperature. The three spectra are very similar though the precursor-1 obtained by 2 h hydrothermal time is Zn4CO3(OH)6·H2O not hydrozincite Zn5(CO3)2(OH)6. This should be ascribed to the same chemical bond in the two precursors except the crystalline water. Closer inspection of the broad band in the range of 3200–3500 cm−1 reveals that hydrozincite has three split peaks but Zn4CO3(OH)6·H2O has one symmetrical peak. The three split peaks at 3367, 3310, 3240 cm−1 of precursor-2 and 3376, 3301, 3237 cm−1 of precursor-3 are attributed to the structured OH groups in hydrozincite. It means that the stretching vibrations of the structural OH groups in hydrozincite should show three types of interactions in chemical bonding due to the different hydrogen bond acceptor strengths of the carbonate oxygen atoms.35 The two peaks at 1500, 1394 cm−1 are ascribed to the asymmetric CO32− stretching ν3 mode. The peak at 1049 cm−1 is assigned to the ν1 symmetric CO32− stretching mode. The strong and sharp peak at 835 cm−1 is assigned to the ν2 out-of-plane OCO bending mode and that at 709 cm−1 is assigned to the ν4 asymmetric OCO bending mode.34,36 The carbonate groups in hydrozincite (Zn5(CO3)2(OH)6) and Zn4CO3(OH)6·H2O show three types of interaction in chemical bonding, two oxygen atoms of the carbonate group are bonded to an octahedral and a tetrahedral zinc atom each, whereas the third oxygen atom is hydrogen bonded to the OH groups.37 The peak in the range of 511–518 cm−1 belongs to the Zn–O vibration.
The complex structure of hydrozincite can be described in terms of coordination octahedra and tetrahedra around zinc. The octahedral to tetrahedral zinc ratio in the sheet structure is 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Zinc oxide crystallites are a hexagonal wurtzite structure with tetrahedral coordination of Zn2+ ions and O2−. During the thermal decomposition process from Zn5(CO3)2(OH)6 to ZnO, zinc octahedrons have to collapse and reorganize to form tetrahedrons. Release of water and carbon dioxide from the Zn5(CO3)2(OH)6 nanoflakes could generate holes and decrease flake thickness,38 in agreement with SEM observations and specific surface area and BJH pore size distribution analysis.
2. Zinc oxide crystallites are a hexagonal wurtzite structure with tetrahedral coordination of Zn2+ ions and O2−. During the thermal decomposition process from Zn5(CO3)2(OH)6 to ZnO, zinc octahedrons have to collapse and reorganize to form tetrahedrons. Release of water and carbon dioxide from the Zn5(CO3)2(OH)6 nanoflakes could generate holes and decrease flake thickness,38 in agreement with SEM observations and specific surface area and BJH pore size distribution analysis.
Thermo-gravimetric analysis is a technique by which one can measure the mass loss with respect to the temperature. The typical thermogravimetric (TG) and DTG curves of the precursor hydrozincite under ambient conditions under N2 flow are shown in Fig. 4. The single weight loss step, of 22% between 250 and 300 °C (DTG peak at 275 °C) was observed, which was assigned to the release of carbon dioxide and water from the thermal decomposition of the precursor. Apurba30 reported that hydrozincite has two weight loss steps. Firstly, the hydrozincite forms a Zn5(CO3)2 O3 intermediate by loss of three H2O molecules in the temperature range of 250–325 °C, and then decomposes completely to form ZnO in the range of 325–440 °C. The different TG and DTG curves between here and those reported by Apurba can be ascribed to the thickness and crystallite size of the hydrozincite flakes. The nano size hydrozincite should decompose at lower temperature. The decomposition temperature here is similar to Chen's report where the thickness of the hydrozincite nanoplates was about 19 nm.12
 | ||
| Fig. 4 TGA curves of precursor-2 hydrozincite and the corresponding first-order differential (DTG) of the TGA curve. | ||
To further confirm the inner architectures of ZnO and the precursors, nitrogen adsorption and desorption measurements were performed to estimate the texture properties. A typical nitrogen adsorption and desorption isotherm and the corresponding Barrett–Joyner–Halenda (BJH) pore size distribution plot (inset) of the porous ZnO-2 and precursor-2 hydrozincite (6 h hydrothermal reaction) are shown in Fig. 5. The N2 isotherm corresponds to a type II isotherm in the Brunauer classification.39,40 The characteristic feature of these curves is their hysteresis loop, which does not exhibit any limiting adsorption at high relative pressures. According to the IUPAC classification, the hysteresis loops are almost H3 type, indicating the ZnO is composed of aggregated layer materials.41 According to the BJH pore size distribution curve (inset of Fig. 5), 3D porous ZnO exhibits a mesoporous structure with three types of pore. The first type is fine pores about 3.2 nm, the second type has a narrow pore size distribution centered at 14.8 nm and the last one has a broad size distribution in the range of 30–70 nm.
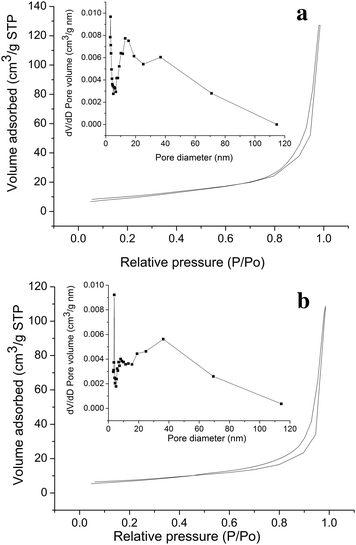 | ||
| Fig. 5 Typical nitrogen adsorption–desorption isotherm and BJH pore size distribution plots (inset) of hierarchically assembled 3D porous ZnO (a) and the precursor hydrozincite (b). | ||
Comparing the pore size distribution curves of ZnO-2 and precursor-2, we find the two curves are similar except the peak at 14.8 nm in ZnO-2. This also implies that the morphology of the resultant ZnO was retained in the process of the hydrozincite calcination to ZnO. The pores of 14.8 nm should be formed when the precursor hydrozincite decomposed.
The specific surface area was determined by the multipoint BET method using the adsorption data in the relative pressure (P/P0) range of 0.05–0.3. The 3D porous ZnO-2 has a specific surface area of 35.8 m2 g−1 (Table 2) and total pore volume of 0.196 cm3 g−1, which are higher than that of the precursor-2, 25.3 m2 g−1, 0.168 cm3 g−1, respectively. Table 2 lists the BET specific surface area and the pore size of ZnO and precursors prepared by different hydrothermal time. It shows the number of fine pores in precursor-3 decreases sharply, and the fine pores were diminished in the ZnO-3. The fine pores should be derived from hydrozincite nanoflake assembling layer by layer like other layered metal hydroxide salts.42,43 The hydrothermal time increase enhanced hydrozincite crystallization and growth, so the nanoflakes thicken (corresponding to crystallite size increase) and the spaces between layers increase to 6.8 nm after 16 h hydrothermal treatment.
| Sample | SBET (m2 g−1) | Fine pore size (nm) | Narrow peak center (nm) | Broad peak range (nm) |
|---|---|---|---|---|
| ZnO-1 | 38.4 | 3.2 | 14.8 | 30–70 |
| ZnO-2 | 35.8 | 3.2 | 14.8 | 30–70 |
| ZnO-3 | 29.3 | No | 15.6 | 30–70 |
| Precursor-1 | 19.6 | 3.8 | 6.6 (a few) | 12–70 |
| Precursor-2 | 25.3 | 3.2 | 7.4 (a few) | 8–70 |
| Precursor-3 | 18.6 | 3.8 (a few) | 6.8 (more) | 20–70 |
3.2. Gas-sensing properties
It is well known that the response of a semiconductor gas sensor is highly influenced by its working temperature.44 In order to determine optimum working temperatures, the responses of 3D porous ZnO gas sensors to 100 ppm acetone, benzene and ethanol in air were tested as a function of working temperature, as shown in Fig. 6. It is clear that the response of three sensors to the three gases varied with working temperature. | ||
| Fig. 6 Gas response versus working temperature of 3D porous ZnO sensors to 100 ppm test gas (a, acetone; b, benzene and c, ethanol). | ||
The responses of the three sensors to acetone and benzene (Fig. 6(a) and (b)) continuously increased when working temperatures varied from 200 to 420 °C, and then decreased. The maximum responses to acetone at 420 °C were 23.5, 77.5 and 49.7 for ZnO-1, ZnO-2, ZnO-3, respectively. The maximum responses to benzene at 420 °C were 5.5, 9.8 and 4.9 for ZnO-1, ZnO-2, ZnO-3, respectively.
For ethanol, the responses of the ZnO-1 and ZnO-2 sensors first increased with temperature, up to 340 °C, and then gradually decreased. The maximum responses of ZnO-1 and ZnO-2 were 41.9 and 29.6 at 340 °C. The response and working temperature were better than the sensor of 2D porous ZnO nanoplates (9 at 380 °C) reported by Jing.38 The maximum response of the ZnO-3 sensor to ethanol was 59.9 at 400 °C. The optimal working temperatures of the three sensors to acetone and benzene were higher than for ethanol, we ascribed it to the lower activation energy of ethanol reacting with oxygen at the nano ZnO surface than that of acetone and benzene.45 Therefore, different operating temperatures were chosen for acetone, benzene and ethanol for further tests.
Fig. 7 shows the response of the three ZnO sensors to acetone, benzene and ethanol with various concentrations at their optimum working temperature. From the curves, it is found that the responses of the three sensors were proportional to the increasing concentration of acetone from 10 to 900 ppm, but slowly tended to saturation when concentrations reached higher levels. The same tendency charts were observed for the three sensors to benzene (in the range 100–1100 ppm) and the ZnO-3 sensor to ethanol. The gas sensor based on ZnO-3 exhibits the highest response to ethanol of the three sensors and its response increased linearly with the concentration of ethanol in the range of 10–1000 ppm at a working temperature of 400 °C. The ZnO-2 sensor had the highest response to acetone and benzene of the three sensors. The ZnO-2 sensor shows a good selectivity to acetone when working at 420 °C (the ratio of Sacetone to Sethanol was about 7). The three sensors response S to ethanol and acetone is higher than to benzene, we attributed it to the low adsorption energies of non-polar benzene.
Response and recovery times are also important parameters in a gas sensor. Both response and recovery times of the three ZnO sensors were less than 20 s to 100 ppm acetone, benzene and ethanol at their optimal working temperature. The rapid response and recovery times of the sensors make them promising candidates for highly sensitive gas sensors.
4. Summary
In summary, hierarchically assembled 3D porous ZnO samples have been successfully synthesized through a simple hydrothermal reaction process after calcination of the precursor hydrozincite at 400 °C for 15 min. The 3D porous ZnO was composed of micrometer spheres which were assembled from nanoflakes. The sensors based on 3D porous ZnO showed improved ethanol response compared to 2D porous ZnO nanoplates. 3D porous ZnO sensor showed high response to 10 ppm ethanol, 10 ppm acetone and 100 ppm benzene at the operation temperatures of 340 °C, 420 °C and 420 °C, respectively. The gas sensor based on 3D porous ZnO-3 obtained after 16 h hydrothermal time exhibits high response to ethanol at working temperature of 400 °C and its response increased linearly with the concentration of ethanol in the range of 10–1000 ppm. The sensor based on 3D porous ZnO-2 obtained after 6 h hydrothermal time shows a high response and a good selectivity for acetone at the operation temperature of 420 °C. All the response and recovery times of the sensors were less than 20 s. The good sensing performance of the 3D porous ZnO sensors indicated that hierarchically assembled 3D porous ZnO could be a promising candidate for highly sensitive gas sensors.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (no. 51072141).References
- X. Wang, W. Liu, J. Liu, F. Wang, J. Kong, S. Qiu, C. He and L. Luan, ACS Appl. Mater. Interfaces, 2012, 4, 817 CAS
.
- J. H. Yang, J. Wang, X. Y. Li, J. H. Lang, F. Z. Liu, L. L. Yang, H. J. Zhai, M. Gao and X. T. Zhao, J. Alloys Compd., 2012, 528, 28 CrossRef CAS PubMed
.
- X. Liu, J. Zhang, L. Wang, T. Yang, X. Guo, S. Wu and S. Wang, J. Mater. Chem., 2011, 21, 349 RSC
.
- Y. Zhang, J. Xu, Q. Xiang, H. Li, Q. Y. Pan and P. C. Xu, J. Phys. Chem. C, 2009, 113, 3430 CAS
.
- W. Guo, T. Liu, H. Zhang, R. Sun, Y. Chen, W. Zeng and Z. Wang, Sens. Actuators, B, 2012, 166–167, 492 CrossRef CAS PubMed
.
- J. Feng, Z. Wang, Y. Li, J. Chen and A. Wang, J. Nanopart. Res., 2013, 15, 1565 CrossRef
.
- Q. Truong, T. Le, T. Kimura, S. Yin, T. Sato and Y. Ling, RSC Adv., 2013, 3, 19154 RSC
.
- K. Keis, C. Bauer, G. Boschloo, A. Hagfeldt, K. Westermark, H. Rensmo and H. Siegbahn, J. Photochem. Photobiol., B, 2002, 148, 57 CAS
.
- S. Jung and K. Yong, Chem. Commun., 2011, 47, 2643 RSC
.
- H. Wang, G. Li, L. Jia, G. Wang and C. Tang, J. Phys. Chem. C, 2008, 112, 11738 CAS
.
- J. X. Wang, C. M. L. Wu, W. S. Cheung, L. B. Luo, Z. B. He, G. D. Yuan, W. J. Zhang, C. S. Lee and S. T. Lee, J. Phys. Chem. C, 2010, 114, 13157 CAS
.
- M. Chen, Y. L. Wang, L. Y. Song, P. Gunawan, Z. Y. Zhong, X. L. She and F. B. Su, RSC Adv., 2012, 2, 4164 RSC
.
- S. Bang, S. Lee, Y. Ko, J. Park, S. Shin, H. Seo and H. Jeon, Nanoscale Res. Lett., 2012, 7, 290 CrossRef PubMed
.
- X. Fang, Y. Bando, U. Gautam, C. Ye and D. Golberg, J. Mater. Chem., 2008, 18, 509 RSC
.
- A. K. Sinha, M. Basu, M. Pradhan, S. Sarkar and T. Pal, Chem.–Eur. J., 2010, 16, 7865 CrossRef CAS PubMed
; J. Liu, F. Liu, K. Gao, J. S. Wu and D. Xue, J. Mater. Chem., 2009, 19, 6073 RSC
.
- D. W. Zhang, X. F. Wu, N. Han and Y. F. Chen, J. Nanopart. Res., 2013, 15, 1580 CrossRef
.
- L. Zhang, J. Zhao, H. Lu, L. Li, J. Zheng, H. Li and Z. Zhu, Sens. Actuators, B, 2012, 161, 209 CrossRef CAS PubMed
.
- J. Li, H. Q. Fan and X. H. Jia, J. Phys. Chem. C, 2010, 114, 14684 CAS
.
- C. Gu, J. Huang, Y. We, M. Zhai, Y. Sun and J. Liu, J. Alloys Compd., 2011, 509, 4499 CrossRef CAS PubMed
.
- J. R. Huang, Y. J. Wu, C. P. Gu, M. H. Zhai, Y. F. Sun and J. H. Liu, Sens. Actuators, B, 2011, 155, 126 CrossRef CAS PubMed
.
- X. Huang, X. H. Xia, Y. F. Yuan and F. Zhou, Electrochim. Acta, 2011, 56, 4960 CrossRef CAS PubMed
.
- Z. Xing, B. Geng, X. Li, H. Jiang, C. Feng and T. Ge, CrystEng Comm., 2011, 13, 2137 RSC
.
- H. Lu, S. Wang, L. Zhao, J. Li, B. Dong and Z. Xu, J. Mater. Chem., 2011, 21, 4228 RSC
.
- Y. Liu, L. Yu, Y. Hu, C. F. Guo, F. M. Zhang and X. W. Lou, Nanoscale, 2012, 4, 183 RSC
.
- Y. Masuda, K. Kato, Y. Masuda and K. Kato, Cryst. Growth Des., 2008, 8, 2633 CAS
.
- C. P. Gua, X. J. Xua, J. R. Huanga, W. Z. Wanga, Y. F. Sunb and J. H. Liu, Sens. Actuators, B, 2012, 174, 31 CrossRef PubMed
.
- R. A. McBride, J. M. Kelly and D. E. McCormack, J. Mater. Chem., 2003, 13, 1196 RSC
.
- S. Bhattacharyya and A. Gedanken, J. Phys. Chem. C, 2008, 112, 659 CAS
.
- X. Gao, X. Li, W. Gao, J. Qiu, X. Gan, C. Wang and X. Leng, CrystEngComm, 2011, 13, 4741 RSC
.
- S. Apurba, K. G. Arnab, P. Provas, S. K. Pahari, H. C. Bajaj and A. B. Panda, J. Mater. Chem., 2012, 22, 17227 RSC
.
- J. Zhang, S. Wang, M. Xu, Y. Wang, B. Zhu, S. Zhang, W. Huang and S. Wu, Cryst. Growth Des., 2009, 9, 3532 CAS
.
- X. Zhou, Z. Hu, Y. Fan, S. Chen, W. Ding and N. Xu, J. Phys. Chem. C, 2008, 112, 11722 CAS
.
- H. Zhang, R. Wu, Z. Chen, G. Liu, Z. Zhang and Z. Jiao, CrystEngComm, 2012, 14, 1775 RSC
.
- R. Wahab, S. G. Ansari, Y. S. Kim and M. A. Dar, J. Alloys Compd., 2008, 461, 66 CrossRef CAS PubMed
.
- S. Music, S. Popovic, M. Maljkovic and D. Dragcevic, J. Alloys Compd., 2002, 347, 324 CrossRef CAS
.
- D. Stoilova, V. Koleva and V. Vassileva, Spectrochim. Acta, Part A, 2002, 58, 2051 CrossRef CAS
.
- S. Ghose, Acta Crystallogr., 1964, 17, 649 CrossRef
.
- Z. H. Jing and J. H. Zhan, Adv. Mater., 2008, 20, 4547 CrossRef CAS
.
- J. C. Yu, A. W. Xu, L. Z. Zhang, R. Q. Song and L. Wu, J. Phys. Chem. B, 2004, 108, 64 CrossRef CAS
.
- C. L. Yan and D. F. Xue, J. Phys. Chem. B, 2006, 110, 11076 CrossRef CAS PubMed
.
- S. J. Gregg and K. S. W. Sing, in Adsorption, Surface Area and Porosity, Academic Press, London, UK, 2nd edn, 1982 Search PubMed
.
- E. Hosono, S. Fujihara, I. Honma and H. S. Zhou, Adv. Mater., 2005, 17, 2091 CrossRef CAS
.
- G. B. Sun, L. N. Sun, H. Wen, Z. Q. Jia, K. L. Huang and C. W. Hu, J. Phys. Chem. B, 2006, 110, 13375 CrossRef CAS PubMed
.
- V. R. Shinde, T. P. Gujar and C. D. Lokhande, Sens. Actuators, B, 2007, 123, 701 CrossRef CAS PubMed
.
- J. A. Manion, R. E. Huie, R. D. Levin, D. R. Burgess Jr, V. L. Orkin, W. Tsang, W. S. McGivern, J. W. Hudgens, V. D. Knyazev, D. B. Atkinson, E. Chai, A. M. Tereza, C.-Y. Lin, T. C. Allison, W. G. Mallard, F. Westley, J. T. Herron, R. F. Hampson and D. H. Frizzell, NIST Chemical Kinetics Database, NIST Standard Reference Database 17, Version 7.0 (Web Version), Release 1.4.3, Data version 2008.12, National Institute of Standards and Technology, Gaithersburg, Maryland, 20899-8320, http://kinetics.nist.gov/ Search PubMed
.
| This journal is © The Royal Society of Chemistry 2014 |



