Selective hydrogenation of cinnamaldehyde over Pt nanoparticles deposited on reduced graphene oxide†
Abstract
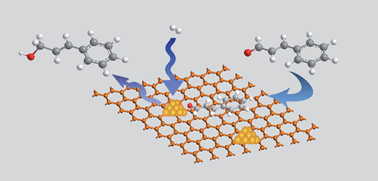
The selective hydrogenation of cinnamaldehyde was investigated with Pt nanoparticles deposited on reduced graphene oxide (Pt/RGO). Compared with carbon nanotubes (CNTs) or activated carbon (AC) as support, Pt/RGO showed the best activity and selectivity for the hydrogenation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond.
O bond.

 Please wait while we load your content...
Please wait while we load your content...