Extracorporeal photochemotherapy as systemic monotherapy of severe, refractory atopic dermatitis: results from a prospective trial†
Peter
Wolf
*a,
Dimitrios
Georgas
b,
Nordwig S.
Tomi‡
b,
Christoph M.
Schempp
c and
Klaus
Hoffmann
b
aResearch Unit for Photodermatology, Department of Dermatology, Medical University of Graz, Graz, Austria. E-mail: peter.wolf@medunigraz.at; Fax: +43 316 385-12466; Tel: +43 316 385-12371
bDepartment of Dermatology and Allergology, St. Josef Hospital, Ruhr University, Gudrunstrasse 56, D-44791 Bochum, Germany
cDepartment of Dermatology, University Medical Center Freiburg, Freiburg, Germany
First published on 20th August 2012
Abstract
Background: Previous work has indicated that extracorporeal photochemotherapy (ECP) may be a safe and effective treatment in patients with severe atopic dermatitis. Methods: We performed a prospective study to investigate the effect of a defined 20-week ECP protocol in patients with severe, refractory atopic dermatitis. The patient inclusion criteria included (i) disease duration of at least 1 year, (ii) SCORAD > 45, and (iii) resistance to first-line therapy, including topical steroids, topical calcineurin inhibitors, and one form of phototherapy (UVA, UVB, or PUVA) or one second-line therapy, including systemic steroids or cyclosporine. Ten patients (4 women and 6 men; age range 29 to 61 years) were enrolled and treated with two sessions of standard ECP in 2-week intervals for 12 weeks and 4-week intervals thereafter until week 20. The patients’ clinical status and response was determined by SCORAD at baseline and every 2 weeks, and quality of life was assessed every 4 weeks using SKINDEX, SF-36, and FACT scores. Results: There was a statistically significant (p = 0.015) reduction of the mean SCORAD by 10.3 (95% CI, 2.5 to 18.0) from 64.8 at baseline to 54.5 (i.e., 15.9% reduction) at week 20. In a subset of patients (all of female sex), the relative reduction in SCORAD after ECP was more than 25% at week 20. Improvement in quality of life measured by SKINDEX, SF-36, and FACT did not reach statistical significance. Conclusions: We detected a small but significant therapeutic effect of ECP in patients with severe, refractory atopic dermatitis.
Introduction
Atopic dermatitis (AD) is a common, chronically relapsing, inflammatory skin disease characterized by itchy eczematous skin lesions which can affect the entire body surface in severe cases.1–3 The exact pathogenesis of AD remains unclear. A multifactorial trait involving gene loci on different chromosomes with high correlations to mutations at the filaggrin gene have been found (cited in ref. 1). These mutations, predisposing to a disturbed epidermal barrier function and a simultaneous over-response of the immune system, characterized by a functional failure of regulatory T cells (Tregs)4,5 and an abnormal Th2/Th17-driven immune response to exogenous and/or endogenous antigens most likely lead to the occurrence of the skin changes in the disease.6,7 Clinical studies have demonstrated a correlation between disease severity of AD and the levels of immunoglobulin (Ig)E, and surrogate markers such as eosinophil cationic protein or soluble E-selectin.8,9AD usually responds adequately to emollients, topical corticosteroids, calcineurin inhibitors, or phototherapy such as UVA-1, 311 nm UVB or broadband UVB, or psoralen plus UVA (PUVA) photochemotherapy.1–3,10–13 However, in some patients standard therapy remains unsatisfactory, in particular when they suffer from chronic disease with wide-spread skin involvement and/or erythroderma. These patients often require immunosuppression with systemic corticosteroids, azathioprine, cyclosporine or mycophenolate mofetil, or methotrexate to prevent severe disability (cited in ref. 1). More recently, systemic third line approaches leading to diminished T cell activation, including alefacept (fusion protein of lymphocyte antigen (LFA)-3 (anti-CD58) antibody), efalizumab (anti-CD11b antibody, no longer available), rituximab (anti-CD20 antibody) or intravenous IgG have been found effective in severe cases of the disease (cited in ref. 1). In addition, anti-TNF treatment with infliximab14 or anti-IL-12/23 antibody15 has been shown to have short term effects in single cases of severe AD. Treatment with the anti-IgE antibody omalizumab (cited in ref. 1) or the anti-IL-5 antibody mepolizumab16 has also revealed promising results in some moderate to severe cases of AD. However, these systemic therapies may be associated with significant risks of adverse effects. The administration of extracorporeal photochemotherapy (ECP; photopheresis) in AD as a safe and likely effective treatment was first described by Prinz et al.17 in 1994. Thereafter, several investigators have administered ECP in open trials to patients with severe AD.17–24 Rubegni et al.25 recently reviewed those trials, some of them retrospective in nature and enrolling AD patients of different disease stages, together with a report on a AD patient series treated with ECP and concomitant systemic medication at their center. We herein report the results of a prospective ECP study with clearly defined treatment protocol and restrictive inclusion criteria of patients with severe, refractory AD.
Methods
Setting
This was a prospective trial conducted at the Department of Dermatology and Allergology, St. Josef Hospital, Ruhr University, Bochum, Germany; Department of Dermatology, University Medical Center Freiburg, Freiburg, Germany; and Department of Dermatology, Medical University of Graz, Graz, Austria.Ethics
The study protocol was approved by the local ethical committees of the participating centers (Bochum: application no. 2284/2004; Freiburg: application no. 142/2004; and Graz: application no. 17-269 ex 05/06). The study was conducted according to the principles of the Declaration of Helsinki. All study participants provided informed written consent for study participation and reporting of results.Patient inclusion and exclusion criteria
The inclusion criteria of the study were as follows: age above 18 years; body weight above 40 kg; adequate veins to provide intravenous access; diagnosis of severe, refractory atopic dermatitis (i) of at least 1 year duration, (ii) SCORAD > 45, and (iii) resistance in the last 12 months to all first-line therapies, including topical steroids, topical calcineurin inhibitors, and one form of phototherapy (UVA, UVB, or PUVA) or resistance to either systemic steroids or cyclosporine as second line therapy; willingness to abstain from therapeutic sunbathing and tanning beds for the duration of the study. Exclusion criteria were as follows: intolerance of extracorporeal volume loss (e.g., due to severe cardiac disease or severe anemia or body weight below 40 kg); recent (within three months) deterioration of renal function with a serum creatinine level above 3.0 mg dL−1, lipemic plasma above 500 ng dL−1, or uncontrolled diabetes; history of liver damage (2.5 times normal aspartate aminotransferase (AST), alanine aminotransferase (ALT)) or porphyria; positive testing for HIV antibody, HCV antibody or hepatitis B surface antigen; severe emotional, behavioral or psychiatric disease that may result in poor compliance with the treatment regimen; childbearing potential; pregnancy and lactation; idiosyncratic or hypersensitivity reactions to 8-methoxypsoralen, heparin, or citrate; treatment with PUVA, UVA or UVB within 4 weeks; systemic immunosuppressant therapy within 4 weeks; treatment with oral steroids above 10 mg unless necessary due to Addison's disease or adrenal insufficiency.Interventions
Standard ECP treatment using UVAR XTS system (Johnson & Johnson, Therakos, Norderstedt, Germany)26 and a standard dose of UVADEX (containing 20 μg 8-methoxypsoralen per 10 ml vial) (Ben Venue Laboratories, Bedford, USA) was administered. UVADEX was applied into the ECP treatment bag using the following formula: treatment volume of the collected plasma/buffy coat in ml multiplied by 0.017. ECP was administered with cycles consisting of treatment on 2 consecutive days every 2 weeks for 12 weeks; thereafter every 4 weeks to end of the study period at week 20. Additional treatment was restricted to the use of emollients, topical steroids, topical calcineurin inhibitors, and/or anti-histamines; no other concomitant treatment was allowed.SCORAD assessment
The patient's clinical status and response was assessed every two week during the study period. The overall response of a patient was based on SCORAD, a validated clinical tool used to assess the severity of AD.1,27 The primary efficacy outcome assessment was SCORAD at week 20.Quality of life
Quality of life was measured using SKINDEX,28,29 SF-36,30–32 and FACT33 at baseline and every 4 weeks during the study period.Laboratory investigations
For laboratory monitoring, blood was taken from each patient before the first ECP treatment of each treatment cycle to determine white and red blood counts, hemoglobin, mean corpuscular hemoglobin (MCH), and platelets; serum uric acid, total protein, bilirubin, alkaline phosphatase, AST, ALT, lactate dehydrogenase (LDH), gamma glutamyl transferase (GGT), BUN, creatinine, and electrolytes; and total serum IgE levels.Statistics
For statistical analysis pretreatment values at baseline (before start of ECP) were compared to values at week 20 by two-tailed paired Student's t test. Normality testing was done by Kolmogoroff–Smirnov test. Ninety-five percent confidence intervals (95% CI) with lower and upper limits were calculated. The p-level of significance was set at ≤0.05. Bonferroni adjustment was made for multiple endpoint testing when appropriate.Results
Patient characteristics
Ten patients (4 women and 6 men) were enrolled from the study centers (4 patients in Bochum; 3 patients in Freiburg; and 3 patients in Graz). The mean age of the patients was 49 years (range, 29 to 61 years). The mean SCORAD in the patients at baseline before ECP treatment was 64.8 (SD, 18.9; range, 46 to 97) (Table 1 and Fig. 1).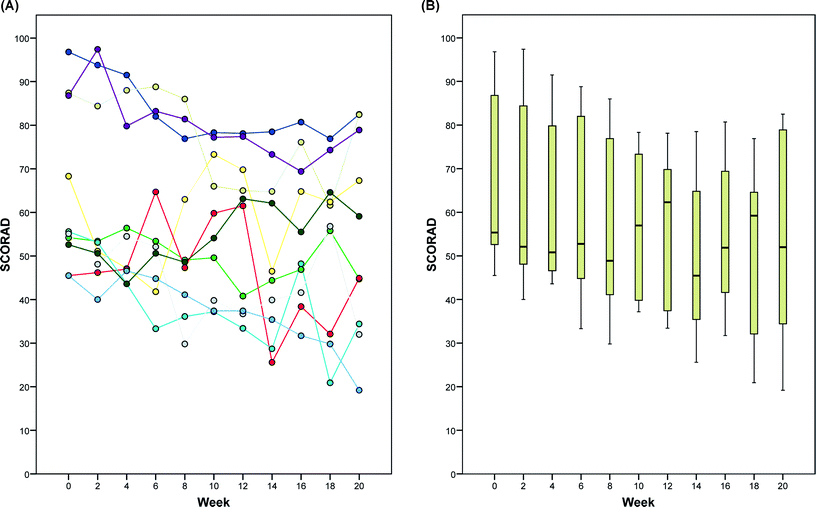 | ||
| Fig. 1 Effect of extracorporeal photochemotherapy on SCORAD in patients with severe, refractory atopic dermatitis during the 20-week study period. (A) Absolute SCORAD values of individual patients and (B) box plots for all patients. | ||
| (A) Data at baseline and week 20 | ||||
|---|---|---|---|---|
| Week 0 (baseline) | Week 20 | |||
| Mean ± SD | Range | Mean ± SD | Range | |
| SCORAD | 64.8 ± 18.9 | 46–97 | 54.5 ± 22.8 | 19–83 |
| SF-36 physical sum | 41.5 ± 3.6 | 37–48 | 43.9 ± 4.9 | 32–50 |
| SF-36 psychological sum | 42.9 ± 8.5 | 31–46 | 45.5 ± 8.7 | 35–52 |
| SKINDEX (symptoms) | 56.8 ± 10.7 | 36–75 | 48.5 ± 18.8 | 14–71 |
| SKINDEX (emotions) | 33.5 ± 15.8 | 13–70 | 30.0 ± 14.8 | 10–60 |
| SKINDEX (function) | 41.5 ± 16.2 | 19–67 | 35.5 ± 14.3 | 10–58 |
| FACT-G (physical well-being) | 22.4 ± 4.0 | 14–27 | 23.6 ± 3.3 | 17–28 |
| FACT-G (social well-being) | 18.2 ± 5.4 | 10–27 | 19.3 ± 4.5 | 13–26 |
| FACT-G (emotional well-being) | 19.3 ± 3.3 | 13–24 | 19.2 ± 3.5 | 13–24 |
| FACT-G (functional well-being) | 16.1 ± 5.7 | 9–25 | 17.8 ± 6.7 | 7–28 |
| FACT-G | 76.0 ± 13.4 | 58–97 | 79.9 ± 14.4 | 63–104 |
| (B) Differences from baseline | ||||
|---|---|---|---|---|
| Type of score | Mean difference from baseline | 95% confidence interval | p-value | |
| Lower limit | Upper limit | |||
| SD, standard deviation. SCORAD,1,27 (A) absolute lower values and (B) negative differences (from baseline) indicate better skin status. SF-36,30–32 (A) absolute higher values and (B) positive differences (from baseline) indicate better quality of life. SKINDEX,28,29 (A) absolute lower values and (B) negative differences (from baseline) indicate better quality of life. FACT,33 (A) absolute higher values and (B) positive differences (from baseline indicate) better quality of life. | ||||
| SCORAD | −10.3 ± 10.8 | −18.0 | −2.5 | 0.015 |
| SF-36 physical sum | 2.3 ± 5.3 | −6.2 | 1.5 | 0.199 |
| SF-36 psychological sum | 2.6 ± 5.4 | −6.4 | 1.3 | 0.167 |
| SKINDEX (symptoms) | −8.3 ± 17.7 | −21.0 | 4.4 | 0.172 |
| SKINDEX (emotion) | −3.5 ± 11.2 | −11.5 | 4.5 | 0.349 |
| SKINDEX (function) | −6.0 ± 15.0 | −16.8 | 4.8 | 0.240 |
| FACT-G (physical well-being) | 1.2 ± 2.3 | −0.4 | 2.8 | 0.133 |
| FACT-G (social well-being) | 1.1 ± 2.6 | −0.8 | 3.0 | 0.221 |
| FACT-G (emotional well-being) | −0.1 ± 1.2 | −1.0 | 0.8 | 0.798 |
| FACT-G (functional well-being) | 1.7 ± 5.5 | −2.2 | 5.6 | 0.355 |
| FACT-G | 3.9 ± 7.5 | −1.5 | 9.3 | 0.134 |
SCORAD
Although there was variability in SCORAD among patients and among treatment visits during the study period (Fig. 1 and 2), a statistically significant (p = 0.015) reduction of the mean SCORAD by 10.3 (SD 10.8; 95% CI, 2.5 to 18.0) from 64.8 at baseline to 54.5 at week 20 was evident (Table 1). This corresponded to an average reduction by 15.9% (95% CI, 3.9 to 27.8), comparing ECP pretreatment to after ECP SCORAD values. The relative SCORAD levels (normalized to 100% at baseline) are shown in Fig. 2. In three patients (no. 7, 8, and 9), the relative reduction in SCORAD after ECP was greater than 25%. This effect was observed at several time points during the ECP treatment regimen and at week 20, as the primary endpoint of the study protocol (Fig. 2A). Remarkably, these well-responders were all of female sex and had the same age distribution (30, 48, and 60 years) as the remaining study population at study entry. As depicted in Fig. 3, significant improvement of skin lesions was observed in certain patients during the study period (Fig. 3A, patient no. 7) or after the 20-week study period under extended ECP treatment (Fig. 3B, patient no. 6).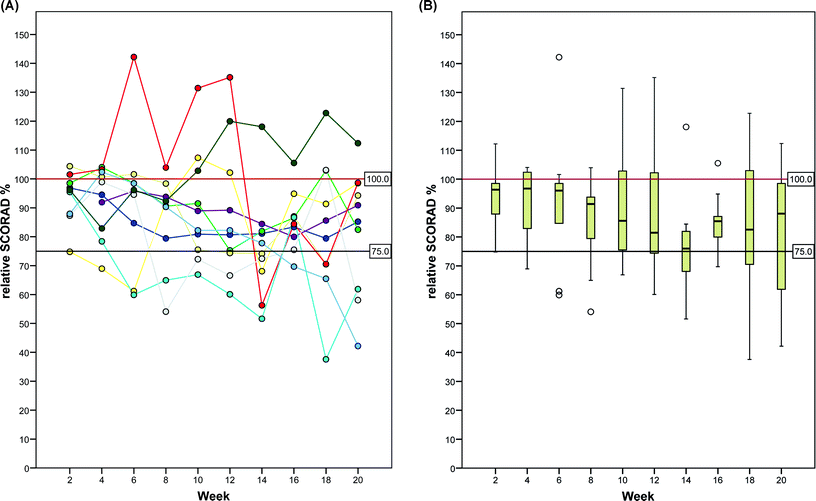 | ||
| Fig. 2 Effect of extracorporeal photochemotherapy on SCORAD in patients with severe, refractory atopic dermatitis during the 20-week study period. (A) Relative SCORAD values (normalized to 100% at baseline) of individual patients and (B) box plots for all patients. | ||
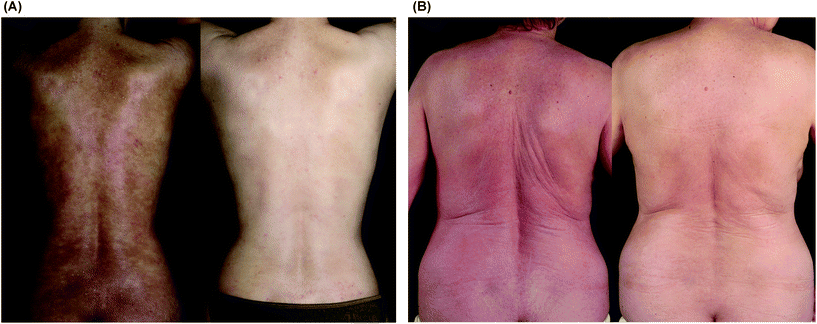 | ||
| Fig. 3 Clinical treatment effect of extracorporeal photochemotherapy in patients with severe atopic dermatitis. (A) 30 year old woman (patient no. 7) with chronic poikilodermic atopic dermatitis before (left photograph) and after 18 weeks of ECP (right photograph). (B) 59 year old woman (patient no. 6) with erythrodermic atopic dermatitis before (left photograph) and 24 weeks after start of ECP (right photograph). Note in both patients a significant improvement of skin lesions was observed after ECP. | ||
Secondary clinical endpoints
Improvement of quality of life was observed after ECP but overall did not reach statistical significance for any of the scores, including SKINDEX,28,29 SF-36,30–32 or FACT33 (Table 1). However, in individual patients substantial improvements were observed comparing baseline vs. after ECP treatment evaluations with these scores (data not shown). No serious side effects of ECP were observed in the study.Laboratory observations
Laboratory monitoring revealed that leucocyte levels (7.1 ± 2.3 vs. 6.3 ± 1.6 × 109 L−1; p = 0.028), MCH (30.0 ± 1.6 vs. 28.7 ± 1.2 pg; p = 0.028), and serum alkaline phosphatase (75 ± 20 vs. 66 ± 14 U L−1; p = 0.028) were significantly decreased, and potassium (4.1 ± 0.5 vs. 4.3 ± 0.4 mmol L−1; p = 0.039) was increased, comparing baseline levels vs. that at week 20. However, when adjusted for multiple endpoint testing by the Bonferroni method these differences were statistically not significant. Three patients had IgE levels increased above laboratory limits (>5000 IU ml−1) at the start of ECP and thereafter; two patients had lacking IgE levels from the visits during the study period; in the remaining five patients IgE levels (321 to 1213 IU ml−1 at baseline) decreased during the ECP treatment period, reaching at week 20 levels of approximately 20 to 40% below baseline (data not shown).Discussion
In this prospective trial we observed a small though statistically significant effect of ECP in patients with severe refractory AD. Patients exhibited after ECP treatment a statistically significant reduction of SCORAD by 10.3 points (95% CI, 2.5 to 18.0) from 64.8 at baseline to 54.5 at week 20 (Table 1), corresponding to an average reduction of 15.9%. In a subset of three patients the reduction of SCORAD was greater than 25% during the 20-week ECP study period and thereafter under extended ECP treatment (Fig. 3). This is consistent with reports by others, indicating that long-term application of ECP treatment can lead to satisfactory treatment results.18,20,25 Notably, on average an improvement in quality of life was not detectable with the standard scores we employed in the study, including SKINDEX,28,29 SF-36,30–32 and FACT33 to the end of the study period at week 20. This indicates that a score such as SCORAD assessing the severity of disease by objective signs (i.e. extent and intensity of skin lesions) and subjective signs (pruritus and insomnia) can be more sensitive than quality of life scores to detect response to treatment.The average response rate measured by SCORAD in this study was lower than that observed with similar ECP treatment protocols in previous studies, in which together more than 80 patients have been treated so far (reviewed in ref. 25). For instance, in the largest study Radenhausen et al.21 administered ECP to 35 patients with severe generalized AD over a period of 6 to 10 cycles. They observed that ECP led to a significant decrease in SCORAD from 74.4 before to 36.8 (i.e., 50% reduction) after a mean of 10 cycles given in 2-weekly intervals. However, we observed, consistent with other reports,17,20 that ECP can be very effective in patients with (first-line-therapy-refractory) erythrodermic AD (Fig. 3) particularly when administered for longer periods of time. It is intriguing to note that, besides in Sezary syndrome,34,35 ECP has also been shown to be effective in erythroderma of other origin, such as red man syndrome,36,37 erythrodermic pityriasis rubra pilaris,38 or photoaccentuated erythroderma associated with CD4+ T lymphocytopenia.39
ECP has also been found to improve laboratory correlates of active AD including elevated levels of IgE, eosinophilic cationic protein, soluble interleukin-2 receptor (sIL-2R), or soluble E-selectin.20–22 However, Radenhausen et al.21 found no significant correlation between a decrease in these levels and values of blood eosinophils. Compared to ECP responders, most non-responders had very high levels of total serum IgE before and during ECP therapy.21 In our study, we observed a trend for decreased IgE levels during and after ECP treatment. Consistent with previous studies, we found that ECP given to AD patients was safe and without any side effects.
The exact mechanisms of the therapeutic action of photochemotherapy40 and ECP34,35,41 remain to be determined in AD. Tregs are crucial in the pathogenesis of AD.4,5 An experimental mouse model of contact hypersensitivity (CHS) revealed that ECP inhibited the induction of CHS and induced Tregs.42 In addition, ECP also inhibited the efferent phase of CHS although Tregs only inhibited sensitization. Similar as in CTCL, in which ECP has been shown to down-regulate peripheral Tregs,43 an altered Treg function may be associated with the therapeutic response mechanism in AD. On the other hand, we have found in a TGF-beta transgenic mouse model of an inflammatory psoriasis-like disease that PUVA photochemotherapy does not only induce disease-suppressive Tregs but simultaneously suppresses the Th17 axis,44–46 a potential key pathway in psoriasis and AD.47 In addition, photochemotherapy may also affect homing of Tregs to lymph nodes and the skin.48 Moreover, we recently found that photochemotherapy down-regulated IL-9,49 another cytokine that may play a role in AD by affecting mast cells.50 In another previous study51 we discovered that photochemotherapy had proapoptotic and immunosuppressive properties and induced the formation of platelet-activating factor (PAF), a potent phospholipid mediator. The potential production of PAF in the flow chamber of the ECP apparatus, resulting in a cascade of downstream events,44 may be involved in the therapeutic mechanisms in ECP-responsive diseases such as AD.
Taken together with previous reports,17–25 ECP seems to be effective in severe, refractory atopic dermatitis. However, the effect we detected in this study was on average rather small and not linked to an overall significant detectable improvement in quality of life in the short-term. Weaknesses of the study were that it was not a randomized controlled trial and the amount of additional local treatment was not quantified. Future studies will also have to identify potential predictive markers for AD patients responding favorably to ECP.
Abbreviations
| ECP | Extracorporeal photochemotherapy |
Acknowledgements
The authors thank the patients for participating in this trial, the staff at the study centers for performing the ECP procedures and collecting clinical data, Dr Agnes Bretterklieber, Medical University of Graz, for compiling data, and Dr Franz Quehenberger, Institute for Medical Informatics, Statistics and Documentation, Medical University of Graz, for help in preparing graphical materials. This work was supported by a research grant from Therakos, Johnson & Johnson Co, to the University of Bochum, Germany. The funder had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.References
- U. Darsow, A. Wollenberg, D. Simon, A. Taieb, T. Werfel, A. Oranje, C. Gelmetti, A. Svensson, M. Deleuran, A. M. Calza, F. Giusti, J. Lubbe, S. Seidenari and J. Ring, ETFAD/EADV eczema task force 2009 position paper on diagnosis and treatment of atopic dermatitis, J. Eur. Acad. Dermatol. Venereol., 2010, 24, 317–328 CrossRef CAS.
- H. Saeki, M. Furue, F. Furukawa, M. Hide, M. Ohtsuki, I. Katayama, R. Sasaki, H. Suto and K. Takehara, Committee for guidelines for the management of atopic dermatitis of Japanese dermatological association. Guidelines for management of atopic dermatitis, J. Dermatol., 2009, 36, 563–577 CrossRef.
- T. Werfel, W. Aberer, M. Augustin, T. Biedermann, R. Folster-Holst, F. Friedrichs, U. Gieler, A. Heratizadeh, A. Kapp, B. Przybilla, E. Rietschel, M. Schlaeger, P. Schmid-Grendelmeier, H. Sitters, D. Staab, R. Szczepanski, D. Vieluf, I. Voigtmann and M. Worm, Atopic dermatitis: S2 guidelines, J. Dtsch. Dermatol. Ges., 2009, 7(Suppl 1), S1–46 Search PubMed.
- L. S. Ou, E. Goleva, C. Hall and D. Y. Leung, T regulatory cells in atopic dermatitis and subversion of their activity by superantigens, J. Allergy Clin. Immunol., 2004, 113, 756–763 CrossRef CAS.
- E. M. Ling, T. Smith, X. D. Nguyen, C. Pridgeon, M. Dallman, J. Arbery, V. A. Carr and D. S. Robinson, Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease, Lancet, 2004, 363, 608–615 CrossRef CAS.
- A. Di Cesare, P. Di Meglio and F. O. Nestle, A role for Th17 cells in the immunopathogenesis of atopic dermatitis?, J. Invest. Dermatol., 2008, 128, 2569–2571 CrossRef CAS.
- J. Louten, K. Boniface and R. de Waal Malefyt, Development and function of TH17 cells in health and disease, J. Allergy Clin. Immunol., 2009, 123, 1004–1011 CrossRef CAS.
- G. B. Colver, J. A. Symons and G. W. Duff, Soluble interleukin 2 receptor in atopic eczema, Br. Med. J., 1989, 298, 1426–1428 CrossRef CAS.
- M. Furue, T. Koga and N. Yamashita, Soluble E-selectin and eosinophil cationic protein are distinct serum markers that differentially represent clinical features of atopic dermatitis, Br. J. Dermatol., 1999, 140, 67–72 CrossRef CAS.
- F. J. Legat, A. Hofer, E. Brabek, F. Quehenberger, H. Kerl and P. Wolf, Narrowband UV-B vs. medium-dose UV-A1 phototherapy in chronic atopic dermatitis, Arch. Dermatol., 2003, 139, 223–224 Search PubMed.
- S. Tzaneva, H. Kittler, G. Holzer, D. Reljic, M. Weber, H. Honigsmann and A. Tanew, 5-Methoxypsoralen plus ultraviolet (UV) A is superior to medium-dose UVA1 in the treatment of severe atopic dermatitis: a randomized crossover trial, Br. J. Dermatol., 2010, 162, 655–660 CrossRef CAS.
- S. A. Grundmann and S. Beissert, Modern aspects of phototherapy for atopic dermatitis, J. Allergy (Cairo), 2012, 2012, 121797 Search PubMed.
- T. Gambichler, Management of atopic dermatitis using photo(chemo)therapy, Arch. Dermatol. Res., 2009, 301, 197–203 CrossRef.
- A. Jacobi, C. Antoni, B. Manger, G. Schuler and M. Hertl, Infliximab in the treatment of moderate to severe atopic dermatitis, J. Am. Acad. Dermatol., 2005, 52, 522–526 CrossRef.
- R. Puya, M. Alvarez-Lopez, A. Velez, E. Casas Asuncion and J. C. Moreno, Treatment of severe refractory adult atopic dermatitis with ustekinumab, Int. J. Dermatol., 2012, 51, 115–116 CrossRef.
- J. M. Oldhoff, U. Darsow, T. Werfel, K. Katzer, A. Wulf, J. Laifaoui, D. J. Hijnen, S. Plotz, E. F. Knol, A. Kapp, C. A. Bruijnzeel-Koomen, J. Ring and M. S. de Bruin-Weller, Anti-IL-5 recombinant humanized monoclonal antibody (mepolizumab) for the treatment of atopic dermatitis, Allergy, 2005, 60, 693–696 CrossRef CAS.
- B. Prinz, F. Nachbar and G. Plewig, Treatment of severe atopic dermatitis with extracorporeal photopheresis, Arch. Dermatol. Res., 1994, 287, 48–52 CrossRef CAS.
- K. P. Hjuler, C. Vestergaard and M. Deleuran, A retrospective study of six cases of severe recalcitrant atopic dermatitis treated with long-term extracorporeal photopheresis, Acta Derm.-Venereol., 2010, 90, 635–636 CrossRef.
- G. Mohla, N. Horvath and S. Stevens, Quality of life improvement in a patient with severe atopic dermatitis treated with photopheresis, J. Am. Acad. Dermatol., 1999, 40, 780–782 CrossRef CAS.
- B. Prinz, S. Michelsen, C. Pfeiffer and G. Plewig, Long-term application of extracorporeal photochemotherapy in severe atopic dermatitis, J. Am. Acad. Dermatol., 1999, 40, 577–582 CrossRef CAS.
- M. Radenhausen, S. Michelsen, G. Plewig, F. G. Bechara, P. Altmeyer and K. Hoffmann, Bicentre experience in the treatment of severe generalised atopic dermatitis with extracorporeal photochemotherapy, J. Dermatol., 2004, 31, 961–970 CAS.
- M. Radenhausen, G. von Kobyletzki, S. Hoxtermann, P. Altmeyer and K. Hoffmann, Activation markers in severe atopic dermatitis following extracorporeal photochemotherapy, Acta Derm.-Venereol., 2003, 83, 49–50 CrossRef CAS.
- H. I. Richter, C. Billmann-Eberwein, M. Grewe, H. Stege, M. Berneburg, T. Ruzicka and J. Krutmann, Successful monotherapy of severe and intractable atopic dermatitis by photopheresis, J. Am. Acad. Dermatol., 1998, 38, 585–588 CrossRef CAS.
- M. Sand, F. G. Bechara, D. Sand, M. Radenhausen, N. S. Tomi, P. Altmeyer and K. Hoffmann, Extracorporeal photopheresis as a treatment for patients with severe, refractory atopic dermatitis, Dermatology, 2007, 215, 134–138 CrossRef CAS.
- P. Rubegni, S. Poggiali, G. Cevenini, G. D'Ascenzo, A. Perrone, M. L. Flori, P. Barbini and M. Fimiani, Long term follow-up results on severe recalcitrant atopic dermatitis treated with extracorporeal photochemotherapy, J. Eur. Acad. Dermatol. Venereol., 2012 DOI:10.1111/j.1468-3083.2012.04552.x.
- R. R. Muellegger, A. Hofer, W. Salmhofer, H. P. Soyer, H. Kerl and P. Wolf, Extended extracorporeal photochemotherapy with extracorporeal administration of 8-methoxypsoralen in systemic sclerosis. An Austrian single-center study, Photodermatol., Photoimmunol. Photomed., 2000, 16, 216–223 CAS.
- E. A. Holm, H. C. Wulf, H. Stegmann and G. B. Jemec, Life quality assessment among patients with atopic eczema, Br. J. Dermatol., 2006, 154, 719–725 CrossRef CAS.
- M. Augustin, K. Wenninger, U. Amon, M. J. Schroth, W. Kuster, M. Chren, J. Kupfer and U. Gieler, German adaptation of the Skindex-29 questionnaire on quality of life in dermatology: validation and clinical results, Dermatology, 2004, 209, 14–20 CrossRef.
- M. M. Chren, R. J. Lasek, L. M. Quinn, E. N. Mostow and S. J. Zyzanski, Skindex, a quality-of-life measure for patients with skin disease: reliability, validity, and responsiveness, J. Invest. Dermatol., 1996, 107, 707–713 CAS.
- M. Morfeld, M. Bullinger, J. Nantke and E. Brahler, The version 2.0 of the SF-36 health survey: results of a population-representative study, Soz.- Praeventivmed., 2005, 50, 292–300 CrossRef.
- M. Bullinger, M. Morfeld, T. Kohlmann, J. Nantke, H. van den Bussche, B. Dodt, S. Dunkelberg, I. Kirchberger, A. Kruger-Bodecker, A. Lachmann, K. Lang, C. Mathis, O. Mittag, A. Peters, H. H. Raspe and H. Schulz, SF-36 Health survey in rehabilitation research. Findings from the North German network for rehabilitation research, NVRF, within the rehabilitation research funding program, Rehabilitation (Stuttg), 2003, 42, 218–225 CrossRef CAS.
- M. Bullinger, German translation and psychometric testing of the SF-36 health survey: preliminary results from the IQOLA project. International quality of life assessment, Soc. Sci. Med., 1995, 41, 1359–1366 CrossRef CAS.
- R. Sanchez, M. Ballesteros and B. J. Arnold, Validation of the FACT-G scale for evaluating quality of life in cancer patients in Colombia, Qual. Life Res., 2011, 20, 19–29 CrossRef.
- T. Dani and R. Knobler, Extracorporeal photoimmunotherapy-photopheresis, Front. Biosci., 2009, 14, 4769–4777 CrossRef CAS.
- R. Knobler, M. L. Barr, D. R. Couriel, J. L. Ferrara, L. E. French, P. Jaksch, W. Reinisch, A. H. Rook, T. Schwarz and H. Greinix, Extracorporeal photopheresis: past, present, and future, J. Am. Acad. Dermatol., 2009, 61, 652–665 CrossRef.
- R. Knobler, Photopheresis and the red man syndrome, Dermatology, 1995, 190, 97–98 CrossRef CAS.
- H. Zachariae, P. Bjerring, U. Brodthagen and H. Sogaard, Photopheresis in the red man or pre-Sezary syndrome, Dermatology, 1995, 190, 132–135 CrossRef CAS.
- A. Hofer, R. Mullegger, H. Kerl and P. Wolf, Extracorporeal photochemotherapy for the treatment of erythrodermic pityriasis rubra pilaris, Arch. Dermatol., 1999, 135, 475–476 CAS.
- P. Wolf, R. Mullegger, L. Cerroni, R. Aigner, G. Fueger, G. Hofler, J. Derbaschnig and H. Kerl, Photoaccentuated erythroderma associated with CD4+ T lymphocytopenia: successful treatment with 5-methoxypsoralen and UVA, interferon alfa-2b, and extracorporeal photopheresis, J. Am. Acad. Dermatol., 1996, 35, 291–294 CrossRef CAS.
- S. A. Grundmann and S. Beissert, Regulation of cellular immunity by photo(chemo)therapy, Front. Biosci., 2009, 14, 4326–4336 CrossRef CAS.
- U. Just, E. Dimou, R. Knobler, G. Klosner, E. Ivancic-Brandenberger, H. Greinix, A. Becherer and F. Trautinger, Leucocyte scintigraphy with (111) in-oxine for assessment of cell trafficking after extracorporeal photopheresis, Exp. Dermatol., 2012, 21, 443–447 CrossRef CAS.
- A. Maeda, A. Schwarz, A. Bullinger, A. Morita, D. Peritt and T. Schwarz, Experimental extracorporeal photopheresis inhibits the sensitization and effector phases of contact hypersensitivity via two mechanisms: generation of IL-10 and induction of regulatory T cells, J. Immunol., 2008, 181, 5956–5962 CAS.
- P. Quaglino, A. Comessatti, R. Ponti, A. Peroni, F. Mola, M. T. Fierro, P. Savoia, M. Novelli and M. G. Bernengo, Reciprocal modulation of circulating CD4+CD25+bright T cells induced by extracorporeal photochemotherapy in cutaneous T-cell lymphoma and chronic graft-versus-host-disease patients, Int. J. Immunopathol. Pharmacol., 2009, 22, 353–362 CAS.
- T. P. Singh, B. Huettner, H. Koefeler, G. Mayer, I. Bambach, K. Wallbrecht, M. P. Schon and P. Wolf, Platelet-activating factor blockade inhibits the T-helper type 17 cell pathway and suppresses psoriasis-like skin disease in K5.hTGF-beta1 transgenic mice, Am. J. Pathol., 2011, 178, 699–708 CrossRef CAS.
- T. P. Singh, M. P. Schon, K. Wallbrecht, K. Michaelis, B. Rinner, G. Mayer, U. Schmidbauer, H. Strohmaier, X. J. Wang and P. Wolf, 8-Methoxypsoralen plus ultraviolet A therapy acts via inhibition of the IL-23/Th17 axis and induction of Foxp3+ regulatory T cells involving CTLA4 signaling in a psoriasis-like skin disorder, J. Immunol., 2010, 184, 7257–7267 CrossRef CAS.
- G. Han, F. Li, T. P. Singh, P. Wolf and X. J. Wang, The pro-inflammatory role of TGFbeta1: a paradox?, Int. J. Biol. Sci., 2012, 8, 228–235 CrossRef CAS.
- S. Eyerich, A. T. Onken, S. Weidinger, A. Franke, F. Nasorri, D. Pennino, M. Grosber, F. Pfab, C. B. Schmidt-Weber, M. Mempel, R. Hein, J. Ring, A. Cavani and K. Eyerich, Mutual antagonism of T cells causing psoriasis and atopic eczema, N. Engl. J. Med., 2011, 365, 231–238 CrossRef CAS.
- T. P. Singh, M. P. Schon, K. Wallbrecht and P. Wolf, 8-Methoxypsoralen plus UVA treatment increases the proportion of CLA+ CD25+ CD4+ T cells in lymph nodes of K5.hTGFbeta1 transgenic mice, Exp. Dermatol., 2012, 21, 228–230 CrossRef CAS.
- T. P. Singh, M. P. Schön, A. Gruber-Wackernagel, K. Wallbrecht, X. J. Wang and P. Wolf, Role of IL-9 in Th17-associated inflammation, hyperproliferation, and angiogenesis in psoriasis, J. Invest. Dermatol., 2011, 131, S6 CrossRef (abstract).
- N. Sismanopoulos, D. A. Delivanis, K. D. Alysandratos, A. Angelidou, M. Vasiadi, A. Therianou and T. C. Theoharides, IL-9 induces VEGF secretion from human mast cells and IL-9/IL-9 receptor genes are overexpressed in atopic dermatitis, PLoS One, 2012, 7, e33271 CAS.
- P. Wolf, D. X. Nghiem, J. P. Walterscheid, S. Byrne, Y. Matsumura, C. Bucana, H. N. Ananthaswamy and S. E. Ullrich, Platelet-activating factor is crucial in psoralen and ultraviolet A-induced immune suppression, inflammation, and apoptosis, Am. J. Pathol., 2006, 169, 795–805 CrossRef CAS.
Footnotes |
| † This article is published as part of a themed issue on current topics in photodermatology. |
| ‡ Present address: Norderney Hospital, Allergy and Dermatology Clinic, Lippestrasse 9-11, D-26548 Norderney, Germany. |
| This journal is © The Royal Society of Chemistry and Owner Societies 2013 |
