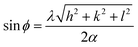Amylamine stabilized platinum(0) nanoparticles: active and reusable nanocatalyst in the room temperature dehydrogenation of dimethylamine-borane
Fatih
Sen
*a,
Yasar
Karatas
b,
Mehmet
Gulcan
b and
Mehmet
Zahmakiran
*b
aDepartment of Chemistry, Science Faculty, Dumlupınar University, 43020, Kütahya, Turkey. E-mail: fatih.sen@dumlupinar.edu.tr
bDepartment of Chemistry, Science Faculty, Yuzuncu Yil University, 65080, Van, Turkey. E-mail: zmehmet@yyu.edu.tr
First published on 28th October 2013
Abstract
Herein, we report the preparation and characterization of platinum(0) nanoparticles stabilized by amylamine (C5H11NH2) ligands plus their catalytic use in the room temperature dehydrocoupling of dimethylamine-borane ((CH3)2NHBH3), which has attracted recent attention as a promising solid hydrogen storage material. Amylamine stabilized platinum(0) nanoparticles were reproducibly generated by an ethanol–superhydride reduction method and their preliminary characterization was done by ICP-OES, XRD, ATR-IR, TEM, HRTEM, and XPS spectroscopies. The sum of their results shows the formation of highly crystalline and colloidally stable platinum(0) nanoparticles. The catalytic performance of these new platinum(0) nanoparticles in terms of activity, isolability and reusability was investigated in the catalytic dehydrocoupling of dimethylamine-borane, in which they were found to be active and reusable heterogeneous catalysts even at room temperature.
Introduction
Over the last ten years, the catalytic dehydrogenation of amine-borane adducts has become increasingly important from the viewpoint of the recent interest in chemical hydrogen storage.1 Efficient and safe hydrogen storage is still one of the key issues in the “Hydrogen Economy”.2 Moreover, the application of catalytic dehydrogenation of amine-borane adducts in materials science3,4 has also made a significant contribution to this field. Of particular interest, recent studies have already showed that the dehydrogenation (or dehydrocoupling) of dimethylamine–borane (borane dimethylamine complex, (CH3)2NHBH3, DMAB) in the presence of suitable catalyst (Scheme 1) potentially releases 3.5 wt% H2.5–13Although, the record activity has been achieved by using a homogeneous [η5-C5H3-1,3-(SiMe3)2Ti]2 (μ2,η1,η1-N2) catalyst,14 the current research has more focused on the development of metal nanoparticle catalysts than that of homogeneous catalysts15,16 because of their advantages such as simple product isolation, catalyst recovery and reusability.17 In this context, we report herein, for the first time, the preparation and preliminary characterization of platinum(0) nanoparticles stabilized by amylamine (C5H11NH2) ligand, hereafter named as Pt(0)/AA. Pt(0)/AA were reproducibly prepared by ethanol–superhydride reduction of PtCl4 in an anhydrous THF solution of amylamine at room temperature. Pt(0) nanoparticles have been as it is well known catalyst for various organic and inorganic transformations.18–20 and well characterized by using ICP-OES, XRD, FTIR, TEM, HRTEM, and XPS spectroscopic methods. The results of these analyses reveal that the formation of highly crysalline well-dispersed amylamine stabilized platinum(0) nanoparticles in the range of 2.4–4.3 nm with a mean diameter of 3.30 ± 0.85 nm. The catalytic application of the resulting Pt(0)/AA in terms of activity, reusability and lifetime was demonstrated in the dehydrogenation of DMAB in THF at room temperature and found that they provide high catalytic activity and notable reusability performance in this important catalytic reaction.
To the best of our knowledge, apart from the APTS-stabilized ruthenium(0) nanoparticles6 these new platinum(0) nanoparticles represent the second example of an isolable and reusable nanocatalyst employed in this important catalytic reaction.
Experimental
Materials
PtCl4 (99% Alfa Aesar), tetrahydrofuran (THF) (99.5%, Merck), lithium triethylborohydride (1.0 M dissolved in THF, Sigma Aldrich), amylamine (Sigma Aldrich), dimethylamine-borane (Sigma Aldrich) were used as received from suppliers. THF was distilled over sodium under argon atmosphere and stored under inert atmosphere. The water was deionized by Millipore water purification system (18 MΩ) in analytical grade.Instrumentation
TEM and HRTEM micrograph was recorded using a JEOL-200 CX 120 kV microscope. Kratos AXIS Ultra spectrometer was used to perform XPS analysis. A Rigaku diffractometer was used for P-XRD. 11B NMR spectra were taken on a Bruker Avance DPX 400 MHz spectrometer (128.2 MHz for 11B NMR). Leeman Ind. ICP was used in the ICP-OES analyses. ATR-IR spectra were taken on a Nicolet Magna-IR 750 spectrometer.Preparation of amylamine stabilized platinum(0) nanoparticles (Pt(0)/AA)
Pt(0)/AA were prepared by following the ethanol–superhydride reduction method.21 In this method, superhydride and ethanol were used to reduce the mixture of 0.25 mmol (0.081 g) of PtCl4 dissolved in small amount of anhydrous tetrahydrofuran and 0.25 mmol of amylamine ligand. The observation of a brown color in the solution indicates the formation of amine stabilized platinum nanoparticles. Besides, excess surfactants should be removed by employing washing process with ethanol, followed by drying process in a vacuum to obtain solid Pt(0)/AA.Catalytic activity of Pt(0)/AA in the dehydrogenation of DMAB
All reactions and manipulations were performed under a dry nitrogen atmosphere using standard Schlenk techniques including a vacuum system unless otherwise specified. Pt(0)/AA catalyzed dehydrogenation of DMAB was performed in a typical jacketed, three-necked reaction flask connected to the water-filled cylinder glass tube. The jacketed reaction flask was kept under vacuum at least for 15 min and filled with nitrogen to remove any trace of oxygen and water present before all catalytic reactions. The catalytic activity of Pt(0)/AA in the dehydrogenation of DMAB was determined by measuring the rate of hydrogen generation. In a vial 31.0 mg (1.0 mmol) of AB was dissolved in 4.0 mL of THF. The solution was transferred into the jacketed reaction flask containing 1.0 mL THF solution of Pt(0)/AA thermostatted at 25.0 ± 0.1 °C. Hydrogen gas generation from the catalytic reaction solution was followed using a typical water-filled gas buret system and recording the displacement of the water level in the gas buret every minute until no more hydrogen evolution was observed. When no more hydrogen generation was observed, the experiment was stopped, the reactor was disconnected from the water-filled tube, and the hydrogen pressure was released. Next, an approximately 0.5 mL aliquot of the reaction solution in the reactor was withdrawn with a glass Pasteur pipet and added to 1 g of CDCl3 in a quartz NMR sample tube (Norell S-500-QTZ), which was subsequently sealed.Reusability of Pt(0)/AA in the dehydrogenation of DMAB
After the first run of the dehydrogenation of 1.0 mmol of DMAB starting with Pt(0)/AA, the jacketed, three-necked reaction flask was detached from the line and connected to a vacuum line. After the evaporation of volatiles, the solid residue was weighed and used again in the dehydrogenation of 1.0 mmol of DMAB under the same conditions (in 5.0 mL THF at 25.0 ± 0.1 °C). This procedure was followed up to four catalytic runs.Mercury (Hg(0)) poisoning of Pt(0)/AA in the dehydrogenation of DMAB
Elemental Hg (300 equiv.) was added into a 5.0 mL THF solution containing Pt(0)/AA in the jacketed, three-necked reaction flask and the mixture stirred for 4 h. Then, this solution was used in the dehydrogenation of 1.0 mmol DMAB under the same conditions given above.Results and discussion
Preparation and characterization of amylamine stabilized platinum(0) nanoparticles (Pt(0)/AA)
Amylamine stabilized platinum(0) nanoparticles (Pt(0)/AA) were readily and reproducibly produced by ethanol–superhydride reduction of PtCl4 in an anhydrous THF solution of amylamine at room temperature. When the reduction of PtCl4 is performed in the absence of stabilizing ligand such as amylamine, the initially formed platinum(0) nanoparticles quickly agglomerate and precipitate out of the THF solution. In the presence of amylamine ligand, the platinum(0) nanoparticles formed from the reduction are found to be stable towards agglomeration over months. This finding demonstrates the role of amylamine ligand as a strong stabilizing agent for platinum(0) nanoparticles. After the washing of the as-prepared Pt(0)/AA with dry ethanol for removing excess amylamine ligand, they can be isolated as solid by evaporation of solvent under vacuum (10−3 Torr). The isolated Pt(0)/AA containing 90 wt% Pt (10 wt% AA) as determined by ICP-OES, were found to be redispersible in THF and retain their colloidal stability over 3 weeks. The preliminary characterization of Pt(0)/AA were done by ICP-OES, XRD, ATR-IR, TEM, HRTEM, and XPS spectroscopies.
Fig. 1 shows powder X-ray diffraction (P-XRD) patterns of Pt(0)/AA, The diffraction lines at about 2θ = 39.90, 46.60, 67.50, 81.20 and 86.70 can be attributed to (111), (200), (220), (311) and (222) planes of the face-centered cubic (fcc) crystal lattice of platinum.21,22 The lattice parameter (aPt) value of the resulting Pt(0)/AA was calculated as 3.922 Å by considering Pt (220) diffraction peak from the following equation:23
 | ||
| Fig. 1 Powder X-ray diffraction pattern of amylamine stabilized platinum(0) nanoparticles in the 2θ range of 10–90°. | ||
Moreover, the average crystalline size (d) of the catalyst was calculated as about 3.20 nm using full width half maximum of the (220) Bragg peak in XRD, from the Scherrer equation:24
The coordination of the amylamine ligand was examined by ATR-IR spectroscopy. ATR-IR spectrum of the isolated solid Pt(0)/AA shows characteristics N–H and C–H stretchings at around 2900 and 1400 cm−1, respectively. The size, morphology, and crystallinity of Pt(0)/AA were investigated by using transmission electron microscopy (TEM) and high resolution-TEM (HRTEM). TEM image (Fig. 2(a)) and corresponding histogram (>100 particles were counted) of Pt(0)/AA (Fig. 2(b)) are given in Fig. 2, they show the presence of Pt(0)/AA in the range of 2.4–4.3 nm with a mean diameter of 3.30 ± 0.85 nm which is in good agreement with P-XRD result. In addition, the selected region HRTEM image of Pt(0)/AA (Fig. 2(a) inset) reveals that the highly crystalline feature of these platinum(0) nanoparticles with a crystalline spacing of 0.227 nm which is very close to nominal Pt(111) spacing of 0.228 nm.
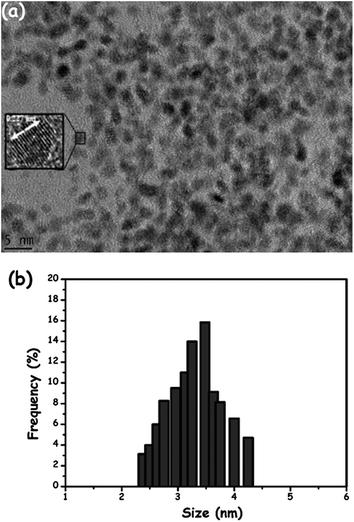 | ||
| Fig. 2 (a) TEM and high resolution-TEM (inset) images, (b) the corresponding size histogram of amylamine stabilized platinum(0) nanoparticles. | ||
The oxidation state of platinum in Pt(0)/AA sample was examined by X-ray photoelectron spectroscopy (XPS). The deconvulation of the high resolution Pt 4f region (Fig. 3) contains two pairs of doublet. The ratio of the 4f7/2/4f5/2 signals for Pt(0)/AA was found to be 4/3, which agrees with the literature.25 The more intense doublet at about 71.0 and 74.3 eV is a signature of metallic platinum (Pt(0))26 and the other doublet at about 74.3 and 77.6 eV can be assigned to Pt(IV) species such as PtO227 and/or Pt (OH)4,28 which might be formed by the surface oxidation of Pt(0)/AA during XPS sampling procedure.
 | ||
| Fig. 3 High resolution X-ray photoelectron spectrum (XPS) of amylamine stabilized platinum(0) nanoparticles. | ||
Catalytic activity of Pt(0)/AA in the dehydrogenation of dimethylamine-borane (DMAB)
Pt(0)/AA was tested as a catalyst in the catalytic dehydrogenation of DMAB at 25 ± 0.1 °C and its catalytic activity in this reaction was determined by measuring the volume of hydrogen gas generated during the reaction and by 11B NMR spectroscopy to identify the reaction products. After the addition of DMAB into a THF solution of Pt(0)/AA the hydrogen evolution starts immediately with an initial turnover frequency of 15 h−1 and continues until 1 equivalent of H2 per mol DMAB is liberated (Fig. 4). When we used PtCl4 as precatalyst under the same conditions in the dehydrogenation of DMAB, after an induction time period (∼7 min.) we observed only 10% conversion. The aggregation of Pt particles and their precipitation out of solution were seen within 10 min. by naked eye, since the sole stabilizer present in that system is the weakly coordinating chloride anion which cannot provide enough stabilization for the platinum(0) nanoparticles.29 The 11B NMR spectrum of the reaction solution taken at the end of the Pt(0)/AA catalyzed dehydrogenation of DMAB (at 1.0 equiv. H2 generation) also shows complete conversion of (CH3)2NHBH3 (δ = −12.8 ppm) to [(CH3)2NBH2]2 (δ = 5 ppm)5,6 even at room temperature (Fig. 5). The heterogeneous nature of Pt(0)/AA catalyst in the dehydrogenation of DMAB was investigated by performing mercury poisoning experiment. It is well-known that, mercury(0) can poison heterogeneous catalysts by amalgamating the metal catalyst or being adsorbed on its surface.30 The suppression of catalysis by mercury(0) is considered to be compelling evidence for heterogeneous catalysis.31 In our case, the performing of mercury (Hg(0)) poisoning experiment, shows that the reaction is entirely ceased by adding 300 equiv. of Hg(0) per Pt, which reveals that Pt(0)/AA act as a heterogeneous catalyst. | ||
| Fig. 4 Plots % conversion versus time graph for the catalytic dehydrogenation of DMAB in THF at room temperature starting with 20% mol of Pt(0)/AA (●) and PtCl4 (○). | ||
 | ||
| Fig. 5 11B NMR spectra of (a) DMAB in THF and (b) reaction solution taken at the end of the Pt(0)/AA catalyzed dehydrogenation of DMAB in THF at room temperature. | ||
Determination of activation parameters (Ea, ΔH#, and ΔS#) for Pt(0)/AA catalyzed dehydrogenation of DMAB
Fig. 6 shows the stoichiometric ratio of generated H2 to (CH3)2NHBH3versus time for the catalytic dehydrogenation of DMAB starting with Pt(0)/AA at four different temperatures. First of all, it is seen that the dehydrogenation rate of DMAB by Pt(0)/AA catalyst increases by the increase of temperature (1.93, 3.65, 5.09 and 6.03 mol H2 per mol DMAB min for 20, 25, 30 and 35 °C, respectively). Secondly, Fig. 6 is showing that Pt(0)/AA can catalyze the dehydrogenation of DMAB at 1.0 equiv. of H2 generation even at low temperature (20 °C). | ||
| Fig. 6 Plots % conversion versus time graph for Pt(0)/AA (7.5% mol) catalyzed dehydrogenation of DMAB in THF at various temperatures as given on the graph. | ||
The observed rate constants (kobs) determined from the nearly linear portions of the plots at four different temperatures were used for the plotting of Arrhenis32 (Fig. 7(a)) and Eyring33 (Fig. 7(b)) graphs to calculate activation parameters: activation energy Ea = 63.9 kJ mol−1, activation enthalpy ΔH# = 51.7 kJ mol−1, and activation entropy ΔS# = −61.7 J mol−1 K−1. The activation energy value (63.9 kJ mol−1) provided by our Pt(0)/AA is higher than Rh(0) nanoparticles (34 kJ mol−1) but comparable with Ru(0) nanoparticles (61 kJ mol−1) catalysts for the same reaction. Additionally, the small value of the activation enthalpy and the large negative value of the activation entropy indicate an associative mechanism in the transition state for the catalytic dehydrogenation of DMAB.
 | ||
| Fig. 7 (a) Arrhenius and (b) Eyring plots for Pt(0)/AA catalyzed dehydrogenation of DMAB at various temperatures. | ||
Reusability performance of Pt(0)/AA in the catalytic dehydrogenation of DMAB
The isolability and reusability of Pt(0)/AA, two crucial measures in heterogeneous catalysis, were also tested in the dehydrogenation of DMAB at room temperature. After complete dehydrogenation of DMAB, Pt(0)/AA were isolated as powder by drying in vacuo and then bottled under an Ar atmosphere. Such isolated Pt(0)/AA were found to be still active in the dehydrogenation of DMAB. They retain >75% of their initial catalytic activity even at the fourth catalytic run (Fig. 8). The decrease in the activity can be attributed to the formation of a precipitate of bulk Pt(0) metal, which becomes visible at the end of the 4th catalytic run, eventually yielding a clear, colorless (i.e., Pt(0) nanoparticle free). P-XRD pattern of the sample harvested from the 4th catalytic run showed that the formation of amorphous phase (no diffraction lines) resulting from the agglomeration of Pt(0) nanoparticles.34 XPS analysis of the same sample, which gives the same XPS spectrum, indicated that Pt(0) nanoparticles do not oxidized throughout the reusability experiments, which is expected as DMAB is a well-known reducing agent.1,3,5,7 | ||
| Fig. 8 Plots % conversion versus time graph for Pt(0)/AA (20% mol) catalyzed dehydrogenation of DMAB in THF at room temperature for 1st and 4th catalytic runs. | ||
Catalytic performance comparison of Pt(0)/AA with the previous catalyst systems tested in the catalytic dehydrogenation of DMAB
The apparent initial TOF value of Pt(0)NPs/AA (15 h−1) is lower than with that of the prior best heterogeneous5 (60 h−1) and homogeneous14 (420 h−1) catalysts but higher than the majority of those of other heterogeneous and homogeneous catalysts reported up to now (Table 1).| Entry | (Pre)catalysts | Conv. (%) | TOF | Ref. |
|---|---|---|---|---|
| 1 | [Rh(1,5-cod)μ-Cl]2 | 100 | 12.5 | 7 |
| 2 | [Ir(1,5-cod)μ-Cl]2 | 95 | 0.7 | 7 |
| 3 | RhCl3 | 90 | 7.9 | 7 |
| 4 | IrCl3 | 25 | 0.3 | 7 |
| 5 | RhCl(PPh3)3 | 100 | 4.3 | 7 |
| 6 | [Cp*Rh(μ-Cl)Cl]2 | 100 | 0.9 | 7 |
| 7 | [Rh(1,5-cod)2]Otf | 95 | 12.0 | 7 |
| 8 | [Rh(1,5-cod)(dmpe)]PF6 | 95 | 1.7 | 7 |
| 9 | HRh(CO)(PPh3)3 | 5 | 0.1 | 7 |
| 10 | trans-RuMe2(PMe3)4 | 100 | 12.4 | 7 |
| 11 | trans-PdCl2(P(o-tolyl)3)2 | 20 | 0.2 | 7 |
| 12 | Pd/C | 95 | 2.8 | 7 |
| 13 | Rh(0)/[Noct4]Cl | 90 | 8.2 | 7 |
| 14 | Cp2Ti | 100 | 12.3 | 8 |
| 15 | [(C5H3-1,3(SiMe3)2)2Ti]2 | 100 | 420.0 | 14 |
| 16 | [RhCl(PHCy2)3] | 100 | 2.6 | 9 |
| 17 | Rh(0)NPs | 100 | 60.0 | 5 |
| 18 | [RuH(PMe3)(NC2H4PPr2)2] | 100 | 1.5 | 10 |
| 19 | (Idipp)CuCl | 100 | 0.3 | 11 |
| 20 | [Cr(CO)5(thf)] | 97 | 13.4 | 12 |
| 21 | [Cr(CO)5(η1-BH3NMe3)] | 97 | 19.9 | 12 |
| 22 | RuCl3·3H2O | 77 | 2.7 | 6 |
| 23 | [Ru(1,5-cod)Cl2]n | 70 | 2.5 | 6 |
| 24 | Ru(cod)(cot) | 40 | 1.6 | 6 |
| 25 | Ru(0)/APTS | 100 | 55.0 | 6 |
| 26 | Ni(skeletal) | 100 | 3.2 | 13 |
| 27 | Pt(0)NPs/AA | 100 | 15.0 |
To the best of our knowledge, apart from the Ru(0)/APTS,6,35 Pt(0)/AA are the second example of an isolable and reusable nanocatalyst used in this important catalytic reaction. More importantly, the reusability performance of our new Pt(0) nanocatalyst is also much better than the previously known most active Rh(0) nanocatalyst,5 which does not have enough stability to isolation and reusability. In this study Rh(0) nanoparticles have been formed under in situ conditions, where the surface adsorbed anions can not provide enough stabilization to these in situ generated nanoparticles. Although they provided the highest catalytic activity among the heterogeneous catalysts, they were not found to be reusable catalyst in this important reaction.
Conclusions
In summary, we have fabricated and characterized, for the first time, isolable and redispersible amylamine stabilized platinum(0) nanoparticles by following a simple and reproducible method. These new Pt(0) nanoparticles were found to be active and reusable catalytic material in the dehydrogenation of dimethylamine-borane, which has been considered as one of the attractive materials for the efficient chemical hydrogen storage.Notes and references
- A. Staubitz, A. P. M. Robertson and I. Manners, Chem. Rev., 2010, 110, 4079 CrossRef CAS PubMed.
- J. Turner, G. Sverdrup, K. Mann, P. G. Maness, B. Kroposki, M. Ghirardi, R. J. Evans and D. Blake, Int. J. Energy Res., 2007, 32, 379 CrossRef.
- A. Staubitz, P. A. Soto and I. Manners, Angew. Chem., Int. Ed., 2008, 47, 6212 CrossRef CAS PubMed.
- D. Jacquemin, C. Lambert and E. A. Perpete, Macromolecules, 2004, 37, 1009 CrossRef CAS.
- M. Zahmakiran and S. Özkar, Inorg. Chem., 2009, 48, 8955 CrossRef CAS PubMed.
- M. Zahmakiran, M. Tristany, K. Philippot, K. Fajerwerg, S. Özkar and B. Chaudret, Chem. Commun., 2010, 46, 2938 RSC.
- C. A. Jaska, K. Temple, A. J. Lough and I. Manners, J. Am. Chem. Soc., 2003, 125, 9424 CrossRef CAS PubMed.
- T. J. Clark, C. A. Russell and I. Manners, J. Am. Chem. Soc., 2006, 128, 9582 CrossRef CAS PubMed.
- M. Sloan, T. J. Clark and I. Manners, Inorg. Chem., 2009, 48, 2429 CrossRef CAS PubMed.
- A. Friendrich, M. Drees and S. Schneider, Chem.–Eur. J, 2009, 15, 10339 CrossRef PubMed.
- R. J. Keaton, J. M. Blacquiere and R. T. Baker, J. Am. Chem. Soc., 2007, 129, 11936 CrossRef PubMed.
- Y. Kawano, M. Uruichi, M. Shiomi, S. Taki, T. Kawaguchi, T. Kakizawa and H. Ogino, J. Am. Chem. Soc., 2009, 131, 14946 CrossRef CAS PubMed.
- A. P. M. Robertson, R. Suter, L. Chabanne, G. R. Whittel and I. Manners, Inorg. Chem., 2011, 50, 12680 CrossRef CAS PubMed.
- D. Pun, E. Lobkovsky and P. J. Chirik, Chem. Commun., 2007, 3297 RSC.
- L. J. Sewell, M. A. Huertos, M. E. Dickinson and A. S. Weller, Inorg. Chem., 2013, 52, 4509 CrossRef CAS PubMed.
- R. T. Baker, J. C. Gordon, C. W. Hamilton, N. J. Henson, P.-H. Lin, S. Maguire, M. Murugesu, B. L. Scott and N. C. Smythe, J. Am. Chem. Soc., 2012, 134, 5598 CrossRef CAS PubMed.
- M. Zahmakiran and S. Özkar, Nanoscale, 2011, 3, 3462 RSC.
- S. Guo, S. Zhang and S. Sun, Angew. Chem., Int. Ed., 2013, 52, 8526 CrossRef CAS PubMed.
- Z. Peng and H. Yang, Nano Today, 2009, 4, 143 CrossRef CAS PubMed.
- J. Wu, J. Zhu, M. Zhou, Y. Hou and S. Gao, CrystEngComm, 2012, 14, 7572 RSC.
- F. Şen, S. Şen and G. Gökağaç, Phys. Chem. Chem. Phys., 2011, 13, 1676 RSC.
- J.-H. Jang, J. Kim, Y.-H. Lee, I. Y. Kim, M.-H. Park, C.-W. Yang, S.-J. Hwang and Y.-U. Kwon, Energy Environ. Sci., 2011, 4, 4947 CAS.
- Z. Liu, X. Y. Ling, X. Su and J. Y. Lee, J. Phys. Chem. B, 2004, 108, 8234 CrossRef CAS.
- H. Klug and L. Alexander, X-ray Diffraction Procedures, Wiley, New York, 1st edn, 1954 Search PubMed.
- J. Huang, H. Yang, Q. Huang, Y. Tang, T. Lu and D. L. Akins, J. Electrochem. Soc., 2004, 151, A1810 CrossRef CAS PubMed.
- F. Şen and G. Gökağaç, J. Phys. Chem. C, 2007, 111, 1467 Search PubMed.
- Z. Ozturk, F. Şen, S. Şen and G. Gökağaç, J. Mater. Sci., 2012, 47, 8134 CrossRef CAS.
- G. Gökağaç, B. J. Kennedy, J. D. Cashion and L. J. Brown, J. Chem. Soc., Faraday Trans., 1993, 89, 151 RSC.
- S. Ozkar and R. G. Finke, J. Am. Chem. Soc., 2002, 124, 5796 CrossRef PubMed.
- G. M. Whitesides, M. Hackett, R. L. Brainard, J. P. Lavalleye, A. F. Sowinski, A. N. Izumi, S. S. Moore, D. W. Brown and E. M. Staudt, Organometallics, 1985, 4, 1819 CrossRef CAS.
- J. A. Widegren and R. G. Finke, J. Mol. Catal. A: Chem., 2003, 198, 317 CrossRef CAS.
- K. J. Laidler, Chemical Kinetics, Benjamin-Cummings, UK, 3rd edn, 1997 Search PubMed.
- H. Eyring, J. Chem. Phys., 1935, 3, 107 CrossRef CAS.
- Y. Sun, L. Zhuang, J. Lu, X. Hong and P. Liu, J. Am. Chem. Soc., 2007, 129, 15465 CrossRef CAS PubMed.
- M. Zahmakiran, K. Philippot, S. Özkar and B. Chaudret, Dalton Trans., 2012, 41, 590 RSC.
| This journal is © The Royal Society of Chemistry 2014 |