Parthenium dermatitis†
Vinod Kumar
Sharma
*,
Parul
Verma
and
K.
Maharaja
Department of Dermatology and Venereology, All India Institute of Medical Sciences, Teaching Block, 4th floor, Ansari Nagar, New Delhi, India. E-mail: aiimsvks@yahoo.com
First published on 30th October 2012
Abstract
Allergic contact dermatitis (ACD) to Parthenium hysterophorus is the most common cause of plant dermatitis in India. Parthenium dermatitis is caused by dry powder of leaves and flowers and hair-like structures (trichomes). Sesquiterpene lactones (SQLs) are the most important allergens responsible for ACD to parthenium. The different patterns include classical airborne contact dermatitis, chronic actinic dermatitis (CAD), exfoliative and widespread dermatitis. There is a definite trend towards a change from an airborne pattern to a CAD pattern in the natural history of parthenium dermatitis. In CAD, there is a reported increased sensitivity to UVB, UVA and even visible light. However, SQLs including parthenin, the major allergen in the Parthenium hysterophorus, has neither documented photoallergic nor phototoxic properties. Recently, the high photoreactivity of α-methylene-γ-butyrolactone ring toward thymidine and resulting photoadducts has been proposed as an explanation of the progressive evolution of allergic contact dermatitis toward chronic actinic dermatitis. However, more data is required to reach a conclusion on the mechanism of photosensitivity in parthenium dermatitis. Sunlight, especially UV radiation, may have a role in increasing the germination capacity and the amount of allergens in the Compositae family, especially in parthenium plants under appropriate conditions like summer and spring, which may contribute to high prevalence of parthenium dermatitis especially in northern India.
 Vinod Kumar Sharma | Vinod K. Sharma MD, FAMS is Professor and Chairman of Dermatology and Venereology at All India Institute of Medical Sciences, New Delhi, India. Has special interest in contact dermatitis and drug eruptions. |
Parthenium dermatitis
Historical aspects
Compositae plants are the second largest family of flowering plants and are the commonest causes of plant dermatitis.1 It affects nearly 1% of the general population and approximately 5% of occupationally exposed groups.2Parthenium hysterophorus is popularly known as “feverfew”, and is a member of the Compositae family. French in 1930 was the first to record contact dermatitis due to this weed and named it “feverfew” in the United States.3Parthenium hysterophorus is a herbaceous plant native to the tropical Americas (the Caribbean islands and the lands bordering the Caribbean) and now grows in Mexico, Central America, South America, the West Indies, East Africa, most of the southern part of the United States, parts of Asia (especially India), and Australia. In Australia it was first recorded in Queensland in 1955 and later rapidly spread to New South Wales and Northern Territory. It causes health problems and economic loss in Queensland.4Parthenium hysterophorus is known as “Congress grass” or “Congress weed” in India.5 Parthenium dermatitis was first reported in 1968, at Pune, India.6 Parthenium, a native of tropical America, caused an epidemic of allergic contact dermatitis after contaminated wheat seed shipments from USA.7 At present, plant dermatitis in India is commonly caused by Parthenium hysterophorus and accounts for 40% of all patients attending contact dermatitis clinics.8Other members of the Compositae family that are in wide use are the ornamental annuals like sunflowers, cosmos, marigold, asters; herbaceous perennials like dahlia, chrysanthemum, marguerites; vegetables like lettuce, chicory, artichokes; herbal medicines like feverfew (Tanacetum parthenium), pot marigold (Calendula); natural insecticides like pyrethrum and weeds like bindii (Soliva pterosperma), ragweed, fleabane, stinkwort and capeweed.9
 | ||
| Fig. 1 Parthenium hysterophorus – the young plant with deeply-dissected leaves and few flower heads. | ||
 | ||
| Fig. 2 Parthenium hysterophorus – the close up view of white flower heads. | ||
Pathogenesis
Parthenium dermatitis is an immuno-inflammatory dermatitis, which starts as contact sensitization with parthenium antigen in a subset of the exposed population and propagates as a type IV (cell-mediated) hypersensitivity immune response (mediated by a series of cellular and molecular mechanisms) with early sensitization phase and a subsequent elicitation phase if antigen exposure persists.13 Epidermal Langerhans cells and other cutaneous antigen-presenting cells transport the allergen from the skin to the regional lymph nodes, where it is presented to T-lymphocytes and T-cell proliferation occurs with production of effector and memory T cells, which finally leads to the development of cutaneous inflammation characterized by the infiltration of T-lymphocytes and other inflammatory cells into the re-exposed skin sites. In all patients with parthenium dermatitis, Akhtar et al. reported significantly elevated pro-inflammatory cytokine levels of TNF-α, IL-6, IL-8 and IL-17 (P < 0.001) in comparison to healthy controls and decrease in anti-inflammatory cytokines IL-4 (P < 0.217) and IL-10 (P = 0.001) which suggests the role of pro-inflammatory cytokines in the pathogenesis of parthenium dermatitis.14 Genetic predisposition to parthenium dermatitis due to lower-producing genotypes of IL-10 has also been reported in a study done on an Indian cohort.15Positive prick test was reported in 12 out of 14 patients of parthenium dermatitis and serum IgE level was elevated in all patients to varying degrees. It has been postulated that a combined immediate (type I) and delayed (type IV) hypersensitivity mechanism may be operational in pathogenesis of parthenium dermatitis and that in sensitized subjects with atopic diathesis it may also induce exacerbation of lesions.16 Kumar et al. evaluated the presence of immediate hypersensitivity to Parthenium hysterophorus in 70 patients with atopic dermatitis (AD) and in 70 healthy controls, who were patch test negative to Parthenium hysterophorus by skin prick test (SPT). Twenty-five (35.7%) patients with AD had a positive SPT to parthenium, compared to 3 (4.3%) of controls. The mean AEC, the mean total IgE and parthenium specific IgE were significantly elevated in SPT-positive AD patients vis-à-vis SPT negative controls. The study suggests that a third of patients with AD demonstrated a type I hypersensitivity to parthenium.15 A combination of type III and type IV hypersensitivity had also been postulated17 but not universally accepted.16
Mode of sensitisation
Parthenium dermatitis is mainly caused by dried leaves and trichomes, which have been seen electron microscopically on the leaf of Parthenium hysterophorus. Sesquiterpenelactones (SQLs), the major allergens in it, have been identified in the leaves, stem and waxy coat of the pollen. Trichomes and their tiny particles of powdered dried plants are disseminated widely by wind.18 Thus parthenium is capable of producing “dermatitis at a distance” or exacerbating an active outbreak of eczema. Direct or indirect contact sensitization is also possible in view of the rampant growth and handling of the weed. Pollens play a role mainly in respiratory allergy, and more often cause allergic rhinitis rather than bronchial asthma since it may not pass beyond the nasal mucosa.19Allergens in the Parthenium hysterophorus
The most important allergens causing parthenium dermatitis are SQLs which are lipophilic and present mainly in the oleoresin fraction in the leaf, stem, flower and pollen of the plant (Table 1).20 But a significant concentration of SQLs are present in the trichomes (small glandular hairs) present on the under-surface of the leaves and stem. Over 200 skeletal types and 1350 individual types of SQLs have been described having multiple functional groups attached to them.21 Parthenin was found to be the major allergen among the SQLs that belong to the pseudoguinolide class of SQLs and has an alpha methylene group exocyclic to gamma lactone, which is probably essential for the induction of allergy. The other allergens found to be in Parthenium hysterophorus were coronopilin, tetraneurin A, hymenin, hysterin, dihydroisoparthenin, ambrosin etc.22 Paulsen et al. patch tested twelve feverfew allergic patients with extracts and fractions containing volatile monoterpenes and sesquiterpenes as well as extracts of airborne particles from flowering feverfew plants, obtained by fractionation of ether extracts, dynamic headspace and the high-volume air sampler (HIVAS) technique, respectively. Among those, eight had positive patch-test reactions to a HIVAS filter extract, while two tested positive to a headspace extract. Subsequent analysis of the HIVAS extract by gas chromatography and mass spectrometry detected parthenolide (PHL) in a concentration of 510 ng mL−1 in the HIVAS extract. Testing with a dilution series of PHL showed positive reactions down to 8.1 ng in selected patients. None of the 12 patients tested positive to monoterpenes or sesquiterpenes, whether they were oxidized or not. It was concluded that some feverfew-allergic patients are sensitive to airborne particles released from the plant, and isolation of PHL from the particle-containing HIVAS extract in allergenic amounts is strong evidence of PHL as the responsible allergen.23 In India, the plant contains large amounts of parthenin.24 The distribution of parthenin in P. hysterophorus plant parts and amount in percentage wet weight are follows: leaf – 3.4%, trichomes – 1.2%, flower – 1.08%, stem – 0.12% and root – 0%.25 Other compositae plants such as Xanthium strumarium, Helianthus annus, Chrysanthemum coronarium, Magnolia stellata and Laurusnobilis may show cross sensitivity with parthenium and vice versa since they also contain similar SQLs.26 But clinically there is no recognized pattern in these patients co-sensitized to other plants. Patch and photopatch tests may aid in revealing co-sensitizers.| In Parthenium hysterophorus |
| Parthenin |
| Coronopilin |
| Tetraneurin A |
| Hymenin |
| Hysterin |
| Ambrosin |
| Dihidroisoparthenin |
| In Tanacetum parthenium |
| Parthenolide |
Allelopathy and impact on livestock
The phenomenon of allelopathy has been well documented in Parthenium hysterophorus, in which one plant exerts a detrimental effect on another through the production of germination- and growth-inhibiting substances, which may have potential to disrupt natural ecosystems like areas that are very sparse or sometimes where no other vegetation can be seen in their dominated areas.27 Toxicities like alopecia, loss of skin pigmentation, dermatitis and diarrhoea have been documented in animals feeding on Parthenium hysterophorus. Liver and kidney damage have been reported in buffalo and sheep, and their milk and meat quality also deteriorate on consumption of this weed.28 Apart from cytotoxic and phytotoxic activities, SQLs in Compositae also have anti-tumour and anti-microbial activities.29Clinical manifestations
The dust and debris from the plant as well as pollen can cause hay fever, asthma or dermatitis.19 With the aid of questionnaires and skin tests, a random clinical survey conducted in Bangalore city revealed that 7.1% of the 2035 study population was suffering from allergic rhinitis due to exposure to parthenium pollen.30 Since the plant grows more abundantly and contains large amounts of parthenin, the severity of dermatitis is greater in India when compared to USA.24 Middle aged or elderly males are commonly more affected than women (male-to-female ratio 5.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1),31 since males are more engaged in outdoor activities and lightly clothed than women. However parthenium dermatitis has been reported in housewives and indoor workers without direct handling in areas of widespread growth of parthenium, suggesting incidental exposure may also sensitize. Parthenium dermatitis is rare in the teenagers and children. None of the 25
1),31 since males are more engaged in outdoor activities and lightly clothed than women. However parthenium dermatitis has been reported in housewives and indoor workers without direct handling in areas of widespread growth of parthenium, suggesting incidental exposure may also sensitize. Parthenium dermatitis is rare in the teenagers and children. None of the 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 children involved in manual pulling were sensitized. Atopic children have shown more frequent sensitisation to Compositae mix than non-atopics.32
000 children involved in manual pulling were sensitized. Atopic children have shown more frequent sensitisation to Compositae mix than non-atopics.32
Clinical patterns of parthenium dermatitis
The classical pattern,2 also known as airborne contact dermatitis (ABCD) pattern, affects the face, especially eyelids and/or neck, V of chest, cubital and popliteal fossae (Fig. 3). In sensitized individuals, the clinical manifestations usually start within 24 hours of exposure, but it may be delayed for up to 2–3 days or even longer in milder cases. In mild cases, only brief periods of erythema and itching occur, which subside within a few hours or days. Moderate dermatitis is characterised by erythema, swelling, papules or papulovesicles with itching and burning and severe dermatitis may exhibit extensive vesiculation and exudation associated with edema. The eruption usually appears in spring and summers and improves during winters. Widespread, extensive and eventually chronic lichenified dermatitis, which may persist throughout the year, results from repeated exposures over many years. The chronic actinic dermatitis (CAD) pattern2 involves the exposed areas (Fig. 4 and 5) such as forehead, rim of ears, cheeks, V of chest, nape of neck, dorsae of forearms and hands as lichenified papules, plaques or papulonodules with relative sparing of non-sun-exposed areas such as eyelids, retro-auricular areas, under-surface of chin and depth of the skin folds. The mixed pattern2 (combination of classical and CAD pattern) manifests as scattered infiltrated scaly papules over the exposed parts and dermatitis over eyelids, flexures of extremities and neck. It may represent a transition phase from classic ABCD to CAD pattern in the natural history of parthenium dermatitis. Exfoliative dermatitis33 presents as scaling, erythema and induration universally all over the skin with often a past history of ABCD and predilection of flexural lichenification in many cases. The photosensitive lichenoid eruption34 pattern presents with pruritic, discrete, flat, violaceous papules and plaques over sun-exposed parts such as forehead, ears, cheek, upper chest and back, extensor aspect of forearms and dorsae of hands simulating photosensitive lichenoid eruptions morphologically also has been described. The prurigo nodularis-like pattern35 presents as multiple hyperkeratotic papules and nodules over extremities (Fig. 6) with characteristic histopathologic features similar to prurigo nodularis with a concurrent or a past history of active dermatitis. The other clinical patterns reported are hands and feet dermatitis, perianal dermatitis, widespread dermatitis of non-airborne contact type, seborrheic dermatitis pattern36 and dermatitis simulating lichen nitidus.37 Patch test to Parthenium hysterophorus is positive in all these variable patterns. Due to vacuolization of Langerhans cells, vitiliginous skin appears to be spared.38Parthenium hysterophorus may precipitate or exacerbate atopic dermatitis.32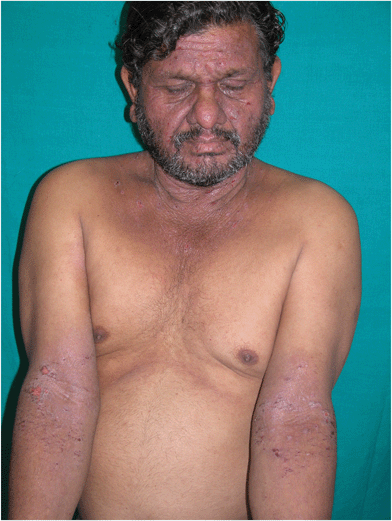 | ||
| Fig. 3 Parthenium dermatitis – airborne contact dermatitis pattern (ABCD). Note the diffuse involvement of the face including eyelids, neck, V of chest, cubital fossa with erythema, erosion, oozing and crusting. | ||
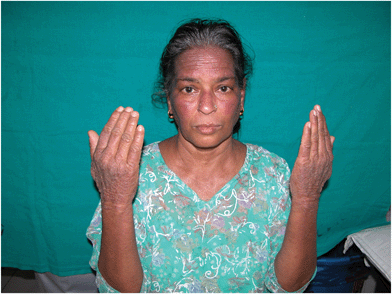 | ||
| Fig. 4 Parthenium dermatitis – chronic actinic dermatitis (CAD) in a female. Note the lichenified papuloplaques over the dorsum of hands, forehead and cheeks. | ||
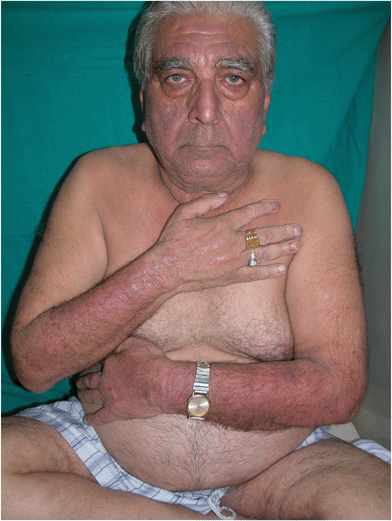 | ||
| Fig. 5 Parthenium dermatitis – elderly male with chronic actinic dermatitis (CAD)-like pattern. Note the lichenified plaques over the dorsum of hands, extensors of forearms, forehead and cheeks. | ||
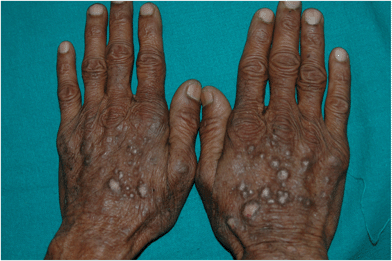 | ||
| Fig. 6 Parthenium dermatitis – prurigo nodularis-like pattern. Note multiple hyperkeratotic prurigo like papules and nodules over the dorsum of hands. | ||
Change of clinical pattern in parthenium dermatitis
In a study conducted by Sharma et al., 74 patients of parthenium dermatitis (60 had ABCD, 9 had CAD and 5 had mixed clinical patterns at onset) with a mean duration of 7.7 years were followed up. Of 60 patients with ABCD, 27 changed to CAD pattern and 11 to mixed pattern after an average period of 4.2 years, which suggests the clinical pattern of parthenium dermatitis undergoes a significant change after the onset, i.e. progresses from ABCD to mixed or CAD pattern.2Photosensitivity and parthenium
Chemical and photochemical reactions
Chemical reaction is a process in which re-arrangement of the constituent atoms of one or more substances, the reactants, are converted to or to create one or more different substances, the products.39Photochemical reaction is a chemical reaction initiated by the absorption of energy in the form of light by molecules leading to creation of transient excited states which can fall apart, change to new structures, combine with each other or other molecules, or transfer electrons, hydrogen atoms, protons, or their electronic excitation energy to other molecules which are stronger acids and reductants than the original ground states.40 Photochemical reactions occur widely in the nature and are involved in photosynthesis, normal vision and vitamin D3 synthesis. In parthenium-induced photosensitivity, photochemical reactions may play a role (Table 2).
| Water stressed Tanacetum parthenium exposed to UV light has increased levels of parthenolide (a SQL) |
| UV light increases germination capacity of Parthenium hysterophorus L. |
Photosensitisation
When a specific chromophore/photosensitizer in a ground state absorbs UV and/or visible radiation of appropriate wavelength, it is transduced into an excited higher energy states which can give off the excess energy as optical radiation (fluorescence/phosphorescence), as heat (internal conversion) or can undergo photochemical reactions. When the excited state molecule returns to the ground state, it can react with ground state oxygen (O2) through excess energy transfer to generate highly reactive singlet oxygen (1O2) and other reactive oxygen species, which rapidly oxidize cellular molecules leading to production of inflammatory mediators through activation of signal transduction pathways that clinically manifest as erythema or inflammation, which is the basis of photosensitisation/phototoxicity. During a photochemical reaction, the chromophore/photosensitizer may be transformed into a new stable molecule called photoproduct, which may also serve as a hapten that conjugates with a carrier and thereby forms a complete antigen (photoantigen/photoallergen) leading to the basis of photoallergic contact dermatitis (type IV hypersensitivity).41,42The relationship between, sunlight, ultraviolet light and parthenium plants has recently been studied, and photosensitivity and parthenium dermatitis has been a mystery. In feverfew (Tanacetum parthenium) plants exposed to water stress, but not in well-watered ones, ultra-violet (UV-A+UV-B) radiation increases the accumulation of parthenolide (a SQL) up to about three times the control.43 The achenes of Parthenium hysterophorus L. germinates well in continuous light and in the dark, but maximum germination was obtained with a 10 hour photoperiod. Increasing duration of light pre-treatment from 6 to 48 hours resulted in greater subsequent germination in the dark. The north Indian populations showed higher percentage germination than those from south India. The patterns of response of achenes of different provenances to light and alternating diurnal temperatures were slightly different.44 A short period of dry storage was sufficient to overcome the light requirement for germination in a minor fraction of the seeds.45 Sunlight, especially the UV radiation, may have a role in increasing the germination capacity and the amount of allergens in Compositae family especially in parthenium plants under appropriate conditions like summer and spring, which may contribute to the high prevalence of parthenium dermatitis especially in northern India.
SQLs are not photo-sensitizers, they have neither phototoxic nor photoallergic properties.46 The absorption spectrum of sesquiterpene lactone of parthenium has not been characterized. There are few reports of phototoxicity due to chrysanthemum extract but it is not from contact sensitizing SQL component.47 There is only one suspected case of photocontact dermatitis to parthenium.48 Fuchs et al. described a study of (+)- and (−)-α-methylene-hexahydrobenzofuranone derivatives with a stereochemically pure cis ring junction used as models of sesquiterpenelactones to study their photoreactivity toward thymidine. After 313 nm irradiation of a deoxygenated acetone solution of lactone models and thymidine, six [2 + 2] photoadducts were isolated for each enantiomer and fully characterized by a combination of NMR experiments. A common syn regioselectivity and exo stereoselectivity were observed for photoadducts. This high photoreactivity of α-methylene-γ-butyrolactone ring toward thymidine was proposed as an explanation of the progressive evolution of allergic contact dermatitis toward chronic actinic dermatitis (Table 3).49 It may be an example how a photochemical reaction may play a role in CAD induced by parthenium. There are reports of aggravation of parthenium dermatitis after exposure to the sun but patients improve if they move away from parthenium-infested areas even if it is sunny. Reduction in the minimal erythema dose of UVB and minimal phototoxic dose to UVA has been described.50 Recent preliminary study at our institute has shown reduced sensitivity to UVB and UVA in CAD due to parthenium (Table 3), which may be responsible for photo-localisation that occurs during the natural course of parthenium dermatitis.51 Similar reduced sensitivity to UVB, UVA and even visible light has been described in CAD due to Compositae plants.46 Similar reduced sensitivity to UVB, UVA and even visible light in CAD-like presentation in parthenium dermatitis still evades proper scientific explanation but it may be considered to be due to ultraviolet radiation/visible light leading to production of an endogenous antigen (photo-induced antigen) in the skin that presents as contact dermatitis-like delayed-type hypersensitivity.
Course of the disease
The disease runs a chronic and recurrent course with exacerbation during summers, since contact sensitivity to parthenium is persistent which may result in lichenified lesions in the flexural areas. An initial ABCD pattern may subsequently over years develop localization to the exposed parts that resembles CAD or mixed pattern in a subset of cases. Due to tolerance, better precautions and treatment taken by the patient, there may be a reduction in the severity of the disease. In patients with parthenium contact dermatitis, there are no documented studies of development of tolerance except by oral hyposensitization, which can be an alternative treatment modality.52Diagnosis
The simplest way of confirming parthenium contact allergy is by patch testing with plant allergens, which is not recommended with parts of fresh, frozen and dried plants as it carries a risk of high false positive irritant reactions and test sensitization. The 0.1% mix of equimolar concentrations of three different SQLs (alantolactone, costunolide and dehydrocostuslactone) had been used for screening Compositae allergy which was able to detect only 42.2% of those cases that yielded positive results with ethanolic dilutions of ether extracts of the plant, and hence it is not an adequate screen.53 Similar results have been reported by Paulsen et al. in a series of 76 patients.54 The plant allergens can be extracted in various solvents such as acetone, alcohol, ether, acid, alkali and water, and patch testing can identify most patients with parthenium allergy at which acetone extract is significantly better than aqueous extract.55 Prick test can be done with parthenium antigen as in the patch test series or with leaf “as is” or plant materials crushed and diluted with saline by recording both immediate reaction at 15 minutes and late-phase reaction at 24–48 hours.16 RAST (radio allegro sorbent test) for parthenium-specific antibodies is less specific than prick testing.16 Due to frequent overlap in histopathological findings in different stages of parthenium dermatitis, its contribution is limited in diagnosis, but it can help in ruling out other suspected diagnoses. Acute dermatitis reveals epidermal spongiosis with perivascular lymphohistiocytic dermal infiltrate. Prurigo-like lesions may show pronounced hyperkeratosis, irregular acanthosis along with spongiosis, and papillary dermis may show lymphocytic infiltrate with vertically oriented collagen bundles. In early CAD, epidermal spongiosis with superficial and deep dermal perivascular lymphohistiocytic infiltrate is seen, but in older lesions often marked acanthosis of epidermis with focal spongiosis and lymphocytic infiltrate is seen. In severe cases prominent infiltrate is seen with marked focal epidermal lymphocyte exocytosis and a degree of irregularity of nuclear outline simulating cutaneous T cell lymphoma. Photopatch test is rarely positive in parthenium dermatitis confined to the exposed parts. A recent study at our institute has shown photopatch positivity in 5 out of 34 CAD due to parthenium. All the patients who were positive had photopatch positive to only a single allergen, which were parthenium (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, 1
100, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 acetone extract), Compositae mix 5%, perfume mix 6%, balsam of peru 6% and promethazine hydrochloride 1%.51 Phototesting is an essential tool in the investigation of photodermatoses which can be done with specific irradiation monochromators, solar simulators, and broadband or narrowband UV chambers used to demonstrate reduced minimum erythema/phototoxic dose in photodermatoses according to the skin type, including chronic actinic dermatitis. It not only determines the presence and severity of any abnormal photosensitivity but also allows definition of the responsible UV and visible wavelengths.56 Phototesting with UVB, UVA and visible light may demonstrate reduced sensitivity to UV radiation and even visible light in CAD-like presentation of parthenium dermatitis. In a study of 10 cases having parthenium dermatitis, MED to UVB was lowered in 9 cases but not detected in 1, whilst MED to UVA was seen in only 1 case. Reduction of MED to UVB is a definite indicator of photosensitivity in parthenium dermatitis.50
200 acetone extract), Compositae mix 5%, perfume mix 6%, balsam of peru 6% and promethazine hydrochloride 1%.51 Phototesting is an essential tool in the investigation of photodermatoses which can be done with specific irradiation monochromators, solar simulators, and broadband or narrowband UV chambers used to demonstrate reduced minimum erythema/phototoxic dose in photodermatoses according to the skin type, including chronic actinic dermatitis. It not only determines the presence and severity of any abnormal photosensitivity but also allows definition of the responsible UV and visible wavelengths.56 Phototesting with UVB, UVA and visible light may demonstrate reduced sensitivity to UV radiation and even visible light in CAD-like presentation of parthenium dermatitis. In a study of 10 cases having parthenium dermatitis, MED to UVB was lowered in 9 cases but not detected in 1, whilst MED to UVA was seen in only 1 case. Reduction of MED to UVB is a definite indicator of photosensitivity in parthenium dermatitis.50
Management
General measures
The degree of contact hypersensitivity and the quantity of antigen to which the patient is exposed dictates the severity of dermatitis. A change of residence or job is not a suitable option since Parthenium hysterophorus is ubiquitous and it would also lead to adverse social and economic consequences. Removing as much of the causative plant as possible from the immediate environment of the patient and advising the patient to cover as much of the skin as possible by clothing, drying of clothes indoors and frequently washing the uncovered areas reduce the quantity of the antigen to which the patient is exposed. Using a barrier cream more frequently to slow down the penetration of the antigen into the skin and combining it with washing each time before reapplication has also been recommended.16 Avoiding exposure to sunlight and use of sunscreens may be helpful, but no documented studies are available in parthenium dermatitis. If patients have photosensitivity, sunscreens can be used. Gloves may not offer protection since the sesquiterpene lactone permeates vinyl, polyethylene and latex gloves.57Treatment
The mainstay of treatment of the parthenium dermatitis is systemic corticosteroids (starting doses of 0.5–1 mg kg−1 day−1 of prednisolone or 2–3 mg day−1 of betamethasone are adequate) especially in acute/severe/extensive disease, and topical steroids can be used for mild to moderate disease. Complete remission of disease activity usually occurs within 3 months. Effort should be made to taper the steroids accordingly and add adjuvants if required, since use of high doses for long periods may lead to adrenocortical axis suppression, diabetes mellitus, hypertension, infections, etc. Calcineurin inhibitors chiefly target activated T lymphocytes and have been successfully used in various inflammatory dermatoses which can be logically used in the treatment of chronic actinic dermatitis. Tacrolimus ointment has been used in management of chronic actinic dermatitis and found to be effective and safe.58Azathioprine has immunosuppressive, anti-inflammatory and steroid-sparing properties and acts mainly by T cell inhibition, and is effective in the treatment of parthenium dermatitis at a dose of 1–2 mg kg−1 day−1. Daily doses of 50–150 mg with and without 300 mg monthly bolus dosing regimens has been tried, with the monthly boluses not providing an additional benefit. Weekly 300 mg azathioprine pulse therapy was found to be as effective as daily therapy with better compliance and reduced cost of therapy.59 For adequate control of parthenium dermatitis, the authors recommend use of daily azathioprine dose which may be continued for 6–12 months after subsidence of disease.60 Azathioprine takes 4–6 weeks to exert its action, hence it is preferred for the treatment of the chronic stage and it should be supplemented with corticosteroids in the beginning during the management of the acute stage. Gastrointestinal intolerance, hepatic dysfunction and bone marrow suppression were the major side effects that generally occur at the beginning of the therapy and people who tolerate it well initially are less likely to have side effects later. Thiopurine methyl transferase (TPMT) levels are recommended before starting azathioprine because administration of azathioprine to a patient with TPMT deficiency results in significant accumulation of thioguanine nucleotides which clinically manifests by increased hematopoietic toxicity. 1 in 300 individuals are homozygous for very low TPMT activity, roughly 10% are heterozygous with intermediate activity, and approximately 90% are homozygous, demonstrating the high methylator phenotype.61 A blinded study by Verma et al. found azathioprine 100 mg daily is almost equally effective to betamethasone 2 mg daily for 6 months (P = 0.0156 vs. 0.0005) in the treatment of parthenium dermatitis, but adverse effects and relapses were more frequent in patients treated with betamethasone.62
In the acute phase of parthenium dermatitis, cyclosporine (immunosuppressive with potent anti-inflammatory actions, 4–5 mg kg−1 day−1) has been reported to be effective in the acute phase of parthenium dermatitis which produces a quicker response and also overcomes the side effects of systemic corticosteroid usage.63 Methotrexate has also been reported to be effective at a dose of 15 mg week−1 in parthenium dermatitis.64 Mycophenolate mofetil (25–50 mg kg−1 day−1), low-dose PUVA have been tried in CAD.65 Narrowband ultraviolet B (NB-UVB) radiation having peak emission of 310–312 nm has been used successfully in patients with ABCD, and the ability to systemically depress the major components of the cell-mediated immune response is likely to be linked to its beneficial effects in several inflammatory skin conditions including eczema.66 Others like topical pimecrolimus, oral pentoxiphylline, chloroquine have been mentioned as treatment modalities but require further evidence.66
Oral hyposensitization (an antigen is introduced into the body by a route different from the natural one, to induce such a change in the immune system so that the body does not develop clinical manifestations when the antigen is introduced into the body through the normal route) was demonstrated to be effective in the 1950s for ragweed dermatitis, but has not been widely accepted since it carries considerable risk of provoking and worsening eczema. Oral hyposensitization with parthenium leaf were not consistently effective and continued therapy may be necessary. Handa et al. evaluated the effect of oral hyposensitization in 24 patients of parthenium dermatitis and found there was a gradual improvement in their clinical status in 70% of those patients who completed the study and 30% had an exacerbation during the course of the study.52 Based on frequent co-sensitization patterns, some of the hybrid proteins have been evolved with the polymerase chain reaction containing all the epitopes from the different allergens in a single protein, and these have been used for vaccination against pollen allergy. Immunotherapy with a recombinant protein is administered in cases where patients are co-sensitised with several unrelated pollen allergens. It has been reported useful in hay fever and allergic rhinitis and is under trial for use in ABCD.67 Azathioprine or other adjuvants like methotrexate or cyclosporine can be used in maintenance doses to prevent relapses, combined with covering the exposed parts, removal of the agent from the environment or removal of the patient from the contaminated environment and desensitization methods. Sunscreens are commonly used in the treatment of CAD but have a limited documented role in the treatment of CAD.68
Conclusions
Parthenium dermatitis is a persistent disabling disease and later it may be localised to photoexposed areas. However, SQLs including parthenin have neither documented photoallergic nor phototoxic properties. The reduced sensitivity to UVB, UVA and even visible light in CAD-like presentation in parthenium dermatitis still evades proper scientific explanation but it may be considered to be due to ultraviolet radiation/visible light leading to production of an endogenous antigen (photo-induced antigen) in the skin that presents as contact dermatitis-like delayed-type hypersensitivity.References
- E. Paulsen, Compositae dermatitis: a survey, Contact Dermatitis, 1992, 26, 76–76 CrossRef CAS.
- V. K. Sharma, S. Gomathy and R. Bhat, Evolution of clinical pattern of parthenium dermatitis: a study of 74 cases, Contact Dermatitis, 2005, 53, 84–88 CrossRef.
- M. A. Siddiqui, R. Singh and R. C. Sharma, Contact dermatitis due to Parthenium hysterophorus, Ind. J. Med. Res., 1978, 68, 481–484 CAS.
- V. K. Sharma and G. Sethuraman, Parthenium dermatitis, Dermatitis, 2007, 18, 183–190 CrossRef.
- J. D. Guin, Sesquiterpene-lactone dermatitis, Immunol. Allergy Clin. North Am., 1989, 9, 447–461 Search PubMed.
- V. D. Tiwari, A. S. Sohi and T. R. Chopra, Allergic contact dermatitis due to pathenium hysterophorus, Ind. J. Dermatol., Venereol Leprol., 1979, 45, 392–400 Search PubMed.
- J. C. Mitchell and C. D. Calnan, Scourge of India. Parthenium dermatitis, Int. J. Dermatol., 1978, 17, 303–304 CrossRef CAS.
- V. K. Sharma, Patch testing with the European standard series and compositae extracts in patients with airborne contact dermatitis, Contact Dermatitis, 2001, 44, 49–50 CrossRef CAS.
- R. L. Rietschel and J. F. Fowler Jr., Fisher's Contact Dermatitis, BC Decker Inc., Hamilton, Ontario, 6th edn, 2008, pp. 421–424 Search PubMed.
- R. Nath, Note in the effect of parthenium extract on germination and seedling growth of crops, Ind. J. Agr. Sci., 1988, 51, 601–603 Search PubMed.
- C. Lakshmi and C. R. Srinivas, Parthenium: a wide angle view, Indian J. Dermatol., Venereol. Leprol., 2007, 73, 296–306 CrossRef.
- A. J. Mcconnachie, W. S. Ltrathie, W. Mersie and L. Gebrehiwot, et al. Current and potential geographical distribution of the invasive plant Parthenium hysterophorus (asteraceae) in eastern and southern Africa, Weed Res., 2011, 51, 71–84 CrossRef.
- R. Khatri, K. Mukhopadhyay, K. K. Verma, G. Sethuraman and A. Sharma, Genetic predisposition to parthenium dermatitis in an Indian cohort due to lower-producing genotypes of interleukin-10 (−) 1082 G>A and (−) 819 C>T loci but no association with interferon-γ (+) 874 A>T locus, Br. J. Dermatol., 2011, 165, 115–122 CrossRef CAS.
- N. Akhtar, K. K. Verma and A. Sharma, Study of pro- and anti-inflammatory cytokine profile in the patients with parthenium dermatitis, Contact Dermatitis, 2010, 63, 203–208 CrossRef CAS.
- S. Kumar, S. Khandpur, D. N. Rao, S. Wahaab and N. Khanna, Immunological response to Parthenium hysterophorus in Indian patients with Parthenium sensitive atopic dermatitis, Immunol. Invest., 2012, 41, 75–86 CrossRef CAS.
- C. Lakshmi and C. R. Srinivas, Type I hypersensitivity to Parthenium hysterophorus in patients with parthenium dermatitis, Indian J. Dermatol., Venereol. Leprol., 2007, 73, 103–105 CrossRef.
- V. K. Mahajan, N. L. Sharma and R. C. Sharma, Parthenium dermatitis: is it a systemic contact dermatitis or an airborne contact dermatitis?, Contact Dermatitis, 2004, 51, 231–234 CrossRef.
- N. Hjorth, J. Roed-Petersen and K. Thomsen, Airborne contact dermatitis from Compositae oleoresins simulating photodermatitis, Br. J. Dermatol., 1976, 95, 613–620 CrossRef CAS.
- R. E. McFadyen, Parthenium weed and human health in Queensland, Aust. Fam. Physician, 1995, 24, 1455–1459 CAS.
- A. Lonkar, B. A. Nagasampagi, C. R. Narayanan, A. B. Landge and D. D. Sawaikar, An antigen from Parthenium hysterophorus Linn, Contact Dermatitis, 1976, 2, 151–154 CrossRef CAS.
- E. M. Warshaw and K. A. Zug, Sesquiterpene lactone allergy, Am. J. Contact Dermatitis, 1996, 7, 1–23 CrossRef CAS.
- A. A. Fisher, Esoteric contact dermatitis. Part IV: devastating contact dermatitis in India produced by American parthenium weed (the scourge of India), Cutis, 1996, 57, 297–298 CAS.
- E. Paulsen, L. P. Christensen and K. E. Andersen, Compositae dermatitis from airborne parthenolide, Br. J. Dermatol., 2007, 156, 510–515 CrossRef CAS.
- G. H. Towers, J. C. Mitchell and E. Rodriguez, Biology and chemistry of Parthenium hysterophorus L. A problem weed in India, J. Sci. Ind. Res., 1997, 36, 672–684 Search PubMed.
- P. Oudhia, International Parthenium Research News Group. Available from: http://www.pankajoudhia.com/iprng/IPRNG_Parthenin.html. Accessed on August 2012..
- S. C. Sharma and S. Kaur, Contact dermatitis from compositae plants, Ind. J. Dermatol., Venereol. Leprol., 1990, 56, 27–30 Search PubMed.
- A. Javaid and T. Anjum, Control of parthenium hysterophorus L., by aqueous extracts of allelopathic grasses, Pak. J. Bot., 2006, 38, 139–145 Search PubMed.
- S. Patel, Harmful and beneficial aspects of Parthenium hysterophorus: an update, BioTechniques, 2011, 1, 1–9 Search PubMed.
- E. Rodriguez, G. H. N. Towers and J. C. Mitchell, Biological activities of sesquiterpenelactones, Phytochemistry, 1976, 15, 1573–1580 CrossRef CAS.
- P. Sriramarao, S. Nagpal, B. S. Subbarao, O. Prakash and P. V. Rao, Immediate hypersensitivity to Parthenium hysterophorus. II. Clinical studies on the prevalence of Parthenium rhinitis, Clin. Exp. Allergy, 1991, 21, 55–62 CrossRef CAS.
- K. K. Singh and G. Singh, Air borne contact dermatitis in Varanasi, Ind. J. Dermatol., Venereol. Leprol., 1986, 52, 140–142 Search PubMed.
- E. Paulsen, A. Otkjær and K. E. Andersen, Sesquiterpene lactone dermatitis in the young: is atopy a risk factor?, Contact Dermatitis, 2008, 59, 1–6 CrossRef.
- K. K. Agarwal, A. K. Nath, T. J. Jaisankar and M. D'Souza, Parthenium dermatitis presenting as erythroderma, Contact Dermatitis, 2008, 59, 182–183 CrossRef CAS.
- K. K. Verma, C. S. Sirka, M. Ramam and V. K. Sharma, Parthenium dermatitis presenting as photosensitive lichenoid eruption. A new clinical variant, Contact Dermatitis, 2002, 46, 286–289 CrossRef.
- V. K. Sharma and B. Sahoo, Prurigo-nodularis like lesions in parthenium dermatitis, Contact Dermatitis, 2000, 42, 235 CAS.
- G. Sethuraman, A. Bansal, V. K. Sharma and K. K. Verma, Seborrhoeic pattern of parthenium dermatitis, Contact Dermatitis, 2008, 58, 372–374 CrossRef.
- D. De, R. Jindal and A. J. Kanwar, Contact dermatitis to parthenium simulating lichen nitidus, Indian J. Dermatol., Venereol. Leprol., 2010, 76, 286–287 CrossRef.
- K. K. Singh, C. R. Srinivas, C. Balachandran and S. Menon, Parthenium dermatitis sparing vitiliginous skin, Contact Dermatitis, 1987, 16, 174 CrossRef CAS.
- M. Nic, J. Jirat and B. Kosata, Chemical reaction, IUPAC Compendium of Chemical Terminology, Gold Book Version 2.3.2, 2012, p. 262 Search PubMed.
- M. Nic, J. Jirat and B. Kosata, Photochemical reaction, IUPAC Compendium of Chemical Terminology, Gold Book Version 2.3.2, 2012. p. 1108 Search PubMed.
- H. W. Lim, H. Honigsmann and J. L. M. Hawk, Photodermatology. Informa Inc., New York, 2007, pp. 15–27 Search PubMed.
- K. Wolff, L. A. Goldsmith, S. I. Katz, B. A. Gilchrist, A. S. Paller and D. J. Leffell, Fitzpatrick's Dermatology in General Medicine, McGraw Hill Inc., New York, 7th edn, 2008, pp. 803–805 Search PubMed.
- J. M. Fonseca, J. W. Rushing, N. C. Rajapakse, R. L. Thomas and M. B. Riley, Potential implications of medicinal plant production in controlled environments: the case of feverfew (Tanacetum parthenium), HortScience, 2006, 41, 531–535 CAS.
- H. N. Pandey and S. K. Dubey, Achene germination of Parthenium hysterophorus L.: effects of light, temperature, provenance and achene size, Weed Res., 1988, 28, 185–190 CrossRef.
- T. Tamado, W. Schutz and P. Milberg, Germination ecology of the weed Parthenium hysterophorus in eastern Ethiopia, Ann. Appl. Biol., 2002, 140, 263–270 CrossRef.
- C. Lakshmi and C. R. Srinivas, Parthenium the terminator: an update, Ind. Dermatol. Online J., 2012, 3, 89–100 CrossRef.
- W. Frain-Bell, A. Hetherington and B. E. Johnson, Contact allergic sensitivity to chrysanthemum and photosensitivity dermatitis actinic reticuloid syndrome, Br. J. Dermatol., 1979, 101, 491–501 CrossRef CAS.
- J. K. Bhutani and D. S. Rao, Photocontact dermatitis caused by Parthenium hysterophorus, Dermatologica, 1978, 157, 206–209 CrossRef.
- S. Fuchs, V. Berl and J. P. Lepoittevin, Chronic actinic dermatitis to sesquiterpene lactones: (2 + 2) photo reaction toward thymidine of (+) and (−) alpha-methylene-hexahydrobenzofuranone with cis ring junction, Photochem. Photobiol., 2010, 86, 545–552 CrossRef CAS.
- C. R. Srinivas and S. D. Shenoi, Minimal erythema dose to ultra violet light in parthenium dermatitis, Ind. J. Dermatol., Venereol. Leprol., 1994, 60, 149–150 Search PubMed.
- A. Wadhwani, A Study of Clinical Spectrum of Photodermatoses with Focus on Chronic Actinic Dermatitis, MD Dermatology & Venereology thesis submitted to All India Institute of Medical Sciences, New Delhi, November 2010 Search PubMed.
- S. Handa, B. Sahoo and V. K. Sharma, Oral hyposensitization in patients with contact dermatitis from Parthenium hysterophorus, Contact Dermatitis, 2001, 44, 279–282 CrossRef CAS.
- V. K. Sharma and A. Chakrabarthy, Common contact sensitizers in Chandigarh, India. A study of 200 patients with the European standard series, Contact Dermatitis, 1998, 38, 127–131 CrossRef CAS.
- E. Paulsen and K. E. Andersen, Patch testing with constituents of Compositae mixes, Contact Dermatitis, 2012, 66, 241–246 CrossRef.
- V. K. Sharma, G. Sethuraman and T. Tejasvi, Comparison of patch test contact sensitivity to acetone and aqueous extracts of Parthenium hysterophorus in patients with air borne contact dermatitis, Contact Dermatitis, 2004, 50, 230–232 CrossRef.
- H. W. Lim, H. Honigsmann and J. L. M. Hawk, Photodermatology, Informa Inc., New York, 2007, pp. 433–440 Search PubMed.
- M. Goncalo, R. Mascarenhas, R. Vieira and A. Figueiredo, Permeability o gloves to plant allergens, Contact Dermatitis, 2004, 50, 200–201 CrossRef.
- Y. Ma and Z. Lu, Treatment with topical tacrolimus favors chronic actinic dermatitis: a clinical and immunopathological study, J. Dermatol. Treat., 2010, 21, 171–177 CrossRef CAS.
- K. K. Verma, A. Bansal and S. G. Sethuraman, Parthenium dermatitis treated with azathioprine weekly pulse doses, Indian J. Dermatol., Venereol. Leprol., 2006, 72, 24–27 CrossRef.
- V. K. Sharma, A. Chakrabarti and V. Mahajan, Azathioprine in the treatment of parthenium dermatitis, Int. J. Dermatol., 1998, 37, 299–302 CrossRef CAS.
- A. A. Patel, R. A. Swerlick and C. O. A. McCall, et al. Azathioprine in dermatology: the past, the present, and the future, J. Am. Acad. Dermatol., 2006, 55, 369–389 CrossRef.
- K. K. Verma, R. Mahesh, P. Srivastava, M. Ramam and A. K. Mukhopadhyaya, Azathioprine versus betamethasone for the treatment of parthenium dermatitis: a randomized controlled study, Indian J. Dermatol., Venereol. Leprol., 2008, 74, 453–457 CrossRef.
- C. Lakshmi, C. R. Srinivas and A. Jayaraman, Ciclosporin in parthenium dermatitis – a report of 2 cases, Contact Dermatitis, 2008, 59, 245–248 CrossRef.
- V. K. Sharma, R. Bhat, G. Sethuraman and Y. Manchanda, Treatment of parthenium dermatitis with methotrexate, Contact Dermatitis, 2007, 57, 118–119 CrossRef.
- H. C. Nousari, G. J. Anhalt and W. L. Morison, Mycophenolate in psoralen-UV-A desensitization therapy for chronic actinic dermatitis, Arch. Dermatol., 1999, 135, 1128–1129 CAS.
- S. Dogra, D. Parsad and S. Handa, Narrow band ultraviolet B in air borne contact dermatitis: a ray of hope!, Br. J. Dermatol., 2004, 150, 367–399 CrossRef.
- O. Cromwell, V. Niederberger, F. Horak and H. Fiebig, Clinical experience with recombinant molecules for allergy vaccination, Curr. Top. Microbiol. Immunol., 2011, 352, 27–42 CrossRef.
- P. S. Craig and B. L. Diffey, A prospective longitudinal study of the outdoor behaviour and symptoms of photosensitive patients, Br. J. Dermatol., 1997, 137, 391–394 CrossRef CAS.
Footnote |
| † This article is published as part of a themed issue on current topics in photodermatology. |
| This journal is © The Royal Society of Chemistry and Owner Societies 2013 |
