Nanocomposites of monodisperse nanoparticles embedded in high-K oxide matrices – a general preparation strategy
Abstract
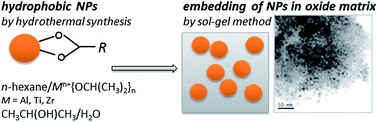
We present a general approach, which enables preparation of multifunctional nanocomposites of monodisperse nanoparticles embedded in oxide matrices. The two-step route has been successfully applied to nanocomposites composed of CoFe2O4 nanoparticles embedded in high-K oxide matrices (ZrO2, Al2O3 and TiO2). First, hydrophobic CoFe2O4 nanoparticles were produced by hydrothermal synthesis and then their incorporation in the oxide matrix was completed by the sol–gel method using the corresponding alcoxides. The as-prepared samples were subjected to annealing at temperatures ranging from 200 to 700 °C, and characterized in detail by the Powder X-Ray Diffraction (PXRD), Energy Dispersive Analysis (EDX), Mössbauer Spectroscopy (MS) and magnetic measurements. The particle size does not change with the annealing temperature, while the amorphous matrices crystallize at temperatures above 400 °C. At much higher annealing temperatures, partial decomposition of the CoFe2O4 occurs accompanied by formation of additional phases. The magnetic measurements also confirmed presence and stability of the uniform CoFe2O4 nanoparticles in the matrices. Thus the proposed method allows preparation of new types of nanocomposites constituted of uniform nanoparticles of the desired type (magnetic, luminescent etc.) embedded in the favored oxide matrix.

 Please wait while we load your content...
Please wait while we load your content...