 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effect of ionic liquids on the conformation of a porphyrin-based viscometer†
Laramie P. Jamesona,
Joseph D. Kimballb,
Zygmunt Gryczynskib,
Milan Balaz*c and
Sergei V. Dzyuba*a
aDepartment of Chemistry, Texas Christian University, Fort Worth, TX 76129, USA. E-mail: s.dzyuba@tcu.edu; Fax: +1 817 257 5851; Tel: +1 817 257 6218
bDepartment of Physics and Astronomy, Texas Christian University, Fort Worth, TX 76129, USA
cDepartment of Chemistry, University of Wyoming, Laramie, WY 82071, USA. E-mail: mbalaz@uwyo.edu; Fax: +1 307 766-2807; Tel: +1 307 766-4330
First published on 5th August 2013
Abstract
Structure of the cationic and anionic counterparts of ionic liquids has a significant impact on the conformational bias of the porphyrin rotor; an apparent correlation between the conformation and the viscosity of ionic liquids was noted, albeit it was found to be distinct and more complex from that found in molecular solvents.
Introduction
Ionic liquids (ILs) are materials comprised exclusively of ions, and have phase-transition temperatures at or below room temperature.1 ILs have found numerous and ever-expanding applications in various areas of science and engineering.1–7Virtually unlimited structural variations of ILs, which can be obtained via well-developed synthetic routes, are likely to give rise to distinct physicochemical properties such as phase transitions, density, viscosity, polarity, hydrogen bonding abilities, etc.8 Arguably, these physical properties could have an impact on solutes, and therefore, the ILs can be designed to control the outcome of a given process by simply varying the structure of cationic and anionic components. This has led to the idea of ILs as designer solvents. The designer solvent ability has been defined as the manipulation of a structure and thus physical property of the IL which can predictably alter the outcome of a given process.9,10 As such, this paradigm has been illustrated through several synthetic transformations.9–11 However, applications demonstrating the designer solvent ability of ILs outside of their use as milieu for synthetic organic transformations appear to be limited. Notably, it was demonstrated that structural changes within either the cation or anion could be used to modulate the conformational flexibility of a small molecule.12,13 Possibilities for using neat ILs to control intermolecular interactions have been suggested primarily for cyanine dye self-assemblies.14
Studies on viscosity of ILs using fluorescent dyes has been a subject of interest,15–17 and the use of fluorescent probes for understanding the structure of ILs, in general, has been recently reviewed.18 In addition, it was shown that the viscosity of molecular organic media could control the conformation of a conjugated porphyrin dimer (PD), resulting in an array of conformations with two distinct extremes: a planar and a twisted conformation (Fig. 1).19,20 This characteristic is unique for this particular probe, as many fluorescent molecular rotors used for viscosity studies report on viscosity primarily through an emission enhancement21 or lifetime measurement,22 rather than a distinct wavelength. A linear dependence was observed between the conformational preferences of PD and viscosity of the media.20 Importantly, planar and twisted conformations exhibited distinct emission maxima: 710 nm for twisted and 780 nm for planar, which allowed for a facile and straightforward evaluation of the conformational preference of PD. Specifically, in non-viscous media, e.g., methanol, the planar conformer was shown to predominate, while in more viscous methanol–glycerol mixtures, the twisted conformation was observed.20 Thus, PD was proposed to be a suitable probe for an investigation of the media's viscosity.
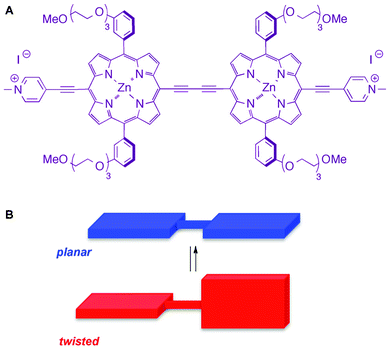 | ||
| Fig. 1 Structure (A) and conformational extremes (B) of PD. | ||
Conversely, viscosity of the media might be viewed as a physical property that could control the conformation of PD. Because the viscosity of ILs can easily be tuned by varying the structure of the cationic and anionic counterparts,8 we investigated whether the conformational preference of PD would be altered as a consequence of changes in the viscosity due to variances in the IL structure. As such, this could also be used as a corroboration of the designer solvent ability of ILs,9,10 as well as a demonstration of the distinct nature of ILs as compared to the molecular solvents.
First, the fluorescence spectra of PD were measured in ethanol, glycerol and their respective mixtures (Fig. 2A, see Table S1 and Fig. S1 for additional information†).
Next, in order to evaluate the scope of single component molecular solvents, the physical properties of more viscous decanol, ethylene glycol and tetraethylene glycol were also measured (Fig. 2B, see Table S2 for additional information†). Qualitatively our results were in complete agreement with the published data:20 the planar conformer of PD dominated in less viscous media, whereas the twisted conformation started to dominate in more viscous media. Specifically, the switch from the planar to the twisted conformation of PD took place at a molecular solvent viscosity of ca. 39 mPa s. Notably, a good linear correlation between the viscosity and a conformational preference of PD was found (Fig. 2C).
![Emission spectra of PD in EtOH–glycerol mixtures of different viscosity (A), single component molecular solvents of different viscosity (B), and effect of media's viscosity on % of twisted PD (C, black diamonds – EtOH–glycerol mixtures, grey diamonds – single component solvents). Conditions: λex = 470 nm, [PD] = 1 μM, all mixtures contain 0.1% DMSO (v/v).](/image/article/2013/RA/c3ra43001d/c3ra43001d-f2.gif) | ||
| Fig. 2 Emission spectra of PD in EtOH–glycerol mixtures of different viscosity (A), single component molecular solvents of different viscosity (B), and effect of media's viscosity on % of twisted PD (C, black diamonds – EtOH–glycerol mixtures, grey diamonds – single component solvents). Conditions: λex = 470 nm, [PD] = 1 μM, all mixtures contain 0.1% DMSO (v/v). | ||
In order to examine the correlation between the conformational preference of PD and the viscosity of ILs, we employed ILs based on one of the most commonly used cationic components, i.e., 1-alkyl-3-methylimidazolium, [Cn-mim]. It is well known that the viscosity of this type of ILs can be altered by varying the length of the alkyl chain as well as the identity of the anion.23–28 Hence, we prepared several sets of [Cn-mim]-based ILs (Scheme 1) in order to investigate the effect of viscosity on PD conformation and potential cation and/or anion effects.
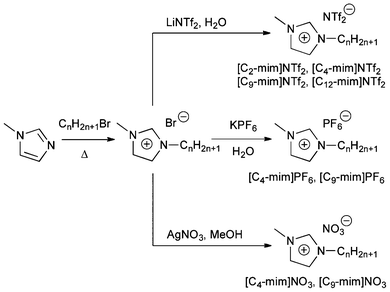 | ||
| Scheme 1 Synthesis and abbreviations of ILs. | ||
ILs were chosen to cover a wide range of viscosities.23–28 In general, for 1-alkyl-3-methylimidazolium-containing ILs, increase of the alkyl chain length leads to more viscous ILs. It is also known that NTf2-based ILs are among the least viscous imidazolium-type ILs, while viscosity of PF6-containing ILs spans over a fairly wide range. In addition, NO3-containing ILs, which are generally much more hydrophilic than the corresponding NTf2- and PF6-containing congeners, were chosen due to similarities in viscosity with the PF6-containing set of ILs.28,29 Collectively, this set of ILs would aid in identifying possible anion and cation effects on the PD conformation.
Furthermore, the viscosity of ILs is known to be greatly affected by the presence of water.27,28 Therefore, the water content of all ILs was measured after the viscosity and spectroscopic measurements were performed (Table S3, ESI†). It could be argued that in order for the ILs to be viable from the practical standpoint, all manipulations involving the ILs must be conducted on a bench top. As such, no attempts to reduce the amount of residual water in ILs, beyond commonly applied azeotropic water removal and vacuum drying were taken (see ESI† for further details).
Concurrent with water content measurements, we examined the conformational preference of PD in ILs using fluorescence spectroscopy (Fig. 3). It should be noted that ILs are intrinsically fluorescent, and the origin of the fluorescence has been debated in the literature.29 However, a number of experimental and theoretical accounts from various groups have suggested that it is likely to be related to the microstructuring within ILs.30 Here, for all fluorescence experiments that feature PD, the fluorescence spectra of ILs were subtracted.
![Fluorescence spectra of PD in [Cn-mim]NTf2 ILs (A), [Cn-mim]PF6 ILs (B), [Cn-mim]NO3 ILs (C), and effect of ILs' viscosity on % of twisted PD (D: [Cn-mim]NTf2 – black symbols; [Cn-mim]PF6 – purple symbols; [Cn-mim]NO3 – grey symbols). Conditions: λex = 475 nm, [PD] = 1 μM, all mixtures contain 0.1% DMSO (v/v).](/image/article/2013/RA/c3ra43001d/c3ra43001d-f3.gif) | ||
| Fig. 3 Fluorescence spectra of PD in [Cn-mim]NTf2 ILs (A), [Cn-mim]PF6 ILs (B), [Cn-mim]NO3 ILs (C), and effect of ILs' viscosity on % of twisted PD (D: [Cn-mim]NTf2 – black symbols; [Cn-mim]PF6 – purple symbols; [Cn-mim]NO3 – grey symbols). Conditions: λex = 475 nm, [PD] = 1 μM, all mixtures contain 0.1% DMSO (v/v). | ||
Viscosities of common imidazolium-based ILs are known to be orders of magnitude higher than those of many common molecular solvents, and as such the twisted conformation of PD was expected to be seen for all ILs with a viscosity of ca. 40 mPa s. However, in contrast to molecular solvents (Fig. 2), IL [C2-mim]NTf2, with η = 38 mPa s significantly promoted the planar conformer of PD (Fig. 3A, see Fig. S1, ESI† for absorption spectra of PD in ILs). As the viscosity of the NTf2-containing ILs increased as a function of the alkyl chain length, the preference for the twisted conformation of PD increased as well. A similar effect was observed for the PF6- and NO3-containing ILs (Fig. 3B and 3C, respectively). Thus, the cation of the ILs might have a significant role in the conformational bias of PD.
Furthermore, when the viscosity was plotted as a function of the twisted conformation of PD, an apparent correlation was observed (Fig. 3D), albeit the linearity was found to be somewhat poorer from that observed in the case of the molecular solvents (Fig. 2C). In addition, since the slope in the case of ILs is smaller than that compared to molecular solvents, i.e., 6.0577 versus 7.8798, respectively, a significantly higher viscosity would be required for ILs to induce the twisted conformation when compared to the molecular solvents. Overall, within each set of ILs with a common anion, the percentage of the planar conformation of PD appeared to decrease as the viscosity of the IL increased (Fig. 3: A, B and C). For example, in all [C4-mim]-containing ILs (Fig. 3; see Fig. S2, ESI,† for overlaid spectra), despite ca. 6-fold viscosity range (60 for [C4-mim]NTf2 to 262 for [C4-mim]NO3 to 381 mPa s for [C4-mim]PF6), the planar conformation of PD was observed (although a linear correlation with viscosity was noted). This was reminiscent of the correlation with viscosity observed in the molecular solvents (Fig. 2).
However, upon closer examination of the aforementioned trend (Fig. 3D), it is evident that the viscosity of the ILs might not be a dominant factor in controlling the conformational bias of PD. Specifically, when comparing the viscosities of [C12-mim]NTf2, [C4-mim]NO3 and [C4-mim]PF6, which progressively increased in viscosity from 202 to 262 to 381 mPa s, no correlation in regard to the PD conformation was observed (Fig. S3, ESI†). An inverse correlation was evident for [C9-mim]NO3 and [C9-mim]PF6 ILs (Fig. 3D, see Fig. S4, ESI† for overlaid spectra): with increasing viscosity of IL, the planar conformation started to dominate.
It is plausible that in ILs specific solvent–solute interactions (as well as the structure of ILs) could control the conformation of a small molecule, rather than the viscosity of the media. Unlike molecular solvents, which interact with solutes via dipole–dipole interactions and hydrogen bonding, ILs have the added ability to interact via ion–, and ion–ion interactions, i.e., via electrostatic interactions. It was also suggested that a significant amount of solute stabilization by ILs comes from the cation,30 which might explain the apparent cation effect observed here.
To gain further support for the notion that viscosity of ILs might not be the dominant factor in controlling PD's conformation in ILs, we examined the relationship between viscosity and conformational bias of PD as a function of temperature. Arguably, if the viscosity were the main factor that modulated the conformation of PD, then changing the temperature (and as a result changing the viscosity of the solvent) should result in a linear correlation between the viscosity and the percent of the twisted conformation of PD. Also, the slope should be similar to that observed for solvents of various viscosities at a fixed temperature. Conversely, if the slope of the viscosity (obtained at different temperatures) as a function of PD conformation would be different from the slope obtained for solvents of various viscosities at a fixed temperature, some specific interactions between the solvents and PD might be present.
To test this hypothesis, we examined the conformation of PD in several molecular solvents (Tables S4–5; Fig. S5, ESI†) and ILs (Tables S6–8; Fig. S6, ESI†) at various temperatures and consequently viscosities (Fig. 4).
![Effect of solvent (A: molecular solvents; B: ILs) viscosity on % of twisted PD measured at 20–60 °C range (see ESI for additional information); conditions: λex = 470 nm for A, λex = 475 nm for B, [PD] = 1 μM, all mixtures contain 0.1% DMSO (v/v).](/image/article/2013/RA/c3ra43001d/c3ra43001d-f4.gif) | ||
| Fig. 4 Effect of solvent (A: molecular solvents; B: ILs) viscosity on % of twisted PD measured at 20–60 °C range (see ESI for additional information†); conditions: λex = 470 nm for A, λex = 475 nm for B, [PD] = 1 μM, all mixtures contain 0.1% DMSO (v/v). | ||
The slope of viscosity as a function of the twisted PD in EtOH–glycerol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 v/v) mixture as well as tetraethylene glycol (Fig. 4A) appeared to be very similar to that obtained for various molecular solvents (Fig. 2C). Arguably, this indicated that viscosity of molecular solvents was the major factor that controlled the conformational bias of PD (See Fig. S7, ESI† for overlaid data points).
8 v/v) mixture as well as tetraethylene glycol (Fig. 4A) appeared to be very similar to that obtained for various molecular solvents (Fig. 2C). Arguably, this indicated that viscosity of molecular solvents was the major factor that controlled the conformational bias of PD (See Fig. S7, ESI† for overlaid data points).
On the contrary, in ILs no apparent correlation among several different ILs was observed (Fig. 4B). Although for PF6-containing ILs ([C4-mim]PF6 and [C9-mim]PF6) somewhat similar slopes were obtained, the two data sets were off-set. When the ILs with the same cation were compared ([C4-mim]NO3 and [C4-mim]PF6), the corresponding slopes were found be distinctly different. Therefore, it is plausible that in ILs, solute–solvent specific interactions are playing a significantly more prominent role than viscosity. This is in contrast to the observation noted for the molecular solvents.
Finally, we decided to investigate the effect of water in [C4-mim]NO3 ILs on the viscosity of the IL (Table S9, ESI†) as well as the conformational preference of PD. Following the aforementioned rationale on the effect of temperature on viscosity and the conformation of PD, we reasoned that the presence of water should alter the PD–[C4-mim]NO3 interactions, which should produce a distinct correlation between IL viscosity and PD conformation from that observed upon variation of temperature (Fig. 4B) as well as the structure of ILs (Fig. 3D). It is worth pointing out that in water alone, PD was found to be non-fluorescent.20 Consistent with this assumption, we observed a linear correlation between the viscosity of [C4-mim]NO3 with various amounts of water and the conformation of PD (Fig. 5). However, the slope was found to be drastically different, i.e., 7.7892 as compared to 5.1156, from that found for the temperature effect of the PD emission in [C4-mim]NO3 (Fig. 4B), for example.
![Emission spectra of PD in [C4-mim]NO3 as a function of the water content. Insert: effect of [C4-mim]NO3 viscosity (red symbol – 10% of H2O; blue symbol – 1.7% of H2O and green – 0.5% of H2O) as a function of PD conformation. Conditions: [PD] = 1 μM, λex = 475 nm, [DMSO] = 0.1% (v/v).](/image/article/2013/RA/c3ra43001d/c3ra43001d-f5.gif) | ||
| Fig. 5 Emission spectra of PD in [C4-mim]NO3 as a function of the water content. Insert: effect of [C4-mim]NO3 viscosity (red symbol – 10% of H2O; blue symbol – 1.7% of H2O and green – 0.5% of H2O) as a function of PD conformation. Conditions: [PD] = 1 μM, λex = 475 nm, [DMSO] = 0.1% (v/v). | ||
Conclusions
Overall, the structure of ILs appeared to have an effect on the conformation of PD. ILs promoted the opposite conformation as molecular solvents with similar viscosities, i.e., planar vs. twisted, respectively, and an order of magnitude larger viscosity of ILs was required to promote a similar amount of twisted PD. Specifically, the viscosity range at which twisted conformation becomes dominant is shifted from ca. 40 (in molecular solvents) to 400 (in ILs) mPa s range. There appeared to be linear correlations between PD's conformation and the viscosity in ILs, molecular solvent mixtures and single component molecular solvents.However, the cation effect (in all ILs with a common cation a similar conformation of PD was present, regardless of viscosity) seemed to indicate that the structure of IL could control the conformation of PD. In this light, it is important to distinguish between properties of ILs that correlate with a given process and those that actually control the process. Hence, in ILs with short alkyl chain lengths i.e. [C4-mim] and [C2-mim], the solvent–solute interactions promoted the planar conformation, regardless of viscosity. Similarly, ILs with longer alkyl chains, i.e., [C9-mim] and [C12-mim], seemed to promote the twisted conformation, which happened to correlate with the increased viscosity of the solvent. However, it was apparent that the structure of ILs plays a more important role in the control of the PD conformation and the correlation between the ILs' viscosity and PD conformation might be coincidental. It should be noted that a microheterogenous nature of the ILs could be playing a role as well.31,32
While the exact nature of the observed phenomena remains to be elucidated, this report highlighted the designer solvent ability of ILs in that ILs of different structures gave rise to different PD conformations. Notably, an order of magnitude higher viscosity was required in ILs to induce a similar conformation of PD, potentially emphasising specific interactions between the ILs and PD.
Acknowledgements
This work was partially supported by the donors of the ACS Petroleum Research Fund (47965-G7 to SVD), by the University of Wyoming Start-up Funds (to MB), and by TCU start-up funds (to ZG). We would like to thank Fidelis N. Ngwa (UW) for the synthesis of the PD precursors, Professor Onofrio Annunziata (TCU-Chemistry) for helpful discussions, and Professor Eric E. Simanek (TCU-Chemistry) for acquisition of and access to Lovis 2000ME/DMA 4500 M instrument.Notes and references
- J. P. Hallet and T. Welton, Chem. Rev., 2011, 111, 3508 CrossRef PubMed.
- M. H. G. Pretchtl and S. Sahler, Curr. Org. Chem., 2013, 17, 220 CrossRef.
- Z. Fei and P. J. Dyson, Chem. Commun., 2013, 49, 2594 RSC.
- A. Brandt, J. Graesvik, J. P. Hallett and T. Welton, Green Chem., 2013, 15, 550 RSC.
- X. Sun, H. Luo and S. Dai, Chem. Rev., 2012, 112, 2100 CrossRef CAS PubMed.
- T. L. Greraves and C. J. Drummond, Chem. Soc. Rev., 2013, 42, 1096 RSC.
- H. Weigawrtner, C. Cabrele and C. Herrmann, Phys. Chem. Chem. Phys., 2012, 14, 415 RSC.
- C. Chiappe and D. Pieraccini, J. Phys. Org. Chem., 2005, 18, 275 CrossRef CAS.
- I. Newington, J. M. Perez-Arlandis and T. Welton, Org. Lett., 2007, 9, 5247 CrossRef CAS PubMed.
- M. J. Earle, S. P. Katdare and K. R. Seddon, Org. Lett., 2004, 6, 707 CrossRef CAS PubMed.
- S. V. Dzyuba and R. A. Bartsch, Tetrahedron Lett., 2002, 43, 4657 CrossRef CAS.
- L. P. Jameson and S. V. Dzyuba, J. Nat. Prod., 2011, 74, 310 CrossRef CAS PubMed.
- L. P. Jameson and S. V. Dzyuba, RSC Adv., 2013, 3, 4582 RSC.
- V. Kumar, G. A. Baker and S. Pandey, Chem. Commun., 2011, 47, 4730 RSC.
- D. C. Khara, J. P. Jaini, N. Mondal and A. Samanta, J. Phys. Chem. B, 2013, 117, 5156 CrossRef CAS PubMed.
- S. Mandal, S. Ghosh, C. Banerjee, J. Kuchlyan and N. Sarkar, J. Phys. Chem. B, 2013, 117, 6789 CrossRef CAS PubMed.
- B. Li, Y. Wnag, X. Wang, S. Vdovic, Q. Guo and A. Xia, J. Phys. Chem. B, 2012, 116, 13272 CrossRef CAS PubMed.
- S. Padney, S. N. Baker, S. Padney and G. A. Baker, J. Fluoresc., 2012, 22, 1313 CrossRef PubMed.
- M. Balaz, H. A. Collins, E. Dahlstedt and H. L. Anderson, Org. Biomol. Chem., 2009, 7, 874 CAS.
- M. K. Kuimova, S. W. Bothchway, A. W. Parker, M. Balaz, H. A. Collins, H. L. Anderson, K. Suhling and P. R. Ogilby, Nat. Chem., 2009, 1, 69 CrossRef CAS PubMed.
- M. A. Haidekker, T. P. Brady, D. Lichlyter and E. A. Theodorakis, J. Am. Chem. Soc., 2006, 128, 398 CrossRef PubMed.
- M. K. Kuimova, Phys. Chem. Chem. Phys., 2012, 14, 12671 RSC.
- A. Ahosseini and A. M. Scurto, Int. J. Thermophys., 2008, 29, 1222 CrossRef CAS.
- H. Shirota, T. Mandai, H. Fukazawa and T. Kato, J. Chem. Eng. Data, 2011, 56, 2453 CrossRef CAS.
- S. V. Dzyuba and R. A. Bartsch, ChemPhysChem, 2002, 3, 161 CrossRef CAS.
- B. Mokhrarani, A. Sharifi, H. R. Mortaheb, M. Mirzaei, M. Mafi and F. Sadeghian, J. Chem. Thermodyn., 2009, 41, 1432 CrossRef PubMed.
- J. G. Huddleston, A. E. Visser, W. M. Reichert, H. D. Willauer, G. A. Broker and R. D. Rogers, Green Chem., 2001, 3, 156 RSC.
- K. R. Seddon, A. Stark and M. Torres, Pure Appl. Chem., 2000, 72, 2275 CrossRef CAS.
- P. K. Mandal and A. Samanta, J. Phys. Chem. B, 2005, 109, 15172 CrossRef CAS PubMed.
- A. Samanta, J. Phys. Chem. Lett., 2010, 1, 1557 CrossRef CAS.
- A. Paul and A. Samanta, J. Phys. Chem. B, 2008, 112, 16626 CrossRef CAS PubMed.
- O. Russina, A. Triolo, L. Gontrani and R. Caminti, J. Phys. Chem. Lett., 2012, 3, 27 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details on the synthesis and purification of ILs, characterization data of PD, sample preparations and physical properties of ILs and molecular solvents and their mixtures as well as additional spectra. See DOI: 10.1039/c3ra43001d |
| This journal is © The Royal Society of Chemistry 2013 |
