DOI:
10.1039/C2RA21481D
(Paper)
RSC Adv., 2013,
3, 253-258
Synthesis of multicomponent sulfide Ag2ZnSnS4 as an efficient photocatalyst for H2 production under visible light irradiation
Received 18th July 2012, Accepted 2nd November 2012
First published on 2nd November 2012
Abstract
Stannite-type multicomponent sulfide (Ag2ZnSnS4) nanoparticles with particle diameters of 100–200 nm were synthesized through a solvothermal treatment combined with a post-annealing process under N2 atmosphere. The obtained Ag2ZnSnS4 was characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), high-resolution transmission electron microscopy (HRTEM), UV-vis diffuse reflectance spectroscopy (DRS) and X-ray photoelectron spectroscopy (XPS). The photocatalytic activities for hydrogen production of Ag2ZnSnS4 were evaluated under visible light (λ ≥ 420 nm) irradiation. It was found that the 1.0 wt% Pt-loaded Ag2ZnSnS4 displayed a photocatalytic hydrogen evolution activity of 580 μmol h−1 with a fairly good apparent quantum efficiency of 15.2% at 420 nm incident light irradiation.
1 Introduction
The development of visible light-responsive photocatalysts for H2 production from water has become a research focus because visible light energy composes around 43% of incoming solar energy.1–5 Among various visible light-driven photocatalysts developed, sulfides have been extensively investigated due to their suitable energy band structures for water splitting.3–5 For example, cadmium sulfide (CdS) is one of the most typical compounds for photocatalytic H2 production among the chalcogenides.6,7 Li and co-workers found that the photoactivity of CdS could be enhanced significantly by loading a small amount of a noble metal (Pt, Pd, Ru, Rh) or its sulfide (PdS, Rh2S3, Ru2S3),8,9 and CdS co-deposited with Pt and PdS demonstrated a significantly high quantum efficiency (∼93%) at 420 nm for photocatalytic H2 production from Na2S–Na2SO3 aqueous solution. The synergistic effect between Pt and PdS co-catalyst could be explained by the fact that the two co-catalysts may facilitate separation of the photogenerated carriers.9 However, a major bottleneck limiting the application of CdS is its high toxicity and photocorrosion. Therefore, it is necessary to develop some other novel metal sulfide photocatalysts with a visible light response to meet the requirements of future energy technologies driven by solar energy.In the past decade, designing sulfide solid solution photocatalysts with controlled energy band structures by coupling wide and narrow bandgap semiconductors was considered as a promising method to develop visible light-responsive photocatalysts. For example, Cd1−xZnxS (0 < x < 1) solid solutions, formed by combining ZnS and CdS, have attracted much research interest for visible light-driven photocatalysis. The more negative reduction potential of the conduction band (CB) leads to more efficient H2 production as compared with CdS.10,11 Since Kudo's group reported a series of ZnS–CuInS2/AgInS2 solid solutions as new highly efficient photocatalysts for H2 evolution under visible light irradiation in 2004,12 it has been proved that the energy band structure of the compounds was controllable by varying the component of the composition, and the apparent quantum efficiencies of Pt-loaded (AgIn)0.22Zn1.56S2 and (CuIn)0.09Zn1.82S2 amounted to 20 and 12.5% at 420 nm incident light irradiation, respectively.12–15
Recently, novel stannite-type complex sulfides AI2–Zn–AIV–S4 (AI = Cu or Ag; AIV = Sn or Ge) solid solutions as excellent light energy-harvesting candidates for solar cell materials and photocatalysts have been widely investigated.16–24 Tsuji and co-workers have prepared a series of AI2–Zn–AIV–S4 solid solutions by calcining the corresponding precursors precipitated by bubbling with H2S gas into the mixed inorganic solution.17 Among these novel photocatalysts, Ru-loaded Ag2ZnSnS4 showed the highest activity for photocatalytic H2 evolution from an aqueous solution containing S2− and SO32− salts under visible light irradiation, with a quantum efficiency of ∼3% at 500 nm incident light irradiation, and its action spectrum for H2 production showed a wide range of visible light up to 620 nm, which agreed well with the diffuse reflection spectrum, indicating that those Ag-containing complex sulfides are promising as an excellent light energy-harvesting photocatalysts for H2 production.17 Herein, a stannite-type Ag2ZnSnS4 nanoparticles were prepared through a solvothermal treatment combined with a post-annealing process under N2 atmosphere. The crystal structure, morphology, optical properties and photocatalytic activity of the Ag2ZnSnS4 products were discussed in detail.
2 Experimental
2.1 Material preparation
All chemicals were of analytical grade and used as received without further purification. In a typical procedure, 2 mmol AgNO3, 1 mmol Zn(NO3)2·6H2O and 8 mmol thioacetamide (TAA) were dispersed in 30 mL of absolute ethanol under vigorous stirring, and then 1 mmol SnCl4·5H2O was added into the mixture. Afterward, the mixed solution was transferred into a 50 mL Teflon-lined stainless steel autoclave, which was sealed and kept at 180 °C for 12 h, and then cooled to room temperature naturally. The precipitate was filtered and washed with distilled water and absolute ethanol several times, and then was dried in vacuo at 80 °C for 12 h to obtain the as-prepared precursor, which was loaded in a quartz boat and annealed at 500 °C for 10 h in a N2 atmosphere with a heating rate of 5 °C min−1. Prior to the photoreaction, Pt co-catalyst was loaded on the Ag2ZnSnS4 by an in situ photoreduction of H2PtCl6 solution by using a 500 W high pressure Hg lamp.252.2 Material characterization
X-Ray powder diffraction (XRD) patterns were obtained using a Bruker D8 Advance X-ray diffractometer with Cu-Kα irradiation (λ = 0.154178 nm) at 40 kV and 40 mA. A scan rate of 0.1° s−1 was applied to record the XRD patterns for 2θ in the range of 10–70°. The morphology of the samples was investigated by scanning electron microscopy (SEM) using a HITACHI S-3000N microscope operated at 20 kV. The high-resolution transmission electron microscopy (HRTEM) measurements were conducted using a JEOL JEM 2100F microscope working at 200 kV. X-Ray photoelectron spectroscopy (XPS) measurements were performed on a VG Multilab 2000 X-ray photoelectron spectroscope equipped with a standard and monochromatic source (Al Kα) operated at 300 W. The UV-vis diffuse reflectance spectra (DRS) were obtained using a Shimadzu UV-3600 spectrophotometer equipped with an integrating sphere with BaSO4 as the reference.2.3 Evaluation of photocatalytic H2 production activity
The photoreactions were carried out in an outer irradiation-type photoreactor (Pyrex glass) connected to a closed gas circulation system. Approximately 0.100 g of Pt-loaded Ag2ZnSnS4 was dispersed by a magnetic stirrer in 100 mL of aqueous solution containing 0.25 M Na2SO3 and 0.35 M Na2S as sacrificial reagents. Nitrogen was purged through the cell before reaction to remove oxygen. The photocatalyst suspension was irradiated with visible light (λ ≥ 420 nm) using a cutoff filter from a 300 W Xe-lamp. The photocatalytic H2 evolution amount was analyzed with an online gas chromatograph (GC, SP-6890, TCD detector, 5 Å molecular sieve columns and argon carrier). Control experiments showed no appreciable H2 evolution without light irradiation or photocatalyst.The apparent quantum efficiency (AQE) was measured under the same photocatalytic reaction conditions except for the light wavelength of irradiation. The H2 yields of 1 h photoreaction under different monochromatic light wavelengths (420 or 500 nm) were measured. The energy flux (mW cm−2) of the Xe lamp was measured with a calibrated Si photodiode (SRC-1000-TC-QZ-N, Oriel, USA), and the photon flux absorbed by photocatalyst was obtained by dividing the energy flux by the energy of a photon with a wavelength of 420 or 500 nm. The AQE value at 420 or 500 nm was calculated by eqn (1).
| |  | (1) |
3 Results and discussion
3.1 XRD and microstructure analyses
Fig. 1 shows the XRD patterns of the obtained precursor, Ag2ZnSnS4, and Pt-loaded Ag2ZnSnS4 before and after 15 h photoreaction for H2 production. As shown in Fig. 1, the precursor derived from the solvthermal process shows some diffraction peaks (marked with asterisks) of Ag8SnS6 (JCPDS No. 38-0434) with low crystallinity. The other coexisting very weak peaks indicate this product might be a mixture including certain amorphous phases. After annealing at 500 °C, the product shows an XRD pattern of Ag2ZnSnS4 with stannite-like structure (JCPDS, No. 35-0544), which is in agreement with the literature.16 No diffraction peak for any other crystal phase such as Ag8SnS6 can be detected, indicating that there is no impurity phase existing in the obtained sample. The diffraction peaks at 2θ = 27.4, 30.8, 44.1, 46.1, 52.6, and 55.9° can be attributed to the reflections of the (112), (200), (220), (204), (312), and (224) planes of the tetragonal Ag2ZnSnS4, respectively.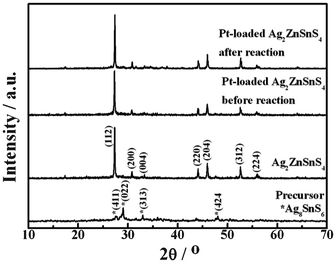 |
| | Fig. 1 XRD patterns of precursor, Ag2ZnSnS4, and Pt-loaded Ag2ZnSnS4 before and after the 15 h photocatalytic reaction. | |
Fig. 2 depicts the SEM and TEM images of the prepared Ag2ZnSnS4. As can been seen, the obtained Ag2ZnSnS4 shows irregular nanoparticles with particle size of 100–200 nm, which are smaller than the reported bulky ones.17 The HRTEM image in Fig. 2c shows the interplanar spacing is measured to be 0.326 nm, which is in accordance with the (112) plane of tetragonal Ag2ZnSnS4. Moreover, it is found that a coverture layer with amorphous structure surrounded the surface of Ag2ZnSnS4 particles, which can be ascribed to the adsorbed organic molecule carbonization during the annealing at 500 °C in the N2 atmosphere.
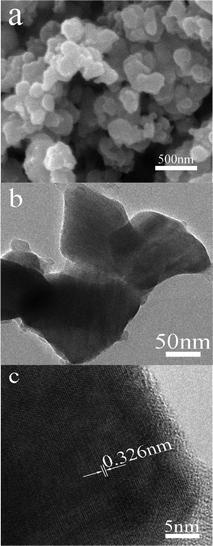 |
| | Fig. 2 SEM (a) and HRTEM (b,c) images of Ag2ZnSnS4. | |
3.2 UV-vis diffuse reflectance spectra analyses
The optical absorption properties of a semiconductor, which are relevant to the electronic structure features, are recognized as key factors in determining its photoactivity. Fig. 3 shows the UV-vis diffuse reflectance spectra (DRS) of the obtained Ag2ZnSnS4.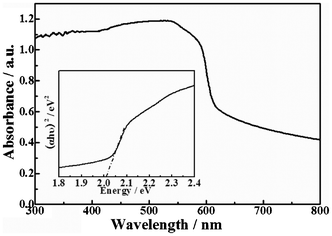 |
| | Fig. 3 UV-vis diffuse reflectance spectra (DRS) of Ag2ZnSnS4 and the band gap value (inset, estimated by a related curve of (αhν)2versus photon energy plotted). | |
As can be seen, the Ag2ZnSnS4 presents a sharp absorption edge at about 620 nm along with enhanced absorption in the whole visible light region detected. The enhanced absorption in the further visible light region may be due to the fact that the Ag2ZnSnS4 contained little carbon derived from calcination treatment at 500 °C in the N2 atmosphere, which can increase the surface electric charge of Ag2ZnSnS4 and cause the modifications of the fundamental process of electron–hole pair formation during light irradiation.26 The experimental result is in good agreement with the above HRTEM investigation. The band gap energy is estimated to be 2.01 eV from the inset in Fig. 3, which is a related curve of (αhν)2versus photon energy (α = absorbance coefficient, h = Planck's constant, ν = frequency).
3.3 XPS analyses
Fig. 4a displays the XPS survey spectrum for Ag2ZnSnS4 nanoparticles, which shows Ag 3d, Zn 2p, Sn 3d, S 2p and C 1s peaks. The regional spectrum (Fig. 4b) of Ag 3d presents two peaks with binding energies of 367.7 eV for Ag 3d5/2 and 373.8 eV for Ag 3d3/2. The peaks (Fig. 4c) at a binding energy of 1045.3 and 1022.1 eV can be attributed to Zn 2p1/2 and Zn 2p3/2, respectively. In addition, binding energy peaks (Fig. 4d) at 486.7 and 495.3 eV are in agreement with the Sn 3d5/2 and Sn 3d3/2, respectively. The binding energy values are very close to the reported ones, indicating that the valence states of Ag, Zn and Sn are +1, +2 and +4,23,27 respectively. The spectrum (Fig. 4e) of S 2p shows a broad peak with binding energy of about 162.0 eV, which is a typical value for metal sulfides.28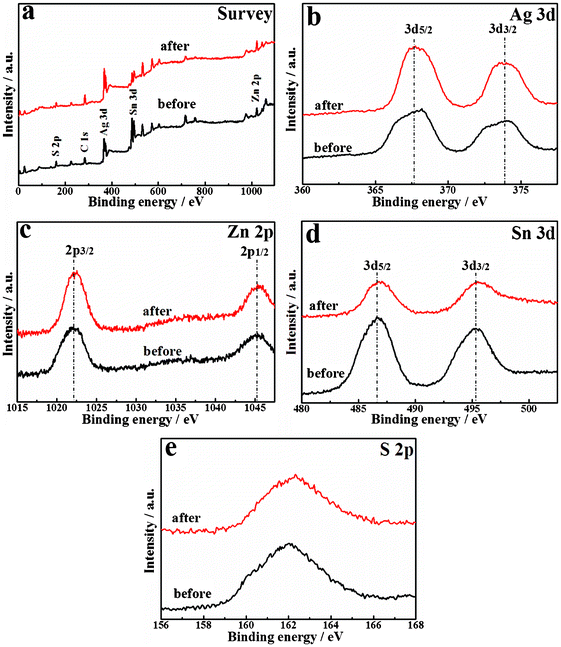 |
| | Fig. 4 XPS spectra of Ag2ZnSnS4 before and after the photocatalytic reaction. | |
3.4 Photocatalytic H2 production activity and stability
Control experiments showed no appreciable H2 evolution over the precursor without post-annealing at 500 °C in N2 atmosphere even in the presence of Na2SO3–Na2S as sacrificial reagent and loaded Pt as cocatalyst. Therefore, the annealed product Ag2ZnSnS4 was used as photocatalyst in the following sections. Fig. 5 shows the effect of Pt-loaded amount on the photocatalytic H2 production over Ag2ZnSnS4 in the Na2SO3–Na2S solution. As can be seen, the H2 production rate of the bare Ag2ZnSnS4 is only 10 μmol h−1. With enhancing the Pt-loaded amount from 0 to 1.0 wt%, the H2 production rate greatly increases, and the 1.0 wt% Pt-loaded Ag2ZnSnS4 reaches a maximum H2 evolution rate of about 580 μmol h−1. While further enhancing the Pt-loaded amount, the H2 production rate slightly decreases. Thus, it can be concluded that the optimum Pt-loaded amount is about 1.0 wt% for Ag2ZnSnS4 under the present photocatalytic system.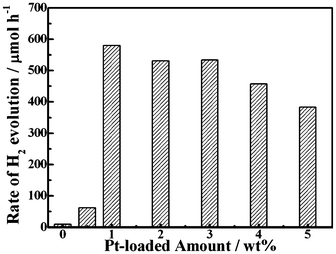 |
| | Fig. 5 Effect of Pt-loaded amount on the photocatalytic H2 evolution rates over Ag2ZnSnS4. | |
The increasing H2 evolution efficiency with enhancing Pt-loading amount could be attributed to the following factors: the Schottky barriers between Pt and Ag2ZnSnS4 nanoparticles would enhance the charge separation of the photogenerated carriers;1,25 Pt-loading is also capable of debasing the overpotential loss for H2 evolution. On the contrary, excessive Pt-loading would result in the growth and agglomeration of Pt nanoparticles on the semiconductor surfaces, which shield the photosensitive Ag2ZnSnS4 surfaces, scatter the light and decrease the light absorption, subsequently diminishing the surface concentration of electrons and holes available for the photochemical reactions.25 Therefore, there is an optimum Pt-loading amount for the photocatalytic H2 production over the present Ag2ZnSnS4.
The apparent quantum efficiencies (AQE) of 1.0 wt% Pt-loaded Ag2ZnSnS4 were measured to be around 15.2, 16.3 and 15.4% at 420, 500 and 520 nm monochromatic light irradiation, respectively. Although these AQE values are relatively low compared with the reported Pt-loaded CdS (60%) and PdS/CdS (93%),1,9 the present Ag2ZnSnS4 displayed a quantum efficiency comparable to the reported Ag-containing complex sulfides such as AgGa2In3S8 (15% at 490 nm), Cu0.25Ag0.25In0.5ZnS2 (7.4% at 520 nm), and AgInZn7S9 (20% at420 nm).1,12–15 Moreover, the AQE values of 16.3 and 15.4% at 500 and 550 nm for the present Pt-loaded Ag2ZnSnS4 is much higher than that (∼3%) of the reported Ru-loaded Ag2ZnSnS4 derived from calcining the precursor precipitated by bubbling with H2S gas into the mixed inorganic solution.17 The above results indicate that the present Ag2ZnSnS4 is promising as a broad and excellent visible light-harvesting photocatalyst.16–18
Fig. 6 shows the time course of 1.0 wt% Pt-loaded Ag2ZnSnS4, which is investigated in three consecutive runs of accumulatively 15 h with fresh sacrificial reagent solution periodically replaced in each run. No noticeable decrease of the photoactivity was found for the present photocatalysts with extending the reaction time. This fairly good stability of the present complex sulfide photocatalyst can be ascribed to the existence of sulfide and sulfite as sacrificial reagents. The scheme of photocatalytic H2 evolution over the photocatalyst in aqueous solution containing sulfide and sulfite anions is shown by eqn (2)–(7).6
| | | Photocatalyst + hv → e− (CB) + h+(VB) | (2) |
| | | H2O + 2e− (CB) → H2 + 2OH− | (3) |
| | | SO32− + H2O +2h+(VB) → SO42− + 2H+ | (4) |
| | | S22− + SO32− → S2O32− + S2− | (6) |
| | | S2− + SO32− + 2h+(VB) → S2O32− | (7) |
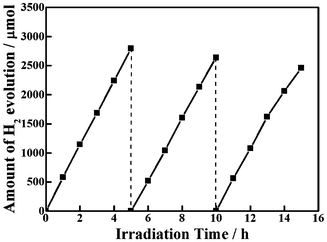 |
| | Fig. 6 Stability study of photocatalytic H2 evolution over Ag2ZnSnS4. | |
As a result, the photogenerated electrons in the CB of the product can reduce the water to form H2. Meanwhile the SO32− and S2− change into SO42− and S22− by the photogenerated holes in the valence band (VB) according to eqn (4) and (5). The production of S22− is efficiently suppressed by reaction with SO32− according to eqn (6). However, the H2 production can drop due to the excess consumption of SO32− and S2−. Therefore, it is necessary to replace fresh sacrificial reagent in each photoreaction run. The production of S2O32− is colorless and doesn't compete with the photocatalyst for light absorption. Moreover, the presence of excessive S2− in the reaction solution can also suppress the formation of sulfur defects and stabilizes the photocatalyst.
Nevertheless, a slight drop in the H2 production rate can still be observed during the second and third runs of the photocatalytic reaction even exchanging the sacrificial reagent solution to a new one as shown in Fig. 6. Since Ag2ZnGeS4, Ag2ZnSnS4, and CuAgZnSnS4 photocatalysts containing monovalent Ag also showed some deactivation during photoreaction conditions similar to ours,17 it can be concluded that some Ag+ cations near the surface might be reduced to form metallic Ag by photogenerated electrons for this Ag-containing photocatalyst, resulting in deactivation.17 The XRD pattern (Fig. 1) of Pt-loaded Ag2ZnSnS4 after 15 h photoreaction is essentially similar to that of the original one, and there is no obvious deviation for the locations of these main diffraction peaks. However, the XRD pattern of the recovered Pt-loaded Ag2ZnSnS4 also includes some additional unconspicuous peaks at 2θ = ∼29, ∼32 and 34–38°, respectively. Since the XRD pattern of Pt-loaded Ag2ZnSnS4 is similar to that of the product without Pt-loading, as shown in Fig. 1, the additional peaks for the recovered Pt-loaded Ag2ZnSnS4 do not originate from the cocatalyst Pt particles. As mentioned above, some of these new peaks might be due to the Ag nanoparticles being reduced by the photogenerated electrons of this Ag+-containing photocatalyst.17 Furthermore, some other complex sulfides might be formed at the photocatalyst surface due to Ag+ reduction, which may also contribute to those additional diffraction peaks shown in Fig. 1 of the recovered photocatalyst, which needs to be further investigated. The XPS spectrum in Fig. 4b for the recovered photocatalyst also indicates the formation of metallic Ag on Ag2ZnSnS4 after 15 h photoreaction. Although the binding energies of Zn 2p, Sn 3d and S 2p before and after reaction are nearly the same whether in the full survey spectrum or the detailed spectra, the binding energy peaks at around 367 eV, corresponding to Ag 3d, apparently decreased and shifted toward a higher binding energy for the recovered Ag2ZnSnS4, indicating some Ag+ cations near the surface might be reduced to form metallic Ag by the photogenerated electrons for the present Ag-containing photocatalyst. These findings are expected to give useful information on the strategy for the development of new metal sulfide-based photocatalysts even though the photostability still requires further improvement.
4 Conclusions
In this study, stannite-type Ag2ZnSnS4 nanoparticles with particle diameter of 100–200 nm are synthesized through a solvothermal treatment combined with a post-annealing process under N2 atmosphere. Ag2ZnSnS4 shows an absorption edge at about 620 nm, with a corresponding bandgap around 2.01 eV. It is found that the Pt-loaded Ag2ZnSnS4 displays significantly high and steady photoactivity for H2 evolution with a fairly good apparent quantum efficiency of 15.2% under 420 nm light irradiation. The present product developed seems to suggest a route for steady and efficient visible light-responsive photocatalysts for photocatalytic hydrogen production.Acknowledgements
This work was supported by the Natural Science Foundation of China (20973128, 21271146), the Program for New Century Excellent Talents in University (NCET-07-0637), and the Fundamental Research Funds for the Central Universities (2081003) of China.References
- X. B. Chen, S. H. Shen, L. J. Guo and S. S. Mao, Chem. Rev., 2010, 110, 6503–6570 CrossRef CAS.
- K. Maeda and K. Domen, J. Phys. Chem. Lett., 2010, 1, 2655–2661 CrossRef CAS.
- X. L. Fu, X. X. Wang, Z. X. Chen, Z. Z. Zhang, Z. H. Li, D. Y. C. Leung, L. Wu and X. Z. Fu, Appl. Catal., B, 2010, 95, 393–399 CrossRef CAS.
- S. H. Shen, L. Zhao and L. J. Guo, Int. J. Hydrogen Energy, 2010, 35, 10148–10154 CrossRef CAS.
- B. Chai, T. Y. Peng, P. Zeng, X. H. Zhang and X. J. Liu, J. Phys. Chem. C, 2011, 115, 6149–6155 CAS.
- N. Z. Bao, L. M. Shen, T. Takata and K. Domen, Chem. Mater., 2008, 20, 110–117 CrossRef CAS.
- D. W. Jing and L. J. Guo, J. Phys. Chem. B, 2006, 110, 11139–11145 CrossRef CAS.
- G. Ma, H. Yan, J. Shi, X. Zong, Z. Lei and C. Li, J. Catal., 2008, 260, 134–140 CrossRef CAS.
- H. Yan, J. Yang, G. Ma, G. Wu, Z. Zong, Z. Lei, J. Shi and C. Li, J. Catal., 2009, 266, 165–168 CrossRef CAS.
- X. W. Wang, G. Liu, Z. G. Chen, F. Li, G. Q (Max). Lu and H. M. Cheng, Electrochem. Commun., 2009, 11, 1174–1178 CrossRef CAS.
- L. Wang, W. Z. Wang, M. Shang, W. Z. Yin, S. M. Sun and L. Zhang, Int. J. Hydrogen Energy, 2010, 35, 19–25 CrossRef CAS.
- I. Tsuji, H. Kato, H. Kobayashi and A. Kudo, J. Am. Chem. Soc., 2004, 126, 13406–13413 CrossRef CAS.
- I. Tsuji, H. Kato, H. Kobayashi and A. Kudo, J. Phys. Chem. B, 2005, 109, 7323–7324 CrossRef CAS.
- I. Tsuji, H. Kato and A. Kudo, Angew. Chem., 2005, 117, 3631–3634 CrossRef.
- I. Tsuji, H. Kato and A. Kudo, Chem. Mater., 2006, 18, 1969–1975 CrossRef CAS.
- S. Ikeda, T. Nakamura, T. Harada and M. Matsumura, Phys. Chem. Chem. Phys., 2010, 12, 13943–13949 RSC.
- I. Tsuji, Y. Shimodaira, H. Kato, H. Kobayashi and A. Kudo, Chem. Mater., 2010, 22, 1402–1409 CrossRef CAS.
- L. Wang, W. Z. Wang and S. M. Sun, J. Mater. Chem., 2012, 22, 6553–6555 RSC.
- J. Zhang, R. G. Xie and W. S. Yang, Chem. Mater., 2011, 23, 3357–3361 CrossRef CAS.
- O. Zaberca, A. Gillorin, B. Durand and J. Y. Chane-Ching, J. Mater. Chem., 2011, 21, 6483–6486 RSC.
- Y. L. Zhou, W. H. Zhou, M. Li, Y. F. Du and S. X. Wu, J. Phys. Chem. C, 2011, 115, 19632–19639 CAS.
- C. Zou, L. J. Zhang, L. L. Zhai, D. S. Lin, J. M. Cao, Q. Li, Y. Yang, X. A. Chen and S. M. Huang, Chem. Commun., 2011, 47, 5256–5258 RSC.
- P. C. Dai, X. N. Shen, Z. J. Lin, Z. Y. Feng, H. Xu and J. H. Zhan, Chem. Commun., 2010, 46, 5749–5751 RSC.
- Y. Cui, G. Wang and D. C. Pan, J. Mater. Chem., 2012, 22, 12471–12473 RSC.
- H. B. Yi, T. Y. Peng, D. N. Ke, D. Ke, L. Zan and C. H. Yan, Int. J. Hydrogen Energy, 2008, 33, 672–678 CrossRef CAS.
- L. W. Zhang, H. Y. Cheng, R. L. Zong and Y. F. Zhu, J. Phys. Chem. C, 2009, 113, 2368–2374 Search PubMed.
- W. J. Zhang, D. Z. Li, M. Sun, Y. Shao, Z. X. Chen, G. C. Xiao and X. Z. Fu, J. Solid State Chem., 2010, 183, 2466–2474 CrossRef CAS.
- J. G. Yu, J. Zhang and S. W. Liu, J. Phys. Chem. C, 2010, 114, 13642–13649 CAS.
|
| This journal is © The Royal Society of Chemistry 2013 |
Click here to see how this site uses Cookies. View our privacy policy here. 






