DOI:
10.1039/C3RA44523B
(Paper)
RSC Adv., 2014,
4, 12506-12513
Leaching of hafnium, zirconium, uranium and other nuclear economic elements from petroleum ash
Received
23rd August 2013
, Accepted 11th December 2013
First published on 17th December 2013
Abstract
To determine the optimum leaching conditions controlling the leaching of petroleum ash residues, the theoretical thermodynamics factors were firstly deduced using the computer program HSC chemistry 5.1. The factors affecting the leaching efficiency, such as the acid type and concentration, helper leaching agent type and amount, leaching temperature, agitation time, solid/liquid ratio, and the effect of grain size, were studied. When applying these optimum leaching conditions more than 95.9, 98.9, 97.1, 97.8, 96.5 and 97.1% of Hf, Zr, U, V, Ni and Fe contents was leached respectively.
Introduction
Fly ash is a powdery solid residue which accumulates on chimney walls, while boiler ash is a solid residue stuck to the outside of boilers in electric power stations that use heavy oil as a fuel source. Petroleum ash contains a relatively high heavy metals content, particularly vanadium and nickel oxides. The residual carbon levels in petroleum ash are also very high.1 Egyptian crude oil contains considerable amounts of vanadium and nickel. These metals are concentrated in the heavy fraction produced during fractional distillation. The heavy fraction is used as a fuel in electric power stations and consequently metals such as vanadium, nickel, etc. are concentrated in the ash.
As a matter of fact, petroleum ash has a deleterious impact on the environment due to the presence of toxic, carcinogenic and nuclear elements (uranium, thorium, hafnium, zirconium and vanadium). Thus, it is essential to remove these elements in order to minimize the risks associated with petroleum ash. Fortunately, the heavy metals in petroleum ash have high economic values which could make the process of their removal quite acceptable from a commercial point of view. For example, vanadium is an important alloying element for improving the mechanical properties of both ferrous and non-ferrous alloys.2 It also has strong potential in energy storage applications and as a catalyst for the chemical and polymer industries.3 Zirconium is employed in the production of optical glasses with high refractive indexes4 and in the ceramic industry to produce enamels.5 Its transparency to thermal neutrons has made Zr a good structural material in nuclear reactors and chemical plants.6 Hf has also been found to be a good absorber of neutrons, leading to its use as a moderator in control rods for nuclear reactors.7
Currently, vanadium is recovered from vanadium containing ash by roasting the ash in a rotary kiln at 800 °C for 3 hours followed by leaching with water to produce soluble sodium vanadate.8–12 This pyro-metallurgical process is technically feasible, but it is not cost-effective. Another technically viable process is the hydrometallurgical method which involves sulfuric acid leaching of ground boiler ash under pressure, followed by electrolytic separation of nickel and vanadium from the sulfate solution.13 However, various aspects of this process need further development.
The present work demonstrates an eco-friendly and affordable hydrometallurgical technique to recover hafnium, zirconium, uranium, vanadium, nickel and iron from petroleum ash. The paper presents detailed leaching studies of these elements, using petroleum ash from an Egyptian electricity power station called El Kriymat as a case study.
Experimental work
The petroleum ash sample was subject to major and minor oxide chemical analysis together with analysis of U, Hf and Zr (Table 1). The major and minor oxides in this sample were analyzed using the conventional chemical technique of Shapiro and Brannock, (1962),14 while a Unicam 969 flame atomic absorption spectrometer (FAAS), produced by Unicam Company, England and fitted with a computer, was used for the estimation of trace elements, as shown in Table 1. In this regard, analysis of hafnium, zirconium, uranium, vanadium, nickel and iron in both the petroleum ash sample and the leaching liquors was performed by high dispersion Inductively Coupled Plasma (Prism ICP) spectroscopy (Teledyne Leeman Labs, USA), at wavelengths of 264.141, 302.952, 256.971, 271.566, 232.003 and 239.563 nm.
Table 1 Complete chemical analysis of the studied petroleum ash sample
| Major oxides (%) |
Trace elements (ppm) |
| Including native sulfur. Total dissolved salts of K and Na. Loss of ignition at 100 °C. Complexed material with a high carbon number. |
| V2O5 |
12.16 |
U |
628 |
| NiO |
8.84 |
Th |
555 |
| SO4a |
9.01 |
Ti |
200 |
| Fe2O5 |
7.94 |
Ba |
180 |
| CaO |
21.00 |
Y |
120 |
| MgO |
2.7 |
Sr |
324 |
| T.D.Sb |
3.06 |
Mn |
2200 |
| Al2O3 |
1.56 |
Si |
1300 |
| L.O.Ic |
8.89 |
Cd |
920 |
| Tard |
13.10 |
Co |
5000 |
| Cr2O3 |
5.81 |
Cu |
810 |
| ZnO |
4.7 |
Pb |
2700 |
| As2O5 |
0.83 |
Zr |
5645 |
| Total |
99.61 |
Hf |
1751 |
To perform studies on hafnium, zirconium, uranium, vanadium, nickel and iron leaching from the petroleum ash sample, the theoretical thermodynamics factors were firstly deduced using the computer program HSC chemistry 5.1. The ΔH, ΔS and ΔG of the reactions of the concerned element oxides with HCl, HNO3 and H2SO4 were studied to choose the best leaching acid as well as to determine the complex nature of these elements.
The theoretical thermodynamics inputs into the computer program HSC chemistry 5.1 are based on the metal oxides concentration percentages presented in Table 1. To determine the phases formed at temperatures ranging from 500 to 1500 °C, 100 kg of ash was introduced to the program. Fig. 1 represents the main output phases resulting from this application. However, other phases, such as sulfides and carbides, were also obtained in very small percentages.
 |
| | Fig. 1 The expected phases formed in the fly ash sample studied using the HSC program. | |
Concerning the hydrometallurgical processing of the petroleum ash samples, many sets of experiments were performed in order to study the factors affecting the leaching efficiencies of the concerned elements, such as the type of acid and its concentration, use of the helper leaching agent and its concentration, leaching temperature, agitation time, solid/liquid ratio and the effect of grain size. In general, the ash sample was firstly crushed and ground until it reached a −200 mesh size. A 5 g portion of the ash sample was well mixed with 20 ml of 200 g l−1 sulfuric acid and stirred at 1200 rpm for 4 hours at 50 °C. The solid/liquid ratio was fixed at 1/4 except when otherwise mentioned. The obtained leachates were filtered and the concentrations of the concerned elements in the filtered solutions were determined.
Results and discussion
1. Chemical composition
The results of chemical composition analysis of the studied petroleum ash sample presented in Table 1 reveal that vanadium, nickel, iron and chromium are the main constituents of the ash as their concentrations reached 12.16, 8.84, 7.94 and 5.81% respectively. The ash also contains a significant concentration of valuable and strategic nuclear elements such as uranium, thorium, hafnium and zirconium.
2. Theoretical thermodynamics studies
By using the computer program HSC chemistry 5.1 one can construct a diagram of the major constituent phases in fly ash belonging to El Kriymat Power Station at different temperatures (Fig. 1). It can be seen that nearly all phases are in the oxide form however, these phases can also form as sulfides or carbides at lower concentrations. This means that, in the leaching experiments, the dissolution of sulfides requires the addition of an oxidant whereas the dissolution of oxides requires acid and temperature only.15 The dissolution reactions for oxides and sulfides are different, as shown below:| | |
V2O5 + H2SO4 → (VO2)2SO4 + H2O
| (1) |
The free energy of the reactions of different oxides in HCl, H2SO4 and HNO3 was also calculated at different temperatures with the assistance of the HSC program (Fig. 2). One expects the use of H2SO4 to be more effective than HCl and HNO3 because ΔG for all oxides is highly negative.
 |
| | Fig. 2 Free energy, ΔG (kJ), of the reactions of the concerned element oxides with HCl, HNO3 and H2SO4 at 50 °C. | |
The results in Fig. 3 show that the ΔG of vanadium oxide dissolution in sulfuric acid is most negative and vanadium oxide is therefore the most soluble while zirconium is the hardest to dissolve. The free energy of the other element oxides varies in the sequence Fe > U > Ni.
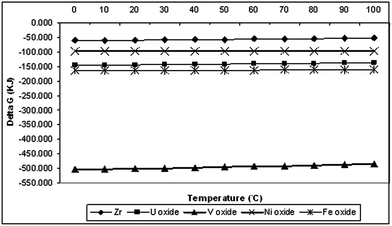 |
| | Fig. 3 Free energy, ΔG (kJ), of the reactions of the concerned element oxides with H2SO4 at temperatures ranging from 0 to 100 °C. | |
3. Leaching results
Effect of acid type. To study the effect of acid type on hafnium, zirconium, uranium, vanadium, nickel and iron element leaching, three experiments were carried out using 5 g ore in 50 ml of 200 g l−1 HNO3, HCl and H2SO4, while stirring the contents at 50 °C for about 3 hours. From the obtained results (Fig. 4), it can be seen that sulfuric acid is much more effective for leaching of the studied elements than nitric or hydrochloric acids. The leaching efficiencies of hafnium, zirconium, uranium, vanadium, nickel and iron by the sulfuric acid leachant reached 30.5, 33.5, 34.8, 40.5, 30.3 and 32.5% respectively. These results match the results obtained from the theoretical thermodynamics studies.
 |
| | Fig. 4 Effect of acid type on the leaching efficiencies of the concerned elements. | |
Effect of acid concentration. The effect of acid concentration on leaching the corresponding elements was studied by applying different concentrations of H2SO4, ranging from 10 to 200 g l−1, as well as leaching with only water. Constant factors included a solid mass/liquid volume ratio of 1/4 and stirring for 4 hours at room temperature. From the obtained results (Fig. 5), one can conclude that the leaching efficiencies increase slightly with increasing acid concentration until 200 g l−1 H2SO4, which is quite sufficient. The extractive leaching reaction mechanisms for vanadium, zirconium and uranium as examples are presented as follows:
| V2O5 + H2SO4 → (VO2)2SO4 + H2O |
| UO2SO4 + SO42− → [UO2(SO4)2]2− |
| [UO2(SO4)2]2− + SO42− → [UO2(SO4)3]4− |
| ZrO2 + 2H2SO4 → Zr(SO4)2 + 2H2O |
| ZrO2+ + SO42− → [ZrOSO4]2− |
 |
| | Fig. 5 Effect of acid concentration on leaching efficiencies. | |
Only hexavalent uranium and V5+ dissolve in H2SO4 while tetravalent uranium and V3+ must first be oxidized to soluble forms using any oxidizing agent. At acid concentrations higher than 200 g l−1, the leaching efficiencies are not sound from an economic point of view, which states that excessive acid consumption should be avoided on an industrial scale. We planned to study this factor using experiments at room temperature as we hoped to leach uranium first and extract other elements later on to facilitate the extraction and separation of each element individually. Unfortunately, other elements were leached in the same comparative order and consequently, other leaching factors should be investigated.
Effect of temperature. The effect of temperature on the leaching efficiencies of the concerned elements was studied by carrying out a series of experiments at room temperature, 50, 60, 70 and 80 °C while keeping other factors constant: 200 g l−1 H2SO4 and a solid/liquid ratio of 1/10. The obtained data (Fig. 6) indicate that raising the temperature from room temperature up to 80 °C improved the leaching efficiencies of hafnium, zirconium, uranium, vanadium, nickel and iron to 59.5, 63, 76.2, 76, 57.2 and 60.2% respectively. In fact, higher temperatures increase the speed of molecular motion and also increase collisions between the atoms and H+. Thus, increasing the temperature increased the leaching efficiencies, indicating that dissolution is controlled by a chemical reaction. Although the leaching efficiencies are increased by increasing the temperature up to 80 °C, the authors do not favour this increase of temperature because of the high acid consumption due to the increased water evaporation rate and difficulty of filtration. So 80 °C was chosen to be the optimum temperature for saving energy.
 |
| | Fig. 6 Effect of temperature on the leaching efficiencies of the concerned elements. | |
Effect of the helper leaching agent. The effect of the helper agent upon the leaching efficiencies of the concerned elements was studied by carrying out a series of experiments using 4% (solid/solid ratio) FeSO4, NaClO3 and MnO2 or the application of 10% H2O2 (volume/H2SO4 volume ratio) at 80 °C, while keeping the other factors constant (solid/liquid ratio 1/10 and 200 g l−1 sulfuric acid). The obtained data (Fig. 7) show the effect of the helper agent on the leaching efficiencies. It is clear that the leaching efficiencies of hafnium, zirconium, uranium, vanadium, nickel and iron with the MnO2 helper agent (oxidant) are highest and reached 81.5, 82.5, 84.2, 86.4, 75.5 and 77.5% respectively. The leaching efficiencies of the studied elements with the NaClO3 helper agent are also high, but slightly lower than with the MnO2 helper agent, so the authors proposed using either MnO2 or NaClO3 for later leaching applications. Although the FeSO4 helper agent has Fe2+ reducing ions which logically decrease the leaching efficiencies, it was found that the leaching efficiencies of the concerned elements are higher than or equal to those with the NaClO3 helper agent. This may be explained according to the fact that ferrous ions can be oxidized in air (or oxygen) at high temperatures to form ferric ions (oxidizing agents) according to the equation:
| 2Fe2+ + 2H+ + 1/2O2 → 2Fe3+ + H2O (ref. 16) |
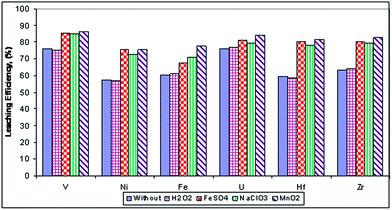 |
| | Fig. 7 Effect of the helper agent on the leaching efficiencies of the concerned elements. | |
Ferric ions act as the principal oxidants of tetravalent uranium in acid leaching. Iron can be generally present either in the ore or introduced as ferrous sulphate. The leaching mechanism depends on the conversion of undissolved tetravalent uranium U(IV) to soluble hexavalent uranium U(VI) and Fe3+ is converted to Fe2+ again according to the following equation:
| UO2 + 2Fe3+ → UO22+ + 2Fe2+ (ref. 17) |
On the contrary, the leaching efficiencies of the concerned elements with the helper leaching agent H2O2 are less than or equal to the data obtained by processing petroleum ash without using a helper agent due to the fact that H2O2 is easily decomposed at higher temperatures.
Effect of the helper leaching agent concentration. The effect of the helper leaching agent (oxidant) concentration upon hafnium, zirconium, uranium, vanadium, nickel and iron leaching efficiencies was studied by constructing a series of different ratios ranging between 2 and 8% (solid/solid ratio) of either (a) the MnO2 helper agent or (b) the NaClO3 helper agent while keeping the other factors constant (solid/liquid ratio of 1/10 and 200 g l−1 sulfuric acid). The obtained results (Fig. 8 and 9) show that the leaching efficiencies of the concerned elements are directly related to the increase in oxidant amount. Thus, 4–6% of helper leaching agent is sufficient to create the necessary oxidizing medium. The oxidizing agent has two effects in the leaching mechanism; firstly it oxidizes the lower valence elements (insoluble) to higher valence elements (soluble form), such as uranium from U4+ to U6+. The second effect is the oxidation of ferrous ions, present in the fly ash, to ferric ions that also act as oxidizing leaching agents according to the following reaction mechanisms:
| UO2 + H2SO4 + 1/2O2 → UO2SO4 + H2O (ref. 17) |
| 6Fe2+ + NaClO3 + 6H+ → 6Fe3+ + NaCl + 3H2O |
| 2Fe2+ + MnO2 + 4H+ → 2Fe3+ + Mn2+ + 2H2O |
 |
| | Fig. 8 Effect of the MnO2 helper leaching agent concentration on the leaching efficiencies of the concerned elements. | |
 |
| | Fig. 9 Effect of the NaClO3 helper leaching agent concentration on the leaching efficiencies of the concerned elements. | |
Effect of agitation time. To study the effect of agitation time upon hafnium, zirconium, uranium, vanadium, nickel and iron leaching efficiencies, another series of leaching experiments were performed by increasing the agitation time from 4 to 10 hours and keeping the other factors constant (solid/liquid ratio 1/10 and 200 g l−1 sulfuric acid). From the obtained data (Fig. 10), it can be seen that the leaching efficiency increased as the agitation time increased up to 8 hours and the leaching efficiencies of hafnium, zirconium, uranium, vanadium, nickel and iron reached 93, 95.2, 94.9, 93, 90.8 and 85.8% respectively. The leaching efficiencies then began to decrease after 10 hours of agitation. The reason could be that when the leaching time reaches more than 8 hours, VOSO4 covers the surfaces of unreacted particle cores. Consequently, the leaching process starts to slow down.2
 |
| | Fig. 10 Effect of agitation time on the leaching efficiencies of the concerned elements. | |
Effect of the solid/liquid ratio. In order to study the effect of the solid (mass)/liquid (volume) ratio upon the leaching efficiencies of hafnium, zirconium, uranium, vanadium, nickel and iron, a series of experiments were performed. In this series, the solid/liquid ratios were decreased from 1/4 to 1/10 while keeping the other factors fixed at 200 g l−1 H2SO4 and stirring for 8 hours. The obtained results shown in Fig. 11 indicate that the leaching efficiency increases linearly with decreasing solid/liquid ratio. At the low solid/liquid ratio of 1/10 and 200 g l−1 H2SO4 the leaching efficiencies of hafnium, zirconium, uranium, vanadium, nickel and iron reached 98, 98.5, 98.5, 99.5, 98.4 and 95.5% respectively.
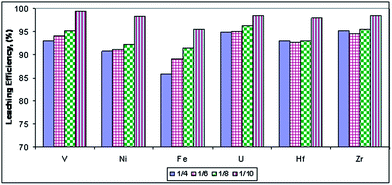 |
| | Fig. 11 Effect of the solid/liquid ratio on the leaching efficiencies of the concerned elements. | |
Effect of grain size. A series of leaching experiments were then performed to study the effect of grain size on the leaching efficiencies of hafnium, zirconium, uranium, vanadium, nickel and iron from petroleum ash. In these experiments different grain size fractions were used, namely >60, −60, −120 and −200 mesh. The other leaching factors were kept fixed at a solid/liquid ratio of 1/10, 200 g l−1 H2SO4, stirring for 8 hours and 80 °C. The obtained results (Fig. 12) show that the smaller particles of the concerned elements were leached more readily from the petroleum ash due to their increased surface areas.
 |
| | Fig. 12 Effect of grain size (mesh) on the leaching efficiencies of the concerned elements. | |
Optimum leaching factors. To determine the optimum factors that control the experimental conditions, for the maximum leaching efficiencies of hafnium, zirconium, uranium, vanadium, nickel and iron from petroleum ash, three series of experiments were performed, changing the agitation time from 4 to 8 hours. The first series was conducted at low sulfuric acid concentration (100 g l−1) with 6% MnO2 oxidant as a helper leaching agent, the second series was performed at high acid concentration (200 g l−1) without a helper leaching agent while the third series was performed at high acid concentration (200 g l−1) with 6% MnO2 oxidant as a helper leaching agent. The other factors were fixed at a 1/10 solid/liquid ratio, −200 mesh grain size and 80 °C. From the obtained results (Fig. 13), it is clear that the highest values of leaching efficiencies of hafnium, zirconium, uranium, vanadium, nickel and iron were obtained at 8 hours when applying low sulfuric acid concentration and reached 82.5, 83.8, 78.4, 86.2, 70.5 and 75.8% respectively. On the other hand, at high acid concentration, without an oxidant and with 8 hours agitation time, the same elements achieved leaching efficiencies of 98, 98.5, 98.5, 99.5, 98.2 and 95.5%. Almost identical leaching efficiencies were obtained for the treated elements when applying a leaching agitation time of 6 hours at high acid concentration with a helper leaching agent. Thus it is clear that applying either high sulfuric acid concentration with 6% oxidant after 6 hours agitation or applying high sulfuric acid concentration without oxidant after 8 hours agitation can be considered to be optimal conditions.
 |
| | Fig. 13 Effect of the experimental conditions on the leaching efficiencies of the concerned elements. | |
Leaching kinetics. To shed some light on the leaching kinetics of V, Ni, Fe, U, Hf and Zr, herein, the effect of reaction temperature on the leaching rate of the concerned elements is presented. This study is performed in the range 30–80 °C under conditions of −200 mesh grain size, 200 g l−1 H2SO4 acid concentration, 4% (w/w) NaClO3 as an oxidizing agent with 1/10 solid/liquid ratio at time intervals ranging from 2 to 8 hours (Fig. 14). In order to obtain the kinetic equations for the dissolution of vanadium, uranium and hafnium as examples of the concerned elements in the presence of an NaClO3 oxidizing agent, the experimental data in Fig. 14a–c are correlated to various kinetic models for solid–liquid reactions, including:18,19| | |
Kt = 1 − 2/3(X) − (1 − X)2/3
| (b) |
where K is the apparent reaction rate constant (min−1), t is the leaching time and X is the reacted fraction which is expressed as X = % metal extraction/100.
 |
| | Fig. 14 Effect of temperature on the leaching efficiencies of (a) vanadium, (b) uranium, and (c) hafnium. | |
With vanadium, (Fig. 14a), there are two stages in the process of leaching from petroleum fly ash. During the first 8 hours there was a sharp increase in the percentage of extracted vanadium, then at longer times the vanadium leaching efficiency decreased slightly. This result is in accordance with the results presented in Fig. 10 as well as in agreement with the published data.2,20 This is the reason vanadium oxide reacts with sulfuric acid according the following equation:
| V2O5 + H2SO4 → (VO2)2SO4 + H2O |
But at prolonged times, the following reaction may occur:
| V2O4 + 2H2SO4 → 2VOSO4 + 2H2O (ref. 21) |
The relationship between 1 − (1 − X)1/3 and the leaching time for vanadium at various temperatures is plotted in Fig. 15. The R2 values for all lines are greater than 0.99 and there is a good agreement between the proposed kinetic model and the experimental data. This indicates that the linear relationship is significant and suggests that the leaching rate of vanadium from fly ash is chemically controlled. Based on the experimental data in Fig. 14a, a plot of 1 − 2/3X − (1 − X)2/3 from eqn (b) versus vanadium leaching time is given in Fig. 16. As can be seen, there is agreement between the proposed kinetic model and the experimental data at low temperatures and long times, while deviation between the model and the data is evident at high temperatures, as well as at low temperatures and short times. Therefore, it can be concluded that the kinetics of vanadium leaching from petroleum fly ash is controlled by diffusion through the liquid film at long times (>200 min) and low temperatures.
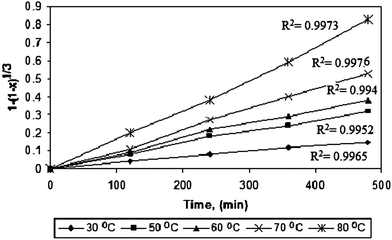 |
| | Fig. 15 Plots of 1 − (1 − X)1/3 versus time for vanadium leaching at various reaction temperatures. | |
 |
| | Fig. 16 Plots of 1 − 2/3X − (1 − X)2/3 versus time for vanadium leaching at various reaction temperatures. | |
However, the results of the effect of temperature upon uranium leaching from petroleum fly ash (Fig. 14b) show that the leaching rate of uranium increases as the temperature increases. The relationships between 1 − (1 − X)1/3 and 1 − 2/3X − (1 − X)2/3 of eqn (a) and (b) respectively and the leaching time for uranium at various temperatures are plotted in Fig. 17 and 18, respectively. As can be seen in Fig. 18, the experimental data do not fit the kinetic model of eqn (b), due to the presence of a sharp deviation. This means that the rate of uranium leaching from fly ash is not controlled by diffusion through the liquid film. Also, the experimental data (Fig. 17) is in good agreement with the proposed kinetic model of eqn (a) and the R2 values for all lines are greater than 0.99. This indicates that the linear relationship is significant and suggests that the leaching rate of uranium from fly ash is chemically controlled. The interaction of hexavalent and tetravalent uranium oxides using a sulfuric acid leaching method is presented in the following equations:
| UO3, solid + 2H+aq → UO22+aq + H2O, dissolution |
| UO2, solid + 0.5O2 + 2H+aq → UO22+aq + H2O, oxidation |
| UO2, solid + 2Fe3+aq → UO22+aq + Fe2+aq, oxidation |
| 3UO2, solid + ClO3aq + 6H+aq → 3UO22+aq + Cl−aq + 3H2O, oxidation |
 |
| | Fig. 17 Plots of 1 − (1 − X)1/3 versus time for uranium leaching at various reaction temperatures. | |
 |
| | Fig. 18 Plots of 1 − 2/3X − (1 − X)2/3 versus time for uranium leaching at various reaction temperatures. | |
Similarly, the results of the effect of temperature on hafnium leaching from petroleum fly ash (Fig. 14c) show that the leaching rate of hafnium follows the same trend as uranium leaching and increases as the temperature increases. Depending upon the experimental data (Fig. 14c), the relationship between 1 − (1 − X)1/3 and the leaching time for hafnium at various temperatures is plotted in Fig. 19. As can be seen, there is a good agreement between the proposed kinetic model and the experimental data and the R2 values for all lines are greater than 0.99. This indicates that the linear relationship is significant and suggests that the leaching rate of hafnium from fly ash is chemically controlled. However, the plots of 1 − 2/3X − (1 − X)2/3 versus time (Fig. 20) show deviation and do not fit the kinetic model of this equation. This means that the rate of hafnium leaching from fly ash is not controlled by diffusion through the liquid film.
 |
| | Fig. 19 Plots of 1 − (1 − X)1/3 versus time for hafnium leaching at various reaction temperatures. | |
 |
| | Fig. 20 Plots of 1 − 2/3X − (1 − X)2/3 versus time for hafnium leaching at various reaction temperatures. | |
Conclusions
From this study one can state that petroleum ash can be considered as a very important, non conventional source of uranium, hafnium and zirconium together with vanadium and nickel. In addition, it was concluded that petroleum ash contains a hafnium/zirconium ratio higher than that in known conventional sources of hafnium and zirconium, and thus the recovery of hafnium and zirconium from petroleum ash avoids the problems facing their recovery from zircon. Nearly all previous studies of petroleum ash leaching concentrated only upon the leaching and extraction of vanadium and nickel because these are the main components of the ash. However, this study focused upon significant economic and nuclear elements such as uranium, hafnium and zirconium as well as vanadium and nickel. This study has achieved the optimum conditions for leaching and investigated a variety of conditions such as the concentration of acid with or without oxidant and temperature. The results show that the optimal conditions of leaching are as follows: 200 g l−1 H2SO4 acid, 6% (solid/solid ratio) of manganese dioxide as a helper agent, 80 °C, 6 hours, 1/10 solid/liquid ratio and −200 mesh grain size to leach more than 95% of all concerned elements. The practical results matched the results obtained from the theoretical thermodynamics studies which helped in determining the type of leaching agent and oxidant.
Acknowledgements
The authors are grateful to the head of the Nuclear Materials Authority, Egypt, Prof. Dr Mohsen M. Ali, for the helpful financial support during the course of this research. They are also gratefully indebted to the members of the Pilot Plant Exp. Dept. Moreover, to the colleagues of the Chemical Analysis Dep., especially the Inductively Coupled Plasma Lab. and the Yellow Cake Purification Dept., for their kind assistance during the performance of the analytical control of the work, and the valuable advice.
Notes and references
- A. Masud and A. Latif, Miner. Eng., 2002, 15, 953–961 CrossRef.
- T. N. Baker, Mater. Sci. Technol., 2009, 25(9), 1083–1107 CrossRef CAS PubMed.
- X.-Y. Chen, X.-Z. Lan, Q.-L. Zhang, H.-Z. Ma and J. Zhou, Trans. Nonferrous Met. Soc. China, 2010, 20, s123–s126 CrossRef CAS.
- A. M. Abdelkader and E. El-Kashif, ISIJ Int., 2007, 47(1), 25–31 CrossRef CAS.
- A. M. Abdelkader, A. Daher, R. A. Abdelkareem and E. El-Kashif, Metall. Mater. Trans. B, 2007, 38(1), 35–44 CrossRef.
- S. Shariati, Y. Yamini and M. K. Zanjani, J. Hazard. Mater., 2008, 156, 583–590 CrossRef CAS PubMed.
- A. M. Abdelkader and D. J. Fray, Electrochim. Acta, 2012, 64, 10–16 CrossRef CAS PubMed.
- A. R. Sastry, Chem. Age India, 1968, 3, 195–201 Search PubMed.
- B. V. Slobodin, Russian publication in Tr. Inst. Khim., Ural. Nauchn. Tsentr, Akad. Nauk SSSR, Sverdlovsk, 1975.
- H. Cheng, Faming Zhuanli Shenging Gongkai Shuomingshu, CN 86,108,218 (CI.CO I 31/02), 1987.
- A. Aydin, Chim. Acta Turc., 1988, 16(2), 153–182 CAS.
- K. S. Chandra and S. Subramanian, Int. J. Miner. Process., 1998, 53, 107–120 CrossRef.
- A. M. Amer, Waste Manage., 2002, 22, 515–520 CrossRef CAS.
- L. Shapiro and W. W. Brannock, U. S. Geol. Surv. Bull., 1962, 1114-A Search PubMed.
- G. Debaraj Mishra, R. Chaudhury, D. J. Kim and J. G. Ahn, Hydrometallurgy, 2010, 101, 35–40 CrossRef PubMed.
- F. Habashi, A textbook of hydrometallurgy, Department of Mining & Metallurgy, Laval University, Quebec City, Canada, 1993, p. 89 Search PubMed.
- R. Venter and M. Boylett, Hydrometallurgy Conference, The Southern African Institute of Mining and Metallurgy, 2009 Search PubMed.
- F. Habashi, Principles of Extractive Metallurgy, Volume 1. General Principles, Gordon and Breach, New York, 1980, vol. 1, pp. 111–252 Search PubMed.
- H. S. Ray, Kinetics of metallurgical reactions, Oxford & IBHC, New Delhi, 1993, pp. 1–75 Search PubMed.
- M. Aarabi-Karasgani, F. Rashchi, N. Mostoufi and E. Vahidi, Hydrometallurgy, 2010, 102, 14–21 CrossRef CAS PubMed.
- Y.-M. Zhang, S.-X. Bao, T. Liu, T.-J. Chen and J. Huang, Hydrometallurgy, 2011, 109, 116–124 CrossRef CAS PubMed.
|
| This journal is © The Royal Society of Chemistry 2014 |
Click here to see how this site uses Cookies. View our privacy policy here. 



















