Facile solution routes for the syntheses of GeTe nanocrystals†
Myeong Ho
Kim ‡
,
Gajendra
Gupta ‡
and
Jinkwon
Kim
*
Department of Chemistry, Nanotechnology Laboratory, Kongju National University, 182 -Shinkwan Kongju-Chungnam, 314-701, Republic of Korea. E-mail: jkim@kongju.ac.kr; Fax: +82 418528496; Tel: +82418568613
First published on 5th October 2012
Abstract
Solution-based synthetic routes are attractive strategies for synthesizing GeTe materials, because they have the potential to impart morphology control on the crystallites and permit liquid-based processing of films and patterned structures. Two liquid phase reaction systems for GeTe nanoparticles (NPs) have been studied using GeCl2·dioxane and trioctylphosphine-tellurium (TOP-Te) in oleylamine (OLA) as solvent and reducing agent and using GeCl2·dioxane and (Et3Si)2Te in trioctylphosphine oxide (TOPO) without the use of any reducing agent. The morphology of the GeTe powders had a strong dependence on the Te source and reaction medium. The SEM image of NPs obtained by the reaction of GeCl2·dioxane and (Et3Si)2Te reveals that with an increase in reaction time (2, 10, 15 and 30 min), the size of the NPs increases and their shape becomes uniform. However, it is interesting to observe that after 30 min, the morphology of the nanoparticles was maintained even after longer reaction times i.e., the duration of heating had no pronounced influence on the size and morphology of the nanocrystals after a particular period of time. Reaction with TOP-Te leads to the formation of irregular GeTe nanocrystals through the so called Ostwald-ripening process. However, with (Et3Si)2Te as Te source, a ligand exchange reaction mechanism has been proposed leading to the formation of well-dispersed GeTe nanoparticles of uniform shape.
Introduction
Germanium telluride (GeTe) has received much attention due to thermally induced behaviours such as amorphous-to-crystalline phase transition,1 ferroelectric phase transition,2 and thermoelectricity.3 A pronounced change of conductivity and reflectivity of GeTe on crystallization enables electrical switching in phase change random access memory (PCRAM)4 and operating optical data storage media.1,5–7 In principle, PCRAM exhibits many potential advantages over conventional electronic memory, including fast access time, low power consumption, low cost, scalability, and nonvolatility;8 consequently, the electronics industry has been actively developing and testing PCRAM for future nonvolatile memory applications. The ferroelectric phase change occurs by a symmetry-breaking distortion from the nonpolar rock salt structure to the polar rhombohedral phase below ∼350 °C.9Solution-based synthetic routes represent an attractive alternative for depositing films and generating dimensionally controlled nanoscale materials, including for quantum-confined binary antimonide and telluride semiconductors. Formation of colloidal faceted GeTe particles and phase change memory materials with distinct properties are recently reported.10–13 However, there have been very few reports describing liquid-phase routes to chalcogenide phase change materials. Recently, R. E. Schaak and co-workers described an important addition to the small number of reports on solution routes to Ge-based phase change materials: a bench top liquid-phase synthesis of crystalline GeTe via reduction of GeI2 with tert-butylaminoborane (TBAB) in the presence of TOP-Te at 180 °C.14 The GeTe product consists of highly faceted microcrystals having cube-shape morphology with a narrow size distribution. More recently, Alivisatos et al. demonstrated size dependent polar ordering by the synthesis of GeTe nanocrystals over a wide range of sizes.15,16 The production of these nanocrystals of widely varying sizes is facilitated by the use of Ge(II) precursors with different reactivities. In all the recent progress, TOP-Te has been generally used for the synthesis of GeTe nanocrystals. On the other hand, alkylsilyl compounds of Te have been exploited as efficient precursors in the atomic layer deposition (ALD) process for Ge2Sb2Te5 (GST) thin film.17 Particularly, (Et3Si)2Te proved to be a better ALD precursor compared to alkyls and alkylamides of Te. Upon reaction with metal halides, the exchange reaction is facilitated by fast bonding of trialkylsilyl moieties and halides. Based on the above considerations, we have conducted two liquid phase syntheses of GeTe nano crystals; one method via reduction of GeCl2·dioxane with OLA in the presence of TOP-Te, another method via the substitution reaction of GeCl2·dioxane and (Et3Si)2Te in TOPO. Smaller GeTe products are obtained with (Et3Si)2Te as Te source as compared to its TOP-Te analogue. These two reactions show that the shape and size are strongly dependent on the Te precursor.
Experimental
Materials and synthesis processes
All chemicals were used as received. Germanium(II) chloride dioxane complex (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), tellurium (99.99%), trioctylphosphine (tech. 90%), oleylamine (tech. 70%), trioctylphosphine oxide (90%), triethylchlorosilane (Aldrich, 99%), sodium (99.9%) were purchased from Sigma-Aldrich. Tetrahydrofuran (THF) was freshly distilled from sodium benzophenone ketyl. Acetone and chloroform (HPLC grade) were purchased from Duksan Chemicals, Korea. The starting precursor (Et3Si)2Te was synthesized by using a standard procedure.17 All syntheses were performed under nitrogen using standard Schlenk techniques and work-ups were performed in air.
1), tellurium (99.99%), trioctylphosphine (tech. 90%), oleylamine (tech. 70%), trioctylphosphine oxide (90%), triethylchlorosilane (Aldrich, 99%), sodium (99.9%) were purchased from Sigma-Aldrich. Tetrahydrofuran (THF) was freshly distilled from sodium benzophenone ketyl. Acetone and chloroform (HPLC grade) were purchased from Duksan Chemicals, Korea. The starting precursor (Et3Si)2Te was synthesized by using a standard procedure.17 All syntheses were performed under nitrogen using standard Schlenk techniques and work-ups were performed in air.
Syntheses of GeTe nano crystals
(a) Synthesis of GeTe nanocrystals using reduction method (sample 1)A clear yellow TOP-Te complex stock solution (0.75 M) was first prepared by dissolving 288 mg Te powder in 3 mL trioctylphosphine (TOP) at 200 °C. GeCl2·dioxane (0.75 mmol, 173.73 mg) was dissolved in 1 mL TOP via sonication. Oleylamine (15 mL) was placed in a three neck flask and dried under vacuum at 70 °C for 1 h. Then the reaction flask was flushed with nitrogen and the temperature was raised to 150 °C and the Ge precursor solution was injected. The solution color immediately changed from colorless to yellow, presumably due to the reduction of Ge2+ to Ge0. After 1 min, 1 mL of 0.75 M TOP-Te solution was injected into the Ge solution and stirred for the desired time and the reaction was allowed to cool down to room temperature. The resulting GeTe precipitate was purified by washing with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 acetone/chloroform and dried under vacuum, yielding a gray powder.
1 acetone/chloroform and dried under vacuum, yielding a gray powder.
(b) Synthesis of GeTe nanocrystals using substitution reaction (sample 2)
GeCl2·dioxane (0.75 mmol, 173.73 mg) was dissolved in 2 mL TOP via ultrasonication. TOPO (4 g) was placed in a three neck flask and dried under vacuum at 70 °C for 1 h. Then the reaction flask was flushed with nitrogen and the temperature was raised to 250 °C and the Ge precursor solution was injected. The Te precursor (Et3Si)2Te (0.75 mmol, 268.1 mg) was dissolved in 2 mL TOP via sonication, and was injected into the reaction mixture and stirred for the desired time and the reaction was allowed to cool down to room temperature. The resulting GeTe precipitate was purified by washing with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 acetone/chloroform and dried under vacuum, yielding a gray powder.
1 acetone/chloroform and dried under vacuum, yielding a gray powder.
Characterization
Powder X-ray diffraction (XRD) data were obtained from a Rigaku DMAX 2000 X-ray diffractometer. Transmission electron microscopy (TEM) images and energy-dispersive X-ray spectroscopy (EDS) data were obtained from a Tecnai G2F30 field emission transmission electron microscope. Scanning electron microscope (SEM) images were obtained from an Ultra-high Resolution FE-SEM S-4800 (Hitachi) microscope. Differential scanning calorimetry (DSC) was performed on a Labsys TG-DSC 1600 machine under flowing nitrogen at a heating rate of 10 °C min−1. The X-ray photoelectron spectra (XPS) were recorded on a MultiLab ESCA 2000 machine after etching the surface oxide layer of samples.Results and discussion
Two samples of GeTe crystals were obtained by different methods. The reaction of GeCl2·dioxane and TOP-Te in the reducing media of olylamine at 150 °C gave a crystalline solid of GeTe (sample 1). Another reaction of GeCl2·dioxane and (Et3Si)2Te in TOPO at 250 °C without the use of any reducing agent resulted in the formation of GeTe nanocrystals (sample 2). The powder XRD analyses of sample 1 and sample 2 obtained at a reaction time of 30 min show the same pattern as displayed in Fig. 1. All the diffraction peaks of each sample correspond to the rhombohedral crystal structure of GeTe (space group R3m (No. 160) and JCPDS No. 47-1079), consistent with the composition of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Ge
1 Ge![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Te found by EDS.
Te found by EDS.
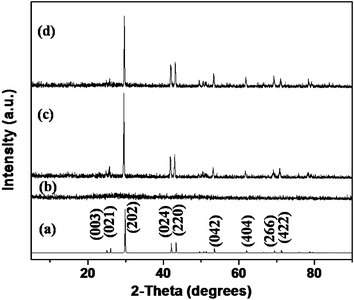 | ||
| Fig. 1 XRD patterns of rhombohedral GeTe crystals composed of (a) reference, (b) amorphous sample, (c) sample 1 and (d) sample 2. | ||
SEM images of sample 1 and sample 2 at different reaction times are displayed in Fig. 2 and Fig. 3, respectively. For sample 1, at the beginning of the reaction, the products are amorphous with a size of about 20 nm as displayed in Fig. 2a, but after 10 min, the amorphous powders start to agglomerate and transform into bigger crystalline particles of different sizes. After 30 min, the final size and shape of the crystals are formed without further significant change. In the case of sample 2, amorphous powders formed in the initial stage of the reaction turned crystalline without significant growth of particles, as shown in Fig. 3a and 3b. Further heating of the reaction mixture produced uniform GeTe crystals.
 | ||
| Fig. 2 SEM images of GeTe crystals (sample 1) produced from reaction of Ge2+ and TOP-Te in OLA at 150 °C and reaction times of (a) 2 min, (b) 10 min, (c) 30 min, (d) 60 min. | ||
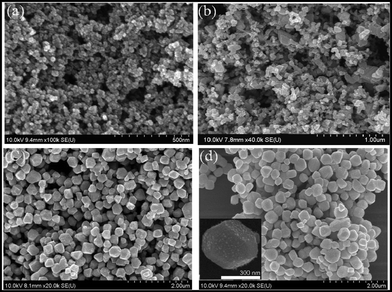 | ||
| Fig. 3 SEM images of GeTe crystals (sample 2) produced from reaction of Ge2+ and (Et3Si)2Te in TOPO at 250 °C and reaction times of (a) 2 min, (b) 10 min, (c) 30 min, (d) 60 min (inset: SEM image of a single GeTe crystal). | ||
On the basis of the above observations, plausible growth mechanisms of GeTe NPs can be suggested as illustrated in Scheme 1. In the reduction method, i.e., for sample 1, the growth process can be explained by two steps, an initial nucleating stage and a subsequent crystal growth process. The Ge atoms were formed by reduction of Ge2+ by oleylamine. At the same time, Te atoms were also formed in solution by thermal decomposition. These two species in solution immediately react to produce GeTe seed crystals. As the reaction time increases, the GeTe seed crystals start to agglomerate together to form much bigger crystals as shown in Fig. 2b. With further increase in reaction time, larger GeTe NPs grow at the expense of smaller ones through the so called Ostwald-ripening process when the temperature is maintained at 150 °C. This prolonged heating can supply enough energy to the system to overcome the energy barrier for ripening.18 During this process, smaller particles will dissolve to feed the growth of larger ones. Thus, only irregular nanocrystals are formed.
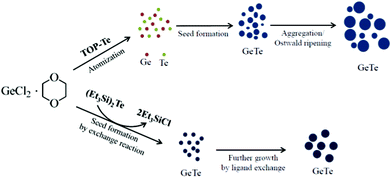 | ||
| Scheme 1 Schematic illustration for the growth processes of GeTe nanocrystals. | ||
However, in the case of NPs formed from the reaction of Ge2+ and (Et3Si)2Te in TOPO, a ligand exchange reaction as shown in eqn (1) takes place to form GeTe seed powders. In this case, we assume that the rate of the substitution reaction might be slower than that in the case of sample 1. Furthermore, the seeds tend to grow bigger crystals by subsequent reaction of Ge2+ and Te2− ions instead of the agglomeration of seeds. SEM images show that the duration of heating had no pronounced influence on the size and morphology of the nanocrystals after a reaction period of 30 min. The completed reaction mixture shows none of the smaller particles. Thus, refluxing for 30 min and 1 h resulted in similar size and shape of the synthesized nanocrystals. A highly practical feature of our ligand exchange method is that the final nanocrystals’ size is highly reproducible. From this above two different growth behaviours, we assume that the Te source plays a very important role in determining the shape and size of the nanocrystals.
 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 53 and 56.6
53 and 56.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43.4 ratios of Ge
43.4 ratios of Ge![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Te, which are in agreement with the nominal 1
Te, which are in agreement with the nominal 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of Ge
1 ratio of Ge![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Te expected in the products, although we could not rule out the possible presence of GeO2 impurities on the surface by the XPS study which is discussed below.
Te expected in the products, although we could not rule out the possible presence of GeO2 impurities on the surface by the XPS study which is discussed below.
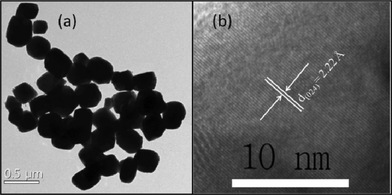 | ||
| Fig. 4 (a) TEM and (b) HRTEM images of the GeTe crystals produced from reaction of Ge2+ and (Et3Si)2Te in TOPO at 250 °C and a reaction time of 1 h. | ||
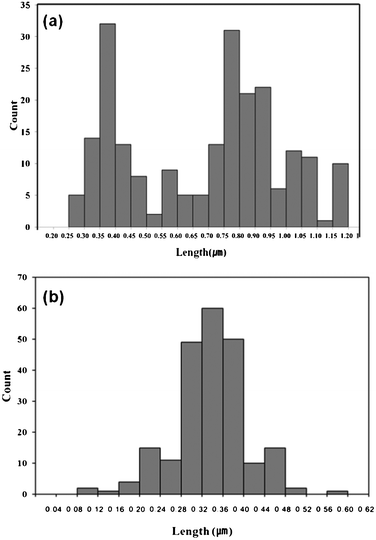 | ||
| Fig. 5 Size distribution histograms for the GeTe crystallites obtained at 1 h using (a) TOP-Te and (b) (Et3Si)2Te. | ||
The thermal behaviour of samples containing GeTe particles was investigated using DSC as shown in Fig. 6. Fig. 6a shows the curve for sample 1 obtained at 1 h, whereas Fig. 6b displays the curve of sample 2 at 1 h. In Fig. 6a, an endotherm is observed at 724.1 °C which agrees well with the melting point of bulk GeTe (725 °C).19 Whereas, in Fig. 6b, an endotherm is observed at 721.8 °C.
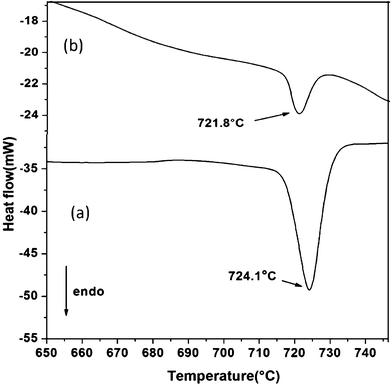 | ||
| Fig. 6 DSC traces for the GeTe crystals for (a) sample 1 and (b) sample 2 at 1 h reaction times. | ||
Inferences of the oxidation state of germanium and tellurium atoms in the nanomaterials were assigned through XPS. Fig. 7 shows the XPS data of sample 1 measured in the Ge 3d and Te 3d regions for GeTe crystals after etching to remove the oxide layer on the surface. A peak binding energy at 29.75 eV for GeTe crystals is attributed to Ge 3d. Peaks for Te 3d5/2 and Te 3d3/2 were observed at 573 eV and 583.45 eV. Peak values of Ge 3d and Te 3d of GeTe nanocrystals are in good agreement with values reported in the literature.19,20 The surface of GeTe crystals were oxidized upon exposure to air for a long time.21–23
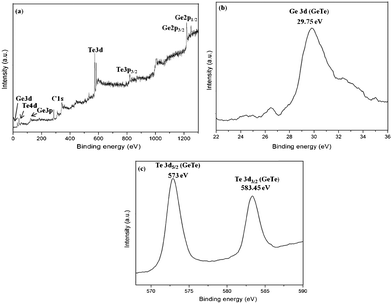 | ||
| Fig. 7 XPS spectra of the GeTe product (sample 1) in the Ge 3d and Te 3d regions. | ||
Conclusions
In summary, we have used two facile liquid phase methods for the synthesis of crystalline GeTe nanoparticles (NPs) using germanium chloride dioxane and elemental tellurium dispersed in tri-n-octylphosphine (TOP) in the presence of olylamine (OLA) which acts as reducing agent as well as solvent and using germanium chloride dioxane and (Et3Si)2Te in the presence of TOPO as solvent without the use of any reducing agent. The GeTe nanoparticles obtained show an interesting phenomenon in the nature of their growth and we assume that this depends on the reaction time and the Te source used. We proposed an Ostwald-ripening mechanism for their growth in the case of reaction with TOP-Te and a ligand exchange reaction in the case of reaction with (Et3Si)2Te as Te source. A highly practical feature of our ligand exchange method is that the final nanocrystals’ size is highly reproducible in good yield. We believe that the nanoparticles synthesized could be desirable for reducing the reset current of a PCRAM device, and hence they can accelerate the speed of memory switching.Acknowledgements
Financial support from the Converging Research Center Program (2012K001295) and the Priority Research Center Program (2012-0006682) and through the National Research Foundation of Korea are gratefully acknowledged.References
- M. Wuttig and N. Yamada, Nat. Mater., 2007, 6, 824 CrossRef CAS.
- M. E. Lines and A. M. Glass, Principles and Applications of Ferroelectrics and Related Materials; Clarendon Press: Oxford, 1977 Search PubMed.
- J. Snyder and E. S. Toberer, Nat. Mater., 2008, 7, 105 CrossRef.
- G. Bruns, P. Merkelbach, C. Schlockermann, M. Salinga, M. Wuttig, T. D. Happ, J. B. Philipp and M. Kund, Appl. Phys. Lett., 2009, 95, 43108 CrossRef.
- N. Yamada, E. Ohno, K. Nishiuchi, N. Akahira and M. Takao, J. Appl. Phys., 1991, 69, 2849 CrossRef CAS.
- N. Yamada, MRS Bull., 1996, 21, 48 CAS.
- M. H. R. Lankhorst, B. Ketelaars and R. A. M. Wolters, Nat. Mater., 2005, 4, 347 CrossRef CAS.
- S. Lai, IEDM Tech. Dig., 2003, 255 Search PubMed.
- E. F. Steigmeier and G. Harbeke, Solid State Commun., 1970, 8, 1275 CrossRef CAS.
- M. A. Caldwell, S. Raoux, R. Y. Wang, H. S. P. Wong and D. J. Milliron, J. Mater. Chem., 2010, 20, 1285 RSC.
- I. U. Arachchige, R. Soriano, C. D. Malliakas, S. A. Ivanov and M. G. Kanatzidis, Adv. Funct. Mater., 2011, 21, 2737 CrossRef CAS.
- H. Y. Tuan and B. A. Korgel, Cryst. Growth Des., 2008, 8, 2555 CAS.
- M. A. Caldwell, R. G. D. Jeyasingh, H. S. P. Wong and D. J. Milliron, Nanoscale, 2012, 4, 4382 RSC.
- M. R. Buck, I. T. Sines and R. E. Schaak, Chem. Mater., 2010, 22, 3236 CrossRef CAS.
- M. J. Polking, H. Zheng, R. Ramesh and A. P. Alivisatos, J. Am. Chem. Soc., 2011, 133, 2044 CrossRef CAS.
- M. J. Polking, J. J. Urban, D. J. Milliron, H. Zheng, E. Chan, M. A. Caldwell, S. Raoux, C. F. Kisielowski, J. W. Ager, R. Ramesh and A. P. Alivisatos, Nano Lett., 2011, 11, 1147 CrossRef CAS.
- V. Pore, T. Hatanpââ, M. Ritala and M. Leskelâ, J. Am. Chem. Soc., 2009, 131, 3478 CrossRef CAS.
- Y. -M. Sung, W. -C. Kwak and T. G. Kim, Cryst. Growth Des., 2008, 8, 1186 CAS.
- N. J. Shevchik, J. Tejeda, D. W. Langer and M. Cardona, Phys. Rev. Lett., 1973, 30, 659 CrossRef CAS.
- B. Yu, X. Sun, S. Ju, D. B. Janes and M. Meyyappan, IEEE Trans. Nanotechnol., 2008, 7, 496 CrossRef.
- O. S. Gogishvili, A. N. Degaltsev, G. G. Kononov, I. P. Lavrinenko and S. P. Lalykin, Inorg. Mater., 1988, 24, 944 Search PubMed.
- T. F. Sheveleva, Y. B. Plaksina and T. P. Markholiya, Inorg. Mater., 1976, 12, 791 Search PubMed.
- L. V. Yashina, S. P. Kobeleva, T. B. Shatalova, V. P. Zlomanov and V. I. Shtanov, Solid State Ionics, 2001, 141, 513 CrossRef.
Footnotes |
| † Electronic Supplementary Information (ESI) available: STEM image and EDS spectrum along with elemental mapping and XPS spectra of the GeTe product. See DOI: 10.1039/c2ra21790b |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2013 |
