Photodermatoses: environmentally induced conditions with high psychological impact†
Muneeza
Rizwan‡
a,
Charlotte Louise
Reddick‡
b,
Christine
Bundy
c,
Rebecca
Unsworth
a,
Helen Louise
Richards
d and
Lesley Elizabeth
Rhodes
*a
aPhotobiology Unit, Dermatological Sciences, University of Manchester, Manchester Academic Health Science Centre, Salford Royal Foundation Hospital, Manchester M6 8HD, UK. E-mail: Lesley.e.rhodes@manchester.ac.uk; Fax: +44 (0)161 206 1156; Tel: +44 (0)161 206 1150
bSchool of Medicine, University of Manchester, Manchester Academic Health Science Centre, Manchester, UK
cInstitute of Brain Behaviour and Mental Health, University of Manchester, Manchester Academic Health Science Centre, Manchester, UK
dDepartment of Clinical Health Psychology, Mercy University Hospital, Cork, Republic of Ireland
First published on 7th September 2012
Abstract
Photodermatoses are a group of skin disorders caused or exacerbated by ultraviolet and/or visible radiation, which collectively affect a high proportion of the population and substantially affect quality of life (QoL). Our objective was to assess the psychological impact of these conditions. Patients with a range of photodermatoses diagnosed at a specialist investigation centre in the UK completed questionnaires evaluating (i) anxiety and (ii) depression, using the Hospital Anxiety and Depression Scale (HADS), (iii) social anxiety, using the Fear of Negative Evaluation measure (FNE), (iv) coping strategies (brief COPE) and (v) QoL, using the Dermatology Life Quality Index (DLQI). Questionnaires were returned by 185 of 260 patients (71.1% response rate). Mean age was 50.2 years (SD 14.5, range 20–85), 80.3% female. Polymorphic light eruption was the most common diagnosis, followed by photoaggravated eczema, other photoaggravated dermatological conditions and solar urticaria. Across the sample, high percentages, i.e. 23% and 7.9% of individuals, showed scores indicating clinical levels of anxiety and depression, respectively. Facial involvement was a strong indicator for depression (t = 2.7, p < 0.01). In regression analyses psychological factors (particularly depression and adaptive coping) were the principle predictors of QoL, accounting for 17.7% of the variance (F = 7.61, p < 0.01), while clinical variables accounted for an additional 10.1% (F = 8.96, p < 0.01), with number of months/year affected exerting a significant effect (p < 0.01). This study demonstrates the high psychological comorbidity of these conditions; more awareness of this is required, with adoption of a biopsychosocial approach to their management.
Introduction
Photodermatoses are a wide group of cutaneous disorders in which there is an abnormal reaction to components of sunlight, i.e. UVB and/or UVA and/or visible radiation, with their action spectrum falling into recognized patterns for specific diagnoses. They are classified into four main categories: (i) immunologically mediated; (ii) DNA repair disorders; (iii) biochemical, and drug/chemical induced and (iv) photoaggravated dermatoses (PAD). Their clinical features are diverse but they share the common feature of skin symptoms and/or eruption induced or exacerbated by sun exposure, particularly occurring in a sun exposed distribution. Artificial light sources including room lighting can exacerbate photosensitivity, and UVA as well as visible light can pass through window glass to affect sufferers when indoors or travelling in a vehicle. Cutaneous manifestations of the photodermatoses vary in severity with season, sun exposure time and climate and include itch, pain and burning sensation, widespread erythema, blistering, scaling, and ultimately scarring in some conditions, whereas increased frequency of skin cancer occurs in certain disorders.1The most common photodermatosis is the apparently immune-mediated condition polymorphic light eruption (PLE), with a prevalence of 10–20% in Europeans.2 Papular and/or vesicular lesions usually develop within hours of UV exposure and typically resolve, without scarring, over a few days.3 Solar urticaria (SU) is a rarer immune-mediated condition, which typically involves widespread uncomfortable erythema and whealing of the skin within minutes of sun exposure.4 Chronic actinic dermatitis (CAD) and photoaggravated eczema (PAE) are immune-based conditions which can appear similarly at a clinical level, involving eczematous skin, with itching and scaling, whereas actinic prurigo (AP) and cutaneous lupus can scar.1 The porphyrias are biochemical disorders attributable to a build up of photoactive porphyrins, with erythropoietic protoporphyria (EPP) causing an acute burning pain. Drug induced photosensitivity (DIP) involves a variety of presentations, a rapid onset burning erythema being the most common. DNA repair disorders are rare, but depending on type they can cause both acute photosensitivity and longer-term skin damage including melanoma and non-melanoma skin cancers.
Skin conditions can have far-reaching consequences including physical discomfort, limitations to daily activities and employment,5 and psychological sequela.6,7 Thus evaluation and management is required at three levels; (i) the physical; (ii) the psychological; (iii) and the social and contextual.8,9 Patients with skin disease may experience impaired quality of life (QoL) and psychological comorbidity,7,10,11 and higher levels of depression and anxiety are found amongst dermatology patients than within the general population.10–12 Additionally, stigmatisation and fear of negative evaluation by others can result from dermatological disease.13 Patient-rated psychological issues relate more closely to health related QoL than clinician-rated disease severity.14
Despite their differing aetiologies and diverse clinical features, the majority of photodermatoses share some common effects on patient lives due to a protracted clinical course;15 disabling symptoms precipitated by sunlight exposure;1 and prophylactic adaptations to lifestyle such as minimizing time spent outdoors, wearing covering clothing and frequently applying sunscreen.5 Thus a range of photodermatoses were found to have a major impact on QoL.16 More in-depth studies in PLE have demonstrated significant psychological distress.9,17 However, the psychological impact of other photodermatoses has not been evaluated. Our objective was therefore to assess the psychological morbidity associated with a range of photosensitivity disorders, and also the relationship between demographic, clinical and psychological variables. We pursued this by applying validated questionnaires during the summer months in 260 patients whose diagnosis had been confirmed at a specialist photosensitivity diagnostic centre.
Methods
Study design and participants
The study was granted ethical approval by Salford and Trafford research ethics committee. All participants provided written informed consent. A cross-sectional survey was undertaken. Eligible patients were aged 18 years and above and had been referred for phototesting to a tertiary diagnostic centre in the UK, where they were assessed and had their photodermatosis confirmed by a consultant dermatologist with expertise in photosensitivity disorders (L.E.R). Patients with porphyria are not included in this sample as they do not undergo phototesting.Procedure
Data collection occurred during the summer months (June–August) 2005–2007 as this is when photosensitive patients are most likely to be experiencing symptoms. A booklet of validated psychological measures (see below) was either administered during the patients’ visit to the Photobiology Unit or was mailed to photosensitive patients who had previously been assessed at the same Photobiology Unit and confirmed to have a photodermatosis.Questionnaires
Clinical parameters
The booklet of questionnaires also included questions on the following: (i) age of onset of photosensitivity; (ii) number of months of the year affected by photosensitivity; (iii) presence or absence of facial involvement; and (iv) use of steroid tablets or injections for the condition. These clinical parameters have been reported to reflect the severity of photosensitivity disorders.9,27Analysis
Statistical analysis was performed using SPSS 14 software (Statistical Package for the Social Sciences: SPSS Inc., Chicago, IL, U.S.A.). With the exception of comparisons between diagnostic groups, parametric statistics were used as data were normally distributed. Non-parametric statistics (Kruskal–Wallis H and Mann–Whitney U test) were used to compare differences between the different diagnostic groups. A series of Pearson's correlations were used to investigate associations between variables. Differences between groups were examined using independent Student t tests. Stepwise multiple regression was used to assess the impact of demographic, clinical and psychological parameters on quality of life. Unless otherwise stated, data are presented as mean and standard deviation (SD).Results
185 of 260 patients returned their questionnaires (71.1% response rate). Five patients had more than 15% missing data and were excluded from further analysis. Two patients with photocontact dermatitis were excluded due to the small number of patients in this diagnostic group. Final analysis was performed on 178 completed data sets.Demographic and clinical characteristics of the entire sample
Patients’ mean age was 50.2 years (SD 14.5, range 20–85 years), 80.3% were female. The ethnicity of patients was primarily white Caucasian (87.6%), with 3.9% describing themselves as Asian and 5.1% as ‘other’. The majority of the sample was employed (53.9%), with 30% retired, 12.8% unemployed and 2.2% in full time education.Mean age at onset of photosensitivity was 35.7 years (SD 14.5, range 2–82 years). The average duration of photosensitivity manifestation per year was 7.7 months (SD 3.9, range 1–12). Facial involvement was reported by 117 participants (65.7%) and 78 (43.8%) had used topical steroids for their photosensitivity disorder. The most common individual photodermatosis in our cohort was PLE, followed by PAE, SU and subacute lupus erythematosus (SCLE). The demographic and clinical characteristic details for the eight diagnostic groups are illustrated in Table 1.
| Entire sample (n = 178) | PLE (n = 63) | PAE (n = 48) | SU (n = 16) | PADa (n = 15) | SCLE (n = 10) | AP (n = 9) | CAD (n = 9) | DIP (n = 8) | |
|---|---|---|---|---|---|---|---|---|---|
| a PAD denotes photoaggravated dermatoses other than PAE and SCLE. | |||||||||
| Age mean (range) years | 50.20 (20–85) | 45.54 (20–72) | 53.83 (20–84) | 42.66 (21–68) | 51.46 (27–75) | 54.77 (43.74) | 47.33 (30–70) | 61.05 (25–85) | 63.19 (49–74) |
| Age of onset mean (range) years | 35.73 (2–82) | 28.70 (2–53) | 42.95 (3–75) | 33.12 (14–58) | 36.33 (5–70) | 37.33 (7–63) | 23 (3–40) | 40 (14–82) | 54.37 (33–70) |
| Sex: females n (%) | 143 (80.3) | 55 (87.3) | 35 (72.9) | 14 (87.5) | 10 (66.7) | 8 (80) | 8 (88.9) | 5 (55.6) | 8 (100) |
| No of months of the year affected mean (SD) | 7.65 (3.93) | 6.54 (3.61) | 8.36 (3.93) | 10.25 (3.33) | 8.16 (4.17) | 7.40 (4.42) | 7.00 (4.00) | 9.42 (3.55) | 5.75 (4.09) |
| Face involved, n (%) | 117 (65.7) | 32 (50.8) | 38 (79.2) | 11 (68.8) | 8 (53.3) | 8 (80) | 8 (88.9) | 7 (77.8) | 5 (62.5) |
Group differences for demographic variables
Significant differences between groups were found for mean age (χ2 = 23.56, p < 0.01), age of onset (χ2 = 31.49, p < 0.01), number of months affected in a year (χ2 = 17.63, p < 0.01) and use of steroids (χ2 = 19.16, p < 0.01). There was a non-significant trend for difference in facial involvement (χ2 = 13.73, p = 0.06) between the groups.Differences were found between PLE and PAE groups with respect to age (z = −2.94, p < 0.01), age of onset (z = −4.55, p < 0.01) and number of months affected (z = −2.36, p = 0.02). Age of onset differed between PLE and CAD (z = −2.23, p = 0.02) and PLE and SCLE (z = −2.16, p = 0.03). The highest levels of facial involvement were found amongst patients with AP, SCLE, PAE and CAD (88.9%, 80%, 79.2% and 77.8%, respectively), while the lowest level of facial involvement was found in patients with PLE (50.8%).
Gender differences
Females showed an earlier age of onset (z = −3.42, p = 0.01), higher levels of anxiety (z = −2.74, p < 0.01) and higher DLQI scores (z = −2.36, p = 0.02) compared to males. There were no other significant relationships between gender and the clinical or psychological variables measured.Psychological variables
The mean HADS anxiety score was 7.0 (SD 4.6, range 0–20) and mean HADS depression score was 5.3 (SD 3.8, range 0–18). Across the sample 19.7% and 19.1% of individuals were identified as possible cases for anxiety and depression respectively and a further 23% and 7.9% were probable cases for anxiety and depression.The FNE score was 13.23 (SD 5.6, range 1–29). Clinical levels of social anxiety were detected in 19 patients (10.7%).
The mean score for adaptive coping strategies was 34.7 (SD 8.9), with acceptance (mean = 5.8, SD 1.8), active coping (mean = 5.3, SD 1.8) and planning (mean = 4.9, SD 1.9) being the most commonly employed strategies. The mean score for maladaptive strategies was 19.2 (SD 6.3), with self-distraction (mean = 3.9, SD 1.8) and venting (mean = 3.4, SD 1.6) being most common.
The mean DLQI score for the entire sample was 8.8 (SD 6.9, range 0–27). Twenty eight percent scored 11–20, indicating a very large effect on QoL, and 6.7% scored 21–30, indicating an extremely large effect. The highest DLQI scores were for DIP (mean 10.84, SD 6.11) followed by CAD (mean 10.71, SD 7.92). PLE scored the lowest (mean 6.28, SD 6.39). The DLQI scores were different across groups (χ2 = 15.26, p < 0.05). Post hoc tests revealed a significant difference between PLE and PAE (z = −3.17, p < 0.01), and PLE and SU (z = 2.15, p < 0.05) in terms of DLQI scores.
There were no significant differences between diagnostic groups with respect to any psychological variables (see Fig. 1a–f).
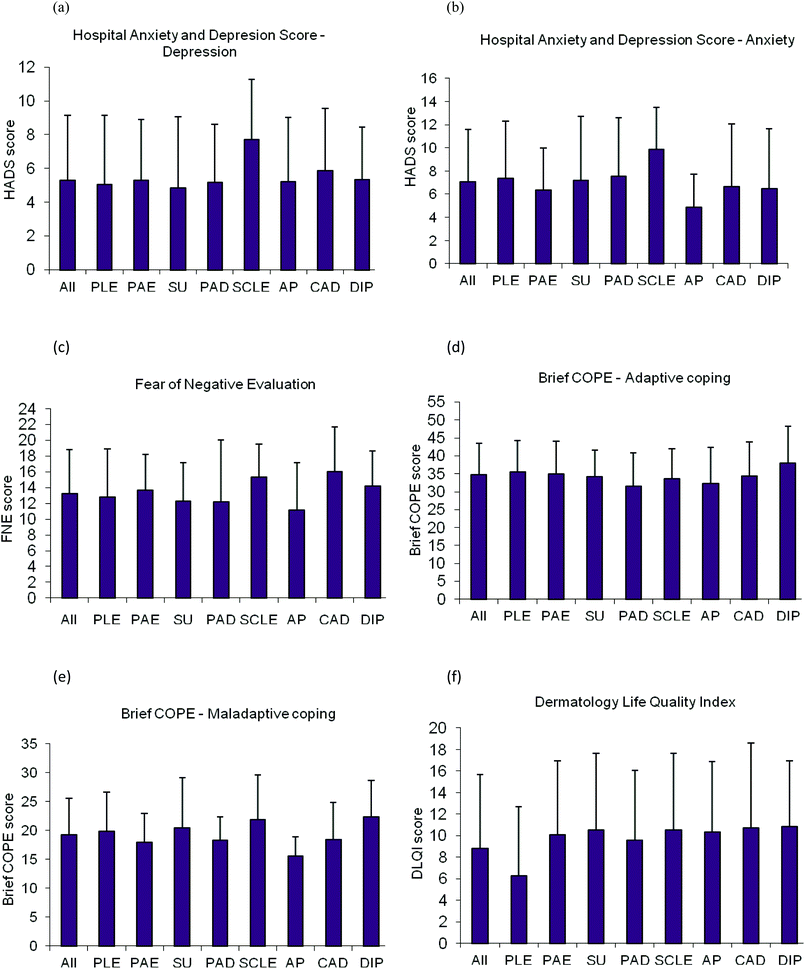 | ||
| Fig. 1 Bar charts showing mean and SD scores for (a) Anxiety (b) Depression (c) Social Anxiety (d) Adaptive Coping (e) Maladaptive Coping (f) Life quality, in 8 categories of photodermatoses. PLE, polymorphic light eruption (n = 63); PAE, photoaggravated eczema (n = 48); SU, solar urticaria (n = 16); PAD, photoaggravated dermatoses other than PAE and SCLE (n = 15); SCLE, subacute cutaneous lupus erythematosus (n = 10); AP, actinic prurigo (n = 9); CAD, chronic actinic dermatitis (n = 9); DIP, drug induced photosensitivity (n = 8). | ||
Associations between demographic, clinical, psychological and quality of life variables
Given the number of tests undertaken a more conservative significance level (p < 0.01) was chosen to control for the possibility of false positive results. Quality of life was significantly associated with anxiety (r = 0.28, p < 0.01), depression (r = 0.41, p < 0.01), adaptive (r = 0.31, p < 0.01) and maladaptive (r = 0.3, p < 0.01) coping strategies. Anxiety and depression scores were correlated (r = 0.69, p < 0.01), suggesting shared variance in distress categories. Depression scores also correlated with maladaptive coping (r = 0.52, p < 0.01) and anxiety scores correlated with adaptive coping (r = 0.26, p < 0.01). Individuals who cited that their photosensitivity disorder affected their face had significantly higher levels of depression (t = 2.7, p < 0.01).A multiple regression analysis (Table 2) was performed to investigate the predictors of QoL in the entire sample using significant correlations of less than p < 0.01 with DLQI scores or a significant difference between groups (e.g. number of months, gender) in the preliminary descriptive analyses. Gender was included in the analysis along with facial involvement and number of months affected. Anxiety, depression, adaptive and maladaptive coping were also included in the model. Psychological factors were the principle predictors of QoL, accounting for 17.7% of the variance (F = 7.61, p < 0.01). Specifically, depression (β = 0.37; t = 3.66; p < 0.01) and adaptive coping (β = 0.24; t = 3.23; p < 0.01) reached significance. The clinical variables accounted for an additional 10.1% of the variance (F = 8.96, p < 0.01), with number of months reaching significance (β = 0.19; t = 2.53; p < 0.01). These findings suggest that psychological factors play a substantial role in predicting QoL in patients with photodermatosis, with clinical variables playing a smaller role.
| Variable | R | %AdjR2 | %R2 change | F change | Df | P | B |
|---|---|---|---|---|---|---|---|
| a B significant at p < 0.01. | |||||||
| Demographic | 0.13 | 1.1 | 1.7 | 2.723 | 1158 | 0.10 | |
| Gender | 0.07 | ||||||
| Clinical | 0.34 | 10.1 | 10.1 | 8.955 | 2156 | <0.001 | |
| Face | −0.11 | ||||||
| No. of months | 0.19a | ||||||
| Psychological | 0.54 | 25.8 | 17.7 | 7.609 | 5151 | <0.001 | |
| FNE | −0.25 | ||||||
| Adaptive coping | 0.24a | ||||||
| Maladaptive coping | 0.06 | ||||||
| Depression | 0.37a | ||||||
| Anxiety | −0.11 | ||||||
Discussion
This is the first study to explore the psychological comorbidity in a range of photosensitivity disorders. We found high levels of anxiety and depression amongst photosensitive patients, with 23% of individuals demonstrating anxiety scores suggestive of clinical morbidity and 7.9% of patients showing evidence of clinical depression. These findings in a range of photodermatoses are consistent with those previously reported in the specific condition PLE.17 The current scores are approximately double the British normative data, where 12.6% and 3.6% of the general population demonstrate clinical anxiety and depression, respectively,28 indicating the large psychological impact photodermatoses have upon patients.Depression was the principal predictor of dermatology related QoL with adaptive coping also a major predictor. Whilst adaptive coping strategies, such as sun avoidance by spending time indoors, could help to improve symptoms they may negatively impact upon QoL due to the restrictions they place upon patient lifestyle. Thus, because of the overlap and shared variance between factors, psychological functioning plays a large role in determining the QoL in patients with photodermatoses. Dermatologists frequently underestimate the psychological and psychiatric morbidity association with dermatological conditions.29,30 Ultra-short screening tools (see PHQ-2; PHQ-931,32) i.e. 1–4 items, taking less than 2 minutes to complete, have been shown to accurately detect depression in a primary care setting33 and thus might be used in other settings such as hospital-based care. Identifying and managing these issues may improve QoL in patients with photodermatoses, through provision of more holistic patient care.
Facial involvement was a strong indicator for depression. It has been suggested that lesions on visible areas such as the face and hands is linked to higher psychiatric impact in females.34 Indeed, we found female patients had higher levels of anxiety and DLQI scores as compared to male patients. Possibly females are more aware of physical appearance and hence lesions on visible areas cause greater distress for female patients although this assertion would need to be tested more formally. Clinicians could target screening at known vulnerable groups and pay particular attention to facial involvement and number of months/year affected in photodermatoses as a possible cause for depression and impaired QoL, respectively.
Drug induced photosensitivity appeared to have the highest impact on QoL, and highest brief COPE score for both adaptive and maladaptive coping strategies. This may be anticipated as DIP commonly presents in later life; in our cohort the mean age of onset was 54.4 years, and patients may have difficulties in making adaptations to their usual routine, resulting in impaired QoL. Patients with SCLE showed the highest score for anxiety as well as depression, perhaps due to patient concern regarding possible progression to systemic disease. Indeed, it has previously been reported that patients with SCLE demonstrate concern over a worsening of their symptoms.35 There are also reports of a significant correlation between measures of anxiety and depression and measures of systemic lupus erythematosus activity.36–39 The highest social evaluative anxiety (FNE) scores were found in patients with CAD. Chronic actinic dermatitis is a particularly severe disorder with very low light reactivity thresholds, accompanied by considerable facial involvement and symptoms year round.40
With respect to QoL and psychological parameters the groups only differed significantly in terms of DLQI scores, with statistically significant differences found between PLE vs. PAE and PLE vs. SU. Overall, the DLQI scores for the conditions were broadly in agreement with those reported by Jong et al. (2008).16 Interestingly, PLE showed close to the mean scores for all the diagnoses despite the notably lower DLQI. Thus DLQI may not be a sensitive enough measure for use alone in PLE, with other measures of psychological functioning providing a more comprehensive picture of the true distress associated with PLE. Despite the wide range of photosensitivity disorders examined, they appear to share common effects on patient lives in terms of psychological impact.
Whilst the strength of this study is that participants were recruited from a specialist diagnostic centre and thus had a confirmed diagnosis, caution must be exercised in extrapolating these results to patients managed by family doctors (i.e. in primary care), particularly as conditions such as PLE and PAE have a range of severities. However, high levels of psychological difficulties have also been found in dermatological patients in primary care,41,42 suggesting hospital based studies may not overemphasise the burden these conditions cause, for example, studies show that anxiety and depression are as prevalent in populations of community-based psoriasis patients as in tertiary referral patients.43,44 The low subject numbers in some patient groups may have reduced the ability to detect differences between diagnostic categories. However, the spread of conditions sampled reflects the prevalence of the conditions referred to the centre.
This study has demonstrated that psychological distress is common amongst patients with photodermatoses. Findings highlight the importance of further addressing their psychological co-morbidity during their clinical care. A whole person, i.e. biopsychosocial approach to photosensitivity disorders is important in the management of patients suffering from these conditions. Future changes in weather patterns may further encourage populations to spend increased leisure time outdoors, with anticipated increased negative impact on those suffering from photosensitivity.
References
- H. W. Lim and J. Hawk, Evaluation of the photosensitive patient, in Photodermatology, ed. H. W. Lim, H. Hönigsmann and J. Hawk, Informa Healthcare, USA, 2007, ch. 10, pp. 139–148 Search PubMed.
- L. E. Rhodes, M. Bock, A. S. Janssens, T. C. Ling, L. Anastasopoulou, C. Antoniou, F. Aubin, T. Bruckner, B. Faivre, N. K. Gibbs, C. Jansen, S. Pavel, A. J. Stratigos, F. R. de Gruijl and T. L. Diepgen, Polymorphic light eruption occurs in 18% of Europeans and does not show higher prevalence with increasing latitude: multicenter survey of 6895 individuals residing from the Mediterranean to Scandinavia, J. Invest. Dermatol., 2010, 130, 626–628 CrossRef CAS.
- H. Hönigsmann, Polymorphic light eruption, Photodermatol. Photoimmunol. Photomed., 2008, 24, 155–161 CrossRef.
- N. Uetsu, H. Miyauchi-Hashimoto, H. Okamoto and T. Horio, The clinical and photobiological characteristics of solar urticaria in 40 patients, Br. J. Dermatol., 2000, 142, 32–38 CrossRef CAS.
- R. Stafford, M. D. Farrar, R. Kift, M. T. Durkin, J. L. Berry, A. R. Webb and L. E. Rhodes, The impact of photosensitivity disorders on aspects of lifestyle, Br. J. Dermatol., 2010, 163, 817–822 CrossRef CAS.
- D. G. Fortune, H. L. Richards and C. E. M. Griffiths, Psychological factors in psoriasis: consequences, mechanisms, and interventions, Dermatol. Clin., 2005, 23, 681–694 CrossRef CAS.
- A. Picardi, P. Pasquini, D. Abeni, G. Fassone, E. Mazzotti and G. A. Fava, Psychosomatic assessment of skin diseases in clinical practice, Psychother. Psychosom., 2005, 74, 315–322 CrossRef.
- G. L. Engel, The need for a new medical model: a challenge for biomedicine, Science, 1977, 196, 129–136 CAS.
- H. L. Richards, T. C. Ling, G. Evangelou, R. C. Brooke, K. Huber, N. K. Gibbs, D. G. Fortune and L. E. Rhodes, Psychological distress in polymorphic light eruption and its relationship to patients’ beliefs about their condition, J. Am. Acad. Dermatol., 2007, 56, 426–431 CrossRef.
- M. A. Gupta and A. K. Gupta, Depression and suicidal ideation in dermatology patients with acne, alopecia areata, atopic dermatitis and psoriasis, Br. J. Dermatol., 1998, 139, 846–850 CrossRef CAS.
- M. A. Gupta and A. K. Gupta, Psychiatric and psychological comorbidity in patients with dermatological disorders, Am. J. Clin. Dermatol., 2003, 4, 833–842 CrossRef.
- H. L. Richards, D. G. Fortune, C. J. Main and C. E. M. Griffiths, The contribution of perceptions of stigmatisation to disability in patients with psoriasis, J. Psychosom. Res., 2001, 50, 10–15 CrossRef.
- D. G. Fortune, C. J. Main, T. M. O'Sullivan and C. E. M. Griffiths, Assessing illness-related stress in psoriasis: the psychometric properties of the psoriasis life stress inventory, J. Psychosom. Res., 1997, 42, 467–475 CrossRef CAS.
- A. Picardi, D. Abeni, C. F. Melchi, P. Puddu and P. Pasquini, Psychiatric morbidity in dermatological outpatients: an issue to be recognized, Br. J. Dermatol., 2000, 143, 983–991 CrossRef CAS.
- W. L. Morison and R. S. Stern, Polymorphous light eruption: a common reaction uncommonly recognized, Acta Derm. Venereol., 1982, 62, 237–240 CAS.
- C. T. Jong, A. Y. Finlay, A. D. Pearse, A. C. Kerr, J. Ferguson, E. C. Benton, J. L. M. Hawk, R. P. Sarkany, E. McMullen, L. E. Rhodes, P. M. Farr and A. V. Anstey, The quality of life of 790 patients with photodermatoses, Br. J. Dermatol., 2008, 159, 192–197 CrossRef CAS.
- H. L. Richards, T. C. Ling, G. Evangelou, R. C. C. Brooke, D. G. Fortune and L. E. Rhodes, Evidence of high levels of anxiety and depression in polymorphic light eruption and their association with clinical and demographic variables, Br. J. Dermatol., 2008, 159, 439–444 CrossRef CAS.
- A. Zigmoid and R. Snaith, The hospital anxiety and depression scale, Acta Psychiatr. Scand., 1983, 67, 361–370 CrossRef.
- I. Bjelland, A. A. Dahl, T. T. Haug and D. Neckelmann, The validity of the hospital anxiety and depression scale: an updated literature review, J. Psychosom. Res., 2002, 52, 69–77 CrossRef.
- A. Wittkowski, H. L. Richards, C. J. Main and C. E. M. Griffiths, The impact of psychological and clinical factors on quality of life in individuals with atopic dermatitis, J. Psychosom. Res., 2004, 57, 195–200 CrossRef.
- M. Shah and M. Coates, An assessment of the quality of life in older patients with skin disease, Br. J. Dermatol., 2006, 154, 150–153 CrossRef CAS.
- D. Watson and R. Friend, Measurement of social-evaluative anxiety, J. Consult. Clin. Psychol., 1969, 33, 448–457 CrossRef CAS.
- L. Stopa and D. M. Clark, Social Phobia: comments on the viability and validity of an analogue research strategy and British norms for the fear of negative evaluation questionnaire, Behav. Cogn. Psychother., 2001, 29, 423–430 CrossRef.
- S. Carver, You want to measure coping but your protocol's too long: consider the brief COPE, Int. J. Behav. Med., 1997, 4, 92–100 CrossRef.
- A. Y. Finlay and G. K. Khan, Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use, Clin. Exp. Dermatol., 1994, 19, 210–216 CrossRef CAS.
- Y. Hongbo, C. L. Thomas, M. A. Harrison, M. S. Salek and A. Y. Finlay, Translating the science of quality of life into practice: what do dermatology life quality index scores mean?, J. Invest. Dermatol., 2005, 125, 659–664 CrossRef CAS.
- T. C. Ling, H. L. Richards, A. S. Jassens, L. Anastassopoulou, C. Antoniou, F. Aubin, T. L. Diepgen, R. Fazakerley, F. R. de Gruijl, C. T. Jansen, S. Pavel, A. Smedley, A. J. Stratigos, A. R. Webb, N. K. Gibbs and L. E. Rhodes, Seasonal and latitudinal impact of polymorphic light eruption on quality of life, J. Invest. Dermatol., 2006, 126, 1648–1651 CrossRef CAS.
- J. R. Crawford, J. D. Henry, C. Crombie and E. P. Taylor, Normative data for the HADS from a large non-clinical sample, Br. J. Clin. Psychol., 2001, 40, 429–434 CrossRef CAS.
- F. Sampogna, A. Picardi, C. F. Melchi, P. Pasquini and D. Abeni, The impact of skin diseases on patients: comparing dermatologists’ opinions with research data collected on their patients, Br. J. Dermatol., 2003, 148, 989–995 CrossRef CAS.
- H. L. Richards, D. G. Fortune, A. Weidmann, S. K. T. Sweeney and C. E. M. Griffiths, Detection of psychological distress in psoriasis patients – low consensus between dermatologist and patient, Br. J. Dermatol., 2004, 151, 1227–1233 CrossRef CAS.
- K. Kroenke, R. L. Spitzer and J. B. Williams, The PHQ-9: validity of a brief depression severity measure, J. Gen. Intern. Med., 2001, 16, 606–613 CrossRef CAS.
- K. Kroenke, R. L. Spitzer and J. B. Williams, The Patient Health Questionnaire-2: validity of a two-item depression screener, Med. Care, 2003, 41, 1284–1292 CrossRef.
- A. J. Mitchell and J. C. Coyne, Do ultra-short screening instruments accurately detect depression in primary care? A pooled analysis and meta-analysis of 22 studies, Br. J. Gen. Pract., 2007, 57, 144–151 Search PubMed.
- A. Picardi, D. Abeni, C. Renzi, M. Braga, P. Puddu and P. Pasquini, Increased psychiatric morbidity in female outpatients with skin lesions on visible parts of the body, Acta Derm. Venereol., 2001, 81, 410–414 CrossRef CAS.
- R. Klein, S. Moghadam, L. Taylor, C. Coley, J. Okawa, J. LoMonico, M. Chren and V. P. Werth, Quality of life in cutaneous lupus erythematosus, J. Am. Acad. Dermatol., 2011, 64, 849–858 CrossRef.
- A. Karasz and S. C. Ouellette, Role strain and psychological well – being in women with systemic lupus erythematosus, Women Health, 1995, 23, 41–57 CrossRef CAS.
- R. Omdal, G. Husby and S. I. Mellgren, Mental health status in systemic lupus erythematosus, Scand. J. Rheumatol., 1995, 24, 142–145 CrossRef CAS.
- T. Stoll, C. Gordon, B. Seifert, K. Richardson, J. Malik, P. A. Bacon and D. A. Isenberg, Consistency and validity of patient administered assessment of quality of life by the MOS SF-36: its association with disease activity and damage in patients with systemic lupus erythematosus, J. Rheumatol., 1997, 24, 1608–1614 CAS.
- P. L. Dobkin, P. R. Fortin, L. Joseph, J. M. Esdaile, D. S. Danoff and A. E. Clarke, Psychosocial contributors to mental and physical health in patients with systemic lupus erythematosus, Arthritis Care Res, 1998, 11, 23–31 CrossRef CAS.
- R. Roelandts, Chronic actinic dermatitis, J. Am. Acad. Dermatol., 1993, 28, 240–249 CrossRef CAS.
- G. K. Khan and A. Y. Finlay, Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use, Clin. Exp. Dermatol., 1994, 19, 210–216 CrossRef.
- T. F. Poyner and P. J. Fell, A survey of patients with plaque psoriasis who had not consulted their doctor in the past year, Br. J. Clin. Res., 1995, 6, 201–207 Search PubMed.
- S. K. Kurd, A. B. Troxel, P. Crits-Christoph and J. M. Gelfand, The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study, Arch. Dermatol., 2010, 146, 891–895 Search PubMed.
- D. G. Fortune, H. L. Richards and C. E. M. Griffiths, Psychological factors in psoriasis: consequences, mechanisms, and interventions, Dermatol. Clin., 2005, 23, 681–694 CrossRef CAS.
Footnotes |
| † This article is published as part of a themed issue on current topics in photodermatology. |
| ‡ Joint first author. |
| This journal is © The Royal Society of Chemistry and Owner Societies 2013 |
