Photodermatoses in pigmented skin†
Vinod Kumar
Sharma
*,
Kanika
Sahni
and
Ashok Roopchand
Wadhwani
Department of Dermatology and Venereology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi-110029, India. E-mail: aiimsvks@yahoo.com; Fax: +91-11-26588663; Tel: +91-11-26593217
First published on 9th October 2012
Abstract
Photodermatoses are a group of skin diseases primarily caused by, or exacerbated by exposure to ultraviolet and or visible radiation. The effect of sunlight on skin depends on a number of factors including skin colour, skin phototype and the content and type of melanin in the skin. There are only a few studies describing photodermatoses in populations with dark skin. A PubMed search was conducted to summarize currently available information on differences in biology of melanin in dark and light skin and photodermatoses in dark skin. Dark skin is characterised by higher content of melanin, higher eumelanin to pheomelanin ratio, lower tyrosinase activity, and more effective distribution of melanin for protection against ultraviolet light. Photodermatoses are common in dark skinned patients with some variation in the spectrum of photodermatoses. Polymorphous light eruption (PMLE) is the commonest, followed by chronic actinic dermatitis. Pin-point papular and lichenoid variants of PMLE and actinic lichen planus are more frequent in dark skin whereas actinic prurigo, solar urticaria and hydroa vacciniforme are uncommon. Photodermatoses are common in dark skinned patients despite better natural photoprotection. It is proposed that lichenoid photodermatoses may be added to the classification of photodermatoses in dark skin.
 Vinod Kumar Sharma | Dr Vinod Kumar Sharma MD, FAMS has worked as Professor and Chairman, Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi, since 2001. He has special interest in alopecia, psoriasis and photodermatoses. He served as President of the Indian Association of Dermatologists and Venereologists & Leprologists in 2009, and President of the International Congress of Dermatology scheduled to be held in December 4–7, 2013, at New Delhi, India. |
Introduction
Sunlight is considered to be the source of life and is essential for a general sense of well-being in humans. It also has physiological roles to play in the human body including but not limited to the synthesis of the active form of vitamin D3.1,2 However, it also contributes to the causation and/or exacerbation of many cutaneous disorders. Furthermore, paradoxically, as exemplified by polymorphous light eruption, sunlight can serve as the inciting factor and also a means for relief (i.e., natural hardening with repeated sunlight exposure).3Photodermatoses are a group of cutaneous disorders caused or exacerbated by exposure to ultraviolet and or visible radiation. “Photosensitivity” refers to abnormal cutaneous response to “ordinary” light exposure.4,5 The interaction of sunlight with the skin depends on various factors, one of which is the constitutive skin colour and the skin phototype. This review focuses on the biology of melanin and photodermatoses in dark skin.
Methods
A PubMed search was conducted. The following search terms were used: melanin, photodermatoses, photosensitivity, photosensitive disorders, dark skin, darker skinned, black in various permutations and combinations to search for case series and case reports of description of photodermatoses in both lighter and darker skinned populations with an emphasis on dark skin.Skin colour and skin phototypes6
Skin colour is primarily determined by the amount, the type, and the distribution of melanin. Hemoglobin (oxy and deoxy) and carotenoids also contribute to the skin colour. Variation in the skin pigmentation is not due to the differences in the number of melanocytes in the skin, but due to differences in the melanogenic activity, the type of melanin produced in melanosomes, and the size, number and packaging of melanosomes. The number of melanocytes in the skin is race-independent, but can vary at different body sites with densities between 2000 mm2 in head or forearm skin to 1000 mm2 elsewhere. While total melanin content of the epidermis differs only by ∼2-fold between Asian and white skin, the content of melanin in black skin is ∼3 to 6-fold higher and it contains more eumelanin with larger and more melanosomes than light skin.7,8Skin colour has been described to be of two types: constitutive skin colour which describes the genetically determined level of melanin in the skin that is not influenced by exogenous or endogenous factors and the facultative pigmentation which designates an induced level of increased epidermal melanin content as a result of environmental factors such as solar radiation or hormones. The facultative pigmentation develops in 3 steps. The first, termed immediate pigment darkening (IPD), is a transient phenomenon occurring within minutes of UV exposure and fading to a brown colour over minutes to days. It results from photooxidation of pre-existing melanin and the redistribution of existing melanosomes from a perinuclear to a peripheral dendritic location. This is followed by the second phase of persistent pigment darkening which occurs within hours of UV exposure and persists for 3–5 days. The final stage is the delayed tanning (DT) response, which is the only response which results in the stimulation of melanin synthesis and increases in the number and activity of functional melanocytes, increased synthesis and transfer, as well as altered packaging of melanosomes. The pigmentary response of the skin to UV is determined to a large extent by constitutive pigmentation and is more pronounced in individuals with darker skin colour.9
Constitutive skin pigmentation has been found to vary between different populations of the world. However, in contrast to other genetically determined phenotypic traits, e.g. craniometric traits, which show only 10–15% diversity based on geographic location, for skin pigmentation 88% of the total variation could be accounted for by differences among geographic regions.6 A number of studies have emphasized the remarkable relationship between skin colour and latitude, which has also been linked to the degree of UVR exposure.10–12
Till the 1960s, simple visual assessment of skin colour was used to describe skin types, which was later found to be scientifically and clinically inadequate. It was Fitzpatrick who first proposed the currently widely used concept of skin phototyping, which was based essentially on listening to a patient's own report of skin responses (burning or tanning) after significant sun exposure.13 Fitzpatrick skin types I to III were initially denoted and, later, it was expanded to include skin types IV, V and VI for brown skins.14,15 However, this concept was mainly determined by skin response in susceptible Caucasians, with little data from populations with dark skin. Recent studies in Mongoloid skin indicate that skin response of Asian people does not match Fitzpatrick's skin types.16,17
Role of melanins in photoprotection9
Melanin pigments play several diverse and important roles, including thermoregulation, camouflage and sexual attraction, and, more importantly, photoprotection. Melanin is generally considered to be the perfect protection for the skin against UV-induced photodamage. However, it has been found in a number of studies that melanin can also have toxic properties after exposure to UVR. It has been shown to induce single strand breaks in DNA by producing reactive oxygen species (ROS).18Melanin is not a single compound. Rather, it is a mixture of biopolymers synthesized by melanocytes located in the basal layer of the epidermis, the hair bulb, and the iris. Melanin production takes place inside melanosomes, which are lysosome-like organelles where melanin granules are synthesized using the amino acid tyrosine as the major substrate.19 Melanins are broadly classified into two types based on their chemical composition: the darker melanins or eumelanins, and the lighter melanins or pheomelanins. Pheomelanin differs significantly from eumelanin in its biologic behaviour. The most important of these properties is the ability of pheomelanin to activate oxygen resulting in the formation of the superoxide radical anion.20,21 Using enzymatic extraction, the action spectrum for oxygen consumption by photoexcited pheomelanin shows a clear increase between 338 and 323 nm.22 Additionally, pheomelanin has been found to increase the release of histamine, which contributes to the sun-induced erythema and edema in fair-skinned individuals.23 These properties may be responsible for the high phototoxic potential of pheomelanin, which may contribute to photodermatoses and photo-induced malignancies in lighter skinned individuals. Reactive oxygen species have been proposed to play an important role in photoaging, skin cancers and a number of photodermatoses including polymorphous light eruption.24–26
Various studies have reported that individuals with dark skin have higher total melanin content, and a higher amount of eumelanin than lighter skinned individuals. This has been further supported by studies on cultured human melanocytes which demonstrate that melanocytes derived from dark skin have higher total melanin and eumelanin contents, as well as a higher ratio of eumelanin to pheomelanin, than those derived from light coloured skin.27 Recently, there has been emphasis on the important role of pH in controlling melanogenesis. It has been found that melanosomes in melanocytes from white/light skin are acidic while those from black/dark skin are near neutral.28 Furthermore, skin colour diversity has been linked to mutations in a number of genes including P, MATP and SLC24A5, which result in alteration of the pH of melanosomes.29–31 Data from experimental studies have found that a more acidic pH of melanosomes (as in light skins) leads to a lower activity of tyrosinase and a slower rate of dopaquinone cyclization (but a faster rate of CD-quinone cyclization), the end result of which is the favouring of pheomelanogenesis in acidic melanosomes.32
In addition, in dark skin, larger and heavily-melanized melanosomes, which are resistant to degradation by lysosomal enzymes, are present throughout the epidermis and contribute considerably to photoprotection against UV-induced damage compared to lighter skin.33 Melanosomes in dark skin have much longer axes than do melanosomes in light skin (800 vs. 400 nm). They exist as single entities and it has been hypothesized that they are transferred to the keratinocytes individually (thus absorbing light more efficiently), while melanosomes in light skin tend to form clusters and are packaged and transferred as complexes.34
Epidemiological data strongly support the protective role of melanin in prevention of skin cancer, as there is an inverse correlation between skin pigmentation and the incidence of sun-induced skin cancers, and white skin is approximately 70 times more likely to develop skin cancer than black skin.35 It has been found that melanin acts as a sunscreen with an efficacy of between 1.5–4.0 sun protective factors (SPF), implying that it absorbs 50% to 75% of UVR.9 It has also been demonstrated by Kaidbey et al. that on irradiation of skin with UVA and UVB, five times less UV reaches the upper dermis of black skin compared to white skin, which is possibly due to the increased melanin content and its more efficient distribution.36 De Winter et al. reported that repetitive UV exposure increased skin pigmentation and thickness while decreasing its sensitivity to erythema by 75% and also reducing induced DNA damage.37 This implies that the pigmentation induced by tanning is photoprotective to some extent. Another study has found that DNA damage at day 1 and 7, following irradiation with a single 1 MED dose of UVA/UVB was significantly greater in lighter, more UV-sensitive skin types and significantly lower in darker, more UV-resistant skin. It was also demonstrated that darker skin also has a higher rate of apoptotic cell formation, implying that darker skin is also more efficient at removing damaged cells. Hence darker skinned populations with higher eumelanin content tend to have higher intrinsic photoprotection, which may have an impact on the susceptibility to photodermatoses.9
The molecular and cellular mechanism of photoprotection by melanin is not fully understood. There are two types of photoreactions mediated by melanin. An anaerobic process, induced by visible or ultraviolet radiation with wavelengths >300 nm (or >330 nm in case of pheomelanin), is a reversible reaction occurring even in the absence of any external electron donors or acceptors. The 2nd process is an aerobic reaction which occurs when eumelanin absorbs photons with higher energy (240–300 nm) and results in photoionization and photohomolysis.38
The intrinsically photoprotective property of melanins is possibly related to the ability of melanin pigments to absorb light with an efficiency that increases inversely with the light wavelength. Importantly, due to the very fast photodynamics of eumelanin, there is almost complete conversion of the energy of the absorbed photons into heat, allowing only very few excited melanin molecules to participate in photochemical reactions. The energy of the absorbed photons is rapidly and safely utilized in non-photochemical processes as the eumelanin gets non-radiatively de-excited. These studies clearly show that eumelanin, at least, is a system in which a very efficient thermal relaxation occurs, preventing the occurrence of toxic and damaging photochemical reactions. By these means, melanin is able to quench the excited states of photosensitizing dye molecules and singlet oxygen and scavenge reactive radicals, and this acts as an important mechanism for the protective action of melanin against oxidative damage. However, a number of studies have also emphasized the ability of photoexcited melanin to generate superoxide anion and hydrogen peroxide, which, under appropriate conditions, could induce oxidation of key cellular components such as nucleic acids and proteins. However, it seems that the net pro-oxidant action of melanin is not likely to be expressed under normal conditions, when the antioxidant and photoprotective efficiency of melanin is not compromised.38
Sunlight and its interaction with the human skin
Sunlight that reaches the surface of the earth (terrestrial sunlight) predominantly consists of electromagnetic radiation above a wavelength of 290 nm, which includes ultraviolet, visible and infrared radiation. Wavelengths below 290 nm are generally completely absorbed by the ozone layer and do not reach the surface of the earth. Ultraviolet light is further divided into ultraviolet C (200–290 nm), ultraviolet B [(UVB); 290 to 320 nm] and ultraviolet A (UVA), comprising UVA-1 (340 to 400 nm) and UVA-2 (320 to 340 nm), while visible light consists of wavelengths from 400–700 nm.39Ultraviolet radiation (UVR) incident on the skin has one of four outcomes: it is either reflected, transmitted, scattered or absorbed by specific chromophores. Chromophores in the skin include urocanic acid, DNA, RNA, tryptophan, tyrosine and melanin. The melanin in the skin has a photoprotective role. It acts as a neutral density filter reducing all the wavelengths of light.40,41 It also affords superior photoprotection to the black epidermis, which is due not only to its increased melanin content but also to the packaging and distribution of the melanosomes. It has been found that the level of transmission of UV light through the epidermis of black skin is about one fifth that of the transmission through the epidermis of white skin.42 Brenner and Hearing reviewed the subject and stated that melanin in black skin is twice as effective compared to white skin in inhibiting UVB radiation from penetrating. While black epidermis allows only 7.4% of UVB and 17.5% of UVA to penetrate, 24% UVB and 55% UVA pass through white skin. Further, melanosomes in dark skin are resistant to degradation by lysosomal enzymes, and remain intact throughout the epidermal layers and form supranuclear caps in keratinocytes and melanocytes which contribute considerably to photoprotection against UV-induced damage. In contrast, in lightly pigmented skin, melanosomes are degraded and only persist as “melanin dust” in the suprabasal layers. This reduction of melanosomes in the upper epidermis is considered to be an important factor in carcinogenesis, as it compromises the photoprotection of the skin.9
Thus it may be expected that photodermatoses are less common in dark skin. In fact, they were initially believed to occur predominantly in people with Fitzpatrick skin types I–IV. Genetic predisposition to developing photosensitive skin diseases is also known, which may also be responsible for the variable prevalence of disease among populations.
Recently, it has been reported from Tanzania that skin colour can have an impact on skin microbial flora. The skin flora in 66 albino individuals and 31 individuals with normal skin pigmentation with a mean age of 30.6 (SD ± 14.9) years was studied. The mean of the colony forming units in albinism were found to be significantly higher (1680 CFU per cm2) as compared with 453.5 CFU per cm2 in those with normally pigmented skin (p = 0.023). The skin type and the severity of sun damage was significantly associated with a higher number of colony forming units (p = 0.038).43
A summary of the differences in the pigmentary system of dark skin compared to light skin is provided in Table 1.
| Characteristic | Dark skin | Light skin |
|---|---|---|
| Melanin content | Higher total melanin | Lower total melanin |
Eumelanin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pheomelanin ratio pheomelanin ratio |
Higher | Lower |
| Distribution and size of melanosomes | Discretely located larger melanosomes (800 nm) throughout the epidermis | Smaller melanosomes (400 nm) in clusters mostly in basal and suprabasal location |
| Resistance of melanosomes to degradation by lysosomal enzymes | Higher | Lower |
| Transfer of melanosomes | Melanosomes transferred to keratinocytes individually | Melanosomes transferred to keratinocytes as complexes |
| pH inside melanosomes | Neutral | Acidic |
| Tyrosinase activity | Lower activity of tyrosinase | Higher activity of tyrosinase |
| Dopaquinone cyclization | Higher rate | Lower rate |
| CD-quinone cyclization | Lower rate | Higher rate |
| Apoptotic cell removal | Higher and more efficient | Less efficient |
| Result of UV exposure | More pronounced tanning response | Usually more prominent burning response |
| Penetration of UVL to upper dermis | Significantly lower compared to lighter skin | Significantly higher compared to dark skin |
| DNA damage after UVL exposure | Less | Higher |
Classification of photodermatoses3
Photodermatoses are broadly classified into idiopathic acquired photodermatoses, porphyrias, photosensitivity secondary to exogenous agents, genodermatoses and photoaggravated dermatoses including collagen vascular disorders. Idiopathic acquired photodermatoses include chronic actinic dermatitis (CAD), polymorphous light eruption (PMLE), actinic prurigo, solar urticaria and hydroa vacciniforme. A number of skin diseases may be photoaggravated, including atopic dermatitis, seborrhoeic dermatitis, rosacea, acne vulgaris, etc, while photosensitivity secondary to exogenous agents may be divided into photoallergic and phototoxic reactions.Prevalence data on photodermatoses in general population
In a population-based study done in four different regions of Yunnan province of China, based on a questionnaire on 4899 patients, it was found that the self-reported incidence of PMLE was 0.65% and that of CAD was 0.18%. In addition, the prevalence of PMLE (but not CAD) was significantly higher in the regions with a higher altitude compared to the regions of lower elevations.44 In a Scottish population-based study, the prevalence of various photodermatoses per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 population were as follows: chronic actinic dermatitis (16.5), porphyria cutanea tarda (7.6), solar urticaria (3.9), actinic prurigo (3.3), erythropoeitic protoporphyria (2.3) and hydroa vacciniforme (0.47).45
000 population were as follows: chronic actinic dermatitis (16.5), porphyria cutanea tarda (7.6), solar urticaria (3.9), actinic prurigo (3.3), erythropoeitic protoporphyria (2.3) and hydroa vacciniforme (0.47).45
Not enough population-based data exists in dark skin to be able to clearly delineate any differences in the prevalence of photodermatoses attributed to skin colour or phototype.
Relative frequency of photodermatoses
Numerous studies have reported on the occurrence of various photodermatoses in both light-skinned and dark-skinned populations.In a study of 1505 patients from Ethiopia in 1995–1997 focused on pattern of skin diseases, photodermatoses were recorded in 22.9% patients. PMLE was most common (80%), followed by hyperpigmentation (14.2%), actinic cheilitis (4%), and porphyria cutanea tarda (1.8%).46
A retrospective review of records over a 7-year period identified 280 patients with photodermatoses in Michigan, USA. Of these patients, 135 (48%) were African-American, 110 (39%) were Caucasian, while the remaining were of other races. This proportion was similar to the relative outpatient attendance of these two races and there was no statistically significant difference, indicating a likelihood of equal prevalence of photodermatoses overall in the two races. In fact it was observed that the proportion of patients with PMLE was statistically greater in the African-American group compared with Caucasians (67.4% vs. 41.8%; P < 0.0001).47 In contrast, porphyrias (21.4% vs. 0.7%; P < 0.0001) and solar urticaria (8.2% vs. 2.2%; P = 0.03) were statistically significantly less in African-Americans.47 The higher incidence of porphyrias in the Caucasian population compared to the African-Americans could be due to the higher racial incidence of hemochromatosis and its alleles in the Caucasian population compared to the African-Americans.48
The percentage of outpatient patients with photodermatoses was found to be 12.3% in Michigan, USA,47 over a 7 year retrospective study and 0.4% in Lagos, Nigeria, over 10 years.49 Data from Singapore in 2 studies conducted 9 years apart found that the incidence of idiopathic photodermatoses was from 0.014%–0.059%.50,51 PMLE was uniformly found to be the most common idiopathic photodermatosis which was also found to be more common in ethnic Indians while actinic prurigo was diagnosed only in ethnic Chinese. Actinic prurigo was observed to have a much later age of onset (in 60s) unlike reports from Americas which report its usual occurrence in adolescents and young adults. In addition, three patients of Fitzpatrick skin types III and IV had associated advanced asymptomatic HIV infection, which is a phenomenon reported previously only in American patients of skin types V and VI.52 In this study, solar urticaria was found to have a later age of onset with more frequent sensitivity to visible light. This was similar to the series from Japan, but unlike the series from Europe, where only a minority reacted to visible light on phototesting.
A study of 362 patients of photodermatoses with skin types IV and V recruited from India found PMLE (59.7%) to be commonest, followed by CAD (13.8%), collagen vascular disorders (7.7%) and photoaggravated atopic dermatitis (6.1%). Actinic lichen planus (ALP) and lichen planus pigmentosus (LPP) were seen in 3.8% patients. No cases of actinic prurigo, solar urticaria or hydroa vacciniforme were recorded in this study.53
A comparison of available studies on photodermatoses in dark and light skin types is summarized in Table 2.
| Author/year | City, country | Inclusion criteria | Race/phototype | No. of patients | Idiopathic (%) | PMLE (%) | CAD (%) | AP (%) | SU (%) | HV (%) | Phototoxicity (%) | Drug induced (%) | Photoallergic CD (%) | Porphyria (%) | Photoaggravated dermatoses other than CTD (%) | CTD (%) | Others (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PMLE: polymorphous light eruption; CAD: chronic actinic dermatitis; AP: actinic prurigo; SU: solar urticaria; HV: hydroa vacciniforme; CD: contact dermatitis; CTD: connective tissue disease; NR: not recorded. | |||||||||||||||||
| Kerr et al., 200747 | Detroit, USA | Photosensitive disorders only (not photoaggravated) | African-American | 135 | 80.7 | 67.4 | 11.1 | — | 2.2 | 0 | 13.3 | NR | 0.7 | 0.7 | NR | NR | 4.4 |
| Caucasian | 110 | 56.2 | 41.1 | 7.1 | — | 8 | 0 | 10.7 | NR | 2.6 | 21.4 | NR | NR | 7.27 | |||
| Wong et al., 200550 | Singapore | Phototested | NR | 141 | 49 | 25 | 14 | 4 | 6 | 0 | NR | 13 | 4 | NR | 23 | NR | — |
| Khoo et al., 199651 | Singapore | Phototested | NR | 152 | 27 | 13 | 5 | 4 | 5 | 0 | NR | 11 | 3 | NR | 32 | 1.7 | — |
| Stratigos et al., 200354 | Greece | 310 | 47 | 30.6 | 4.83 | 0.96 | 8.38 | 0.3 | NR | 4.5 | 5.2 | 4.5 | 27 | 3.2 | 8.1 | ||
| Crouch et al., 200355 | Australia | 397 | 53 | 29.72 | 9.57 | 4.53 | 9.82 | 0 | 0.5 | 6.8 | 1.7 | 0 | 29.4 | 2.51 | 19.8 | ||
| Olumide et al., 198749 | Nigeria | 64 | 3.1 | 3.1 | — | — | — | — | — | — | — | — | — | — | 96.9 | ||
| New York | Phototested | NR | 203 | 47 | 26 | 17 | 0 | 4 | 0 | NR | 7 | 8 | NR | NR | NR | NR | |
| Wadhwani, 201253 | Delhi, India | Fitzpatrick IV–V | 362 | 73.5 | 59.7 | 13.8 | 0 | 0 | 0 | 0 | 2.49 | — | 0.2 | 13.81 | 7.7 | 2.2 | |
Polymorphous light eruption
Polymorphous light eruption is uniformly reported to be the commonest idiopathic photodermatosis. The clinical presentation is varied, including papular, vesiculopapular, plaque-like, vesiculobullous, insect bite-like, erythema multiforme-like, prurigo, erythematous–edematous, and urticarial lesions. Sites of predilection include neck, dorsa of forearms and hands. All racial skin types have been documented as being affected in the medical literature, however it most commonly occurs in fair-skinned individuals of Fitzpatrick skin types I–IV.56,57A peculiar pinpoint papular variant has been described commonly in patients with darker skin types IV–VI (Africans, African-Americans and Asians). Lesions are described as multiple pinpoint erythematous to skin-coloured papules over the sun-exposed areas. Possibly the first description of this variant was from India,58 which described the appearance of lichenoid papular lesions on sun-exposed areas, termed summertime actinic lichenoid eruption (SALE). The lesions described were small (1–5 mm), skin-coloured to slightly hypopigmented lichenoid papules closely aggregated in one area with a tendency to becoming confluent. This description is similar to the entity currently termed the pinpoint papular variant of PMLE, though it was termed differently. This was followed by a similar description in 6 Japanese patients that was called micropapular light eruption.59
The term pinpoint papular variant of PMLE was used for the first time by Kontos et al. who reported the same morphology of lesions in 9 African-American patients.60 Interestingly, all the patients in this series were female. Recent data from Singapore has found that around one third (29.6%) of the total PMLE cases were of the above morphology and more males than females presented with this morphology.61 Indian experience is similar with 30.5% patients having pinpoint (micropapular) PMLE (Fig. 1), and, in addition, papular PMLE (large papules) in 37%, eczematous type in 22.2%, lichenoid type in 5.5%, plaque type in 4.1% and vesicular type in 0.5% patients.53
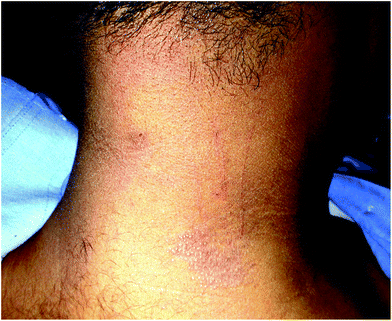 | ||
| Fig. 1 20 year old male (skin type IV) with polymorphous light eruption (PMLE). Grouped pinhead-sized erythematous shiny papules and excoriations over the neck. | ||
Histopathology of pinpoint PMLE lesions has also been studied and Bansal et al. reported two main histologic subtypes of this variant in African-American patients, the histology varying depending on the age of the lesions. Acute lesions (0–3 days) showed focal vesicular formation, spongiosis, RBC extravasation and a perivascular and interstitial lymphocytic infiltrate, while subacute lesions (1–4 weeks) showed parakeratosis, atrophic epidermis overlying a sharply circumscribed lichenoid lymphohistiocytic infiltrate, with epidermal ridges extending in a claw-like fashion and forming the lateral boundaries of the lesion.62
Data from India on the clinicopathologic evaluation of 72 consecutive patients with PMLE presenting with hypopigmented/skin coloured/hyperpigmented/violaceous papules/plaques and lichenified plaques in photoexposed areas showed a histologic pattern of spongiotic and lichenoid features. Hence the term photosensitive spongiotic/lichenoid eruption of micropapules and plaques (PSLEMP) or photosensitive spongiotic/lichenoid eruption (PSLE) was proposed to describe these lesions.63 Recently an Italian study has characterized benign summer light eruption (BSLE) with the help of four criteria, namely predominance of women, shorter than 12-hour latency, lack of involvement of the face and absence of relapse during summer. BSLE was found in 6.1% patients in their series of 346 patients.64
Interestingly, a seasonal exacerbation is noted in Indian patients in the months of March and September and PMLE was found to constitute 0.56% of all patients seen in an outpatient department in Varanasi, India.65
Chronic actinic dermatitis (CAD)
CAD is an immunologically mediated photodermatosis caused by a contact dermatitis-like delayed type hypersensitivity against a photo-induced cutaneous antigen. The spectrum of CAD has variably included chronic CAD-like photosensitive eruption secondary to airborne allergens or drugs. Histologic features include epidermal spongiosis, acanthosis with superficial and deep dermal dense mononuclear cell infiltrate occasionally associated with mitotic figures and large hyperchromatic nuclei.66CAD has been defined by the following criteria: (i) clinically persistent eczematous eruption, with or without infiltrated papules and plaques, predominantly affecting sun-exposed skin; (ii) histopathological changes consistent with chronic eczema with or without cutaneous lymphoma-like changes; and (iii) phototests showing a reduction in the minimal erythema dose (MED) to UVA, UVB and/or visible light.67,68
Lim et al. studied 51 patients in the United States and Japan who were diagnosed CAD on the above criteria and found a mean age of onset of 62.7 years with a mean duration of disease of 5.8 years. Thirty three (65%) of patients had a decrease in MED to both UVA and UVB whereas 14 (27%) patients had reduced MED to UVA only.69
The concept of cutaneous allergy presenting as CAD was suggested by Menage et al. who found positive patch or photopatch tests, most commonly to sesquiterpene lactone mix in 64 of 89 patients with a clinical diagnosis of CAD. This was a heterogeneous population with around 10% patients being dark-skinned.70 In two series of dark-skinned CAD patients from India who were patch tested, parthenium and paraphenylene diamine were found to be the common sensitizers.71,72
In another study on parthenium dermatitis from India, Sharma et al. demonstrated change from the classical airborne contact dermatitis pattern in 38 of the 60 patients to a mixed and CAD pattern over a mean period of 4.2 years (1–15 years). Photopatch testing was done in 19 patients and was found positive to parthenium in 6 patients.73
Recent work from India in type IV and V skin found chronic actinic dermatitis to be the second-most common idiopathic photodermatosis, accounting for 50 out of 266 patients of idiopathic photodermatoses. The mean age at onset of CAD was 44.3 years, which was lower than the usually reported age of onset in Western literature. The most common morphology observed in these patients was lichenified plaques on photoexposed sites (Fig. 2) which extended onto covered sites in 5/50 patients. Interestingly, prurigo-like lesions were a commonly observed morphology occurring in 10/50 (20%) of patients53 (Fig. 3).
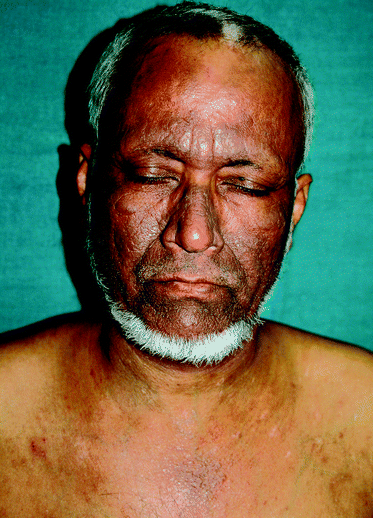 | ||
| Fig. 2 55 year old Indian man (skin type V) with chronic actinic dermatitis (CAD). Hyperpigmented lichenified plaques with prominent skin markings over photoexposed sites on forehead, cheeks, nose and front of chest while sparing the nasolabial folds and upper eyelids. | ||
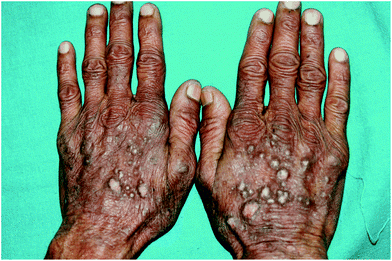 | ||
| Fig. 3 50 year old Indian farmer (skin type V) with chronic actinic dermatitis with prurigo-like lesions on dorsae of hands having patch test and photopatch testing positive to parthenium. | ||
Actinic prurigo
Actinic prurigo is a chronic photodermatosis more commonly seen in Latin American mesitzos (Caucasians and indigenous offspring) with reports of an HLA association (HLA-A24 and HLA-CW4). It has not been reported to be much different in darker skins compared to light-skinned individuals.74 Recently HLA DR 0407 allele has been reported to be associated with a higher risk of actinic prurigo.75 Data from the National Skin Center in Singapore found racial predilection for actinic prurigo which was diagnosed only in ethnic Chinese.50 In comparison to data from the West, they also found actinic prurigo to have a significantly later age of onset (above 60 years) and more persistent course compared to that in Caucasians. It needs to be noted that data from India did not show any patient with actinic prurigo in our study, but 10 out of 50 cases of CAD had prurigo-like lesions over exposed parts. Considering the late age of onset of actinic prurigo and the lack of associated cheilitis in the Singapore study, it needs to be verified whether these patients are indeed actinic prurigo or the above mentioned variant of chronic actinic dermatitis. Adult onset actinic prurigo (mean age: 36.86 years) in type IV and V skin has been described from Thailand.76Solar urticaria
The study from Singapore found a male predisposition, higher mean age of onset and action spectrum with majority of patients reacting positively to visible light.43 This was in contrast to data from Europe and Americas.77 In our study from India we did not record any patient of solar urticaria,46 though in another cohort of patients with urticaria recruited from our center, 3 out of 515 patients were found to have solar urticaria.78 The paucity of reports on solar urticaria in the Indian literature could be due to the rarity of the condition.Hydroa vacciniforme
Hydroa vacciniforme is rare in dark-skinned individuals and no cases were observed in series from Singapore, India and Nigeria except a report of crusts and vacciniforme scars in sun-exposed areas from Pretoria, South Africa.79 It was not observed in the series from India.Actinic lichen planus
Actinic lichen planus (also known as subtropical lichen planus) is a unique and clinically distinct type of lichen planus almost exclusively reported in children or young adults living in tropical countries, especially Middle East, Egypt, Tunisia and India.80,81 It however is not reported from West Africa.82It presents as well-defined annular or discoid hyperpigmented patches with striking peripheral hypopigmentation (Fig. 4) and sun exposure is considered to be central to the pathogenesis of this disease. Actinic lichen planus was observed in 8 out of 362 patients in the series from India. In a case series of patients with lichen planus reported from India, this variant was found to constitute 19.2% of all cases of lichen planus.83
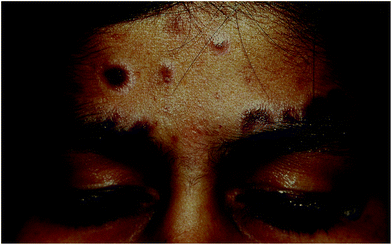 | ||
| Fig. 4 14 year old girl (skin type IV) with actinic lichen planus. Multiple bluish black hyperpigmented plaques, a few showing central atrophy over forehead. Note striking perilesional hypopigmentation. | ||
Phytophotodermatitis
Phytophotodermatitis is a very common skin disease in tropical countries. It is caused by an acute phototoxic reaction when plants, most commonly containing psoralens, come in contact with the skin which is then exposed to UVA light. The presentation is like that of a sunburn which may be severe enough to lead to blistering.84 In dark-skinned individuals the acute reaction may be followed by residual hyperpigmentation which may persist for weeks to months. In fact it may be the only sign of the disease without any previous symptoms of sunburn.Photosensitive nutritional dermatoses
Pellagra is a disorder presenting with eczematous and pigmentary abnormalities in photoexposed distribution occurring due to nutritional deficiency of nicotinic acid, a B-complex vitamin (Fig. 5). It was commonly observed in third world countries with high incidence of malnutrition, particularly where millet or maize was the principal cereal in the diet.85,86 The incidence has fallen remarkably and there was no case of nutritional photosensitive dermatitis in the series from India. Photosensitivity secondary to systemic and occupational exposure to pyridoxine (vitamin B6) has also been reported.87,88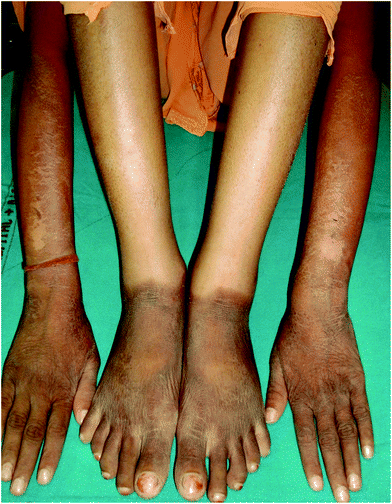 | ||
| Fig. 5 30 year old lady (skin type V) with pellagra. Dry minimally scaly brownish hyperpigmented plaques over photoexposed sites on the forearms and dorsae of hands and feet. Note well defined lateral border on the forearm and sharp cut off at the ankles. | ||
Drug-induced photosensitivity
The phenomenon by which a chemical or a therapeutic drug enhances the photosensitivity of cellular skin components is known as drug-induced photosensitivity. It may be caused by both topically applied and systemically administered drugs and the most commonly implicated systemic drugs include fluoroquinolones, tetracyclines, antifungals, NSAIDs, psoralens and retinoids. Recently hydrochlorothiazide and diltiazem have been reported to produce hyperpigmentation in photodistributed areas.89–91 In our series, 9 patients had drug-induced photosensitivity, from drugs including NSAIDs, doxycycline, antihypertensives and antibiotics.53HIV and photosensitivity92
A number of photosensitivity disorders have been reported in HIV-infected patients such as porphyria cutanea tarda (PCT), photosensitive granuloma annulare (GA), primary facial hyperpigmentation, chronic actinic dermatitis (CAD), lichenoid photoeruption, and erythroderma, of which the last two were thought to be the most common associations.Photodermatoses associated with HIV may present as either lichenoid lesions, chronic actinic dermatitis like presentation, subacute dermatitis or hyperpigmented skin lesions in a photodistributed location. In a study by Gregory et al., lichenoid photoeruptions and photodistributed hyperpigmentation were observed more frequently in African-Americans with advanced HIV infection and CD4 counts of <50 cells per mL.93 Another study conducted to assess risk factors for photosensitivity among HIV-positive individuals found that the overall prevalence of photosensitivity in the recruited HIV-positive patients was 5.4%, whereas in the same cohort HIV-positive African-Americans exhibited a much higher prevalence of 7.3% and that ethnic origin continued to remain a risk factor even after adjusting for CD4 count and HAART (highly active antiretroviral therapy). Photodermatitis was reported in 21 (17.5%) in a series of 137 HIV-positive individuals from Bastar tribal region in India in contrast to isolated case reports in the past.94,95 The exact reason for this predisposition is not known and could be either genetic or behavioural. Other risk factors reported were low CD4 count and patients receiving HAART, particularly saquinavir.92
Phototesting in dark skins
A study in Korean patients by Youn et al. found a weak correlation of MED to UVB with Fitzpatrick skin types in normal individuals. The mean MED for patients with skin types II, III, IV and V were found to be 60.0 ± 14.1, 66.2 ± 14.7, 74.9 ± 16.9 and 74.6 ± 17.7 mJ cm−2, respectively. Significant differences were found between type II versus type IV and V, and also between type III versus type IV and V skin.96 Another study by the same group reported median MED to NBUVB to be 750, 950 and 1075 mJ cm−2 in Korean patients with skin phototypes III, IV and V respectively.97A similar study in Arab patients with skin types III and IV from Bahrain reported a mean MED value of 112.22 ± 32.53 mJ cm−2, which was higher than that quoted in previous studies. This study also found that MED of skin type IV was 25% higher than that for skin type III.98 However, the majority of phototesting guidelines do not mention specifically about phototesting in the types IV–VI skin.99,100
The difficulties faced in phototesting patients with dark skins are highlighted in a study by Mehta et al. from India. They attempted to determine MED to UVA, whole spectrum irradiation (UVA, UVB and visible) and visible light in 100 normal Indian subjects with skin phototypes IV, V and VI. They claimed that were unable to determine MED to UVA in any of the 100 subjects since none developed erythema even after irradiation for 45 minutes (700 J cm−2).101
In patients of chronic actinic dermatitis at our centre, MED to UVA was detectable in only 3 of the 6 patients tested (MED of 2.8, 4 and 5.6 J cm−2).53 A previous study on 50 Indian patients with PMLE, CAD and solar urticaria using solar simulator, found MED to UVA at 30 J cm−2 to be detectable in 29 of the 50 patients compared to 8 of the 25 controls. For UVB, MED was detectable in 19 of 50 patients and none in the control group.102
However, in the study from Singapore which had patients of skin types III–IV, the authors were able to elicit MEDs in most patients who were phototested and found MED to be reduced in all 19 patients of CAD (16 to UVA and UVB, 2 to UVB and 1 to UVA), 17 out of 35 patients of PMLE and all patients of actinic prurigo and solar urticaria.50
The greater resistance of progressively darker skin to the effect of UVR is probably due to the presence of a higher number of, and deeply pigmented, larger melanosomes. Another postulated reason is the differences in epidermal optics and UVR penetration. This creates hurdles in interpretation and classification of individual patients on the photodermatosis spectrum and also their management.
Conclusion
This review highlights the frequent occurrence of photodermatoses in dark-skinned populations with slight variability in the relative incidence of different photodermatoses compared to lighter skinned individuals. Pinpoint papular variant of PMLE occurs in dark skins and histology shows spongiotic/lichenoid reaction. CAD is common in dark-skinned populations, especially in India due to parthenium dermatitis. Actinic prurigo, solar urticaria and hydroa vacciniforme are uncommon in dark-skinned races. We suggest inclusion of lichenoid photodermatoses as a separate entity in the classification of photodermatoses in dark skins because of the relatively frequent incidence of lichenoid PMLE, actinic lichen planus and lichen planus pigmentosus in such populations. Another entity that requires mention is HIV-associated photosensitivity, which has been more commonly reported in darker skins.References
- M. C. LoPiccolo and H. W. Lim, Vitamin D in health and disease, Photodermatol., Photoimmunol. Photomed., 2010, 26, 224–229 Search PubMed.
- J. Moan and A. Juzeniene, Solar radiation and human health, J. Photochem. Photobiol., B, 2010, 101, 109–110 CrossRef.
- J. L. M. Hawk, Photodermatology, Arnold, London, 1999 Search PubMed.
- J. J. Kim and H. W. Lim, Evaluation of the photosensitive patient, Semin. Cutaneous Med. Surg., 1999, 18, 253–256 Search PubMed.
- T. P. Millard and J. L. Hawk, Photosensitivity disorders: cause, effect and management, Am. J. Clin. Dermatol., 2002, 3, 239–246 Search PubMed.
- J. H. Relethford, Apportionment of global human genetic diversity based on craniometrics and skin colour, Am. J. Phys. Anthropol., 2002, 118, 393–398 Search PubMed.
- S. Ito and K. Wakamatsu, Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals: a comparative review, Pigm. Cell Res., 2003, 16, 523–531 Search PubMed.
- G. Szabo, A. B. Gerald, M. A. Pathak and T. B. Fitzpatrick, Racial differences in the fate of melanosomes in human epidermis, Nature, 1969, 222, 1081–1082 Search PubMed.
- M. Brenner and V. J. Hearing, The protective role of melanin against UV damage in human skin, Photochem. Photobiol., 2008, 84, 539–549 CrossRef CAS.
- R. Biasutti, Le razze e i popoli della terra, Unione Tipografico Editrice Torinese, Torino, 1953 Search PubMed.
- J. H. Relethford, Hemispheric difference in human skin color, Am. J. Phys. Anthropol., 1997, 104, 449–457 CrossRef CAS.
- N. G. Jablonski and G. Chaplin, The evolution of human skin coloration, J. Hum. Evol., 2003, 39, 57–106 Search PubMed.
- T. B. Fitzpatrick, Soleil et peau, J. Med. Esthet., 1975, 2, 33–34 Search PubMed.
- S. Sachdeva, Fitzpatrick skin typing: applications in dermatology, Indian J. Dermatol., Venereol. Leprol., 2009, 75, 93–96 Search PubMed.
- T. B. Fitzpatrick, The validity and practicality of sun-reactive skin types I through VI, Arch. Dermatol., 1988, 124, 869–871 CAS.
- J. H. Chung, W. S. Koh and J. I. Youn, Relevance of skin phototyping to a Korean population, Clin. Exp. Dermatol., 1994, 19, 476–478 Search PubMed.
- D. G. Stanford, K. E. Georgouras, E. A. Sullivan and G. E. Greenoak, Skin phototyping in Asian Australians, Australas. J. Dermatol., 1996, 37(s1), S36–S38 Search PubMed.
- E. Wenczl, G. P. Van der Schans, L. Roza, R. M. Kolb, A. J. Timmerman and N. P. Smit, et al., (Pheo)melanin photosensitizes UVA-induced DNA damage in cultured human melanocytes, J. Invest. Dermatol., 1998, 111, 678–682 CrossRef CAS.
- E. J. Parra, Human pigmentation variation: evolution, genetic basis, and implications for public health, Am. J. Phys. Anthropol., 2007, 134(S45), 85–105 Search PubMed.
- M. R. Chedekel, S. K. Smith, P. W. Post and D. L. Vessell, Photodestruction of pheomelanin: role of oxygen, Proc. Natl. Acad. Sci. U. S. A., 1978, 75, 5395–5399 CrossRef CAS.
- T. Sarna, I. A. Menon and R. C. Sealy, Photoinduced oxygen consumption in melanin systems-II. Action spectra and quantum yields for pheomelanins, Photochem. Photobiol., 1984, 39, 805–809 Search PubMed.
- T. Ye, L. Hong, J. Garguilo, A. Pawlak, G. S. Edwards and R. J. Nemanich, et al., Photoionization thresholds of melanins obtained from free electron laser-photoelectron emission microscopy, femtosecond transient absorption spectroscopy and electron paramagnetic resonance measurements of oxygen photoconsumption, Photochem. Photobiol., 2006, 82, 733–737 Search PubMed.
- A. L. Kadekaro, R. J. Kavanagh, K. Wakamatsu, S. Ito, M. A. Pipitone and Z. A. Abdel-Malek, Cutaneous photobiology. The melanocyte vs. the sun: who will win the final round?, Pigm. Cell Res., 2003, 16, 434–447 Search PubMed.
- J. Krutmann, Ultraviolet A radiation-induced biological effects in human skin: relevance for photoaging and photodermatosis, J. Dermatol. Sci., 2000, 23(Suppl 1), S22–S26 CrossRef CAS.
- M. Dalle Carbonare and M. A. Pathak, Skin photosensitizing agents and the role of reactive oxygen species in photoaging, J. Photochem. Photobiol., B, 1992, 14, 105–124.
- G. M. Halliday, S. N. Byrne and D. L. Damian, Ultraviolet A radiation: its role in immunosuppression and carcinogenesis, Semin. Cutaneous Med. Surg., 2011, 30, 214–221 Search PubMed.
- K. Wakamatsu, R. Kavanagh, A. L. Kadekaro, S. Terzieva, R. A. Sturm and S. Leachman, et al., Diversity of pigmentation in cultured human melanocytes is due to differences in the type as well as quantity of melanin, Pigm. Cell Res., 2006, 19, 154–162 Search PubMed.
- D. R. Smith, D. T. Spaulding, H. M. Grenn and B. B. Fuller, The relationship between Na+/H+ exchanger expression and tyrosinase activity in human melanocytes, Exp. Cell Res., 2004, 298, 521–534 CrossRef CAS.
- H. L. Norton, R. A. Kittles, E. Parra, P. McKeigue, X. Mao and K. Cheng, et al., Genetic evidence for the convergent evolution of light skin in Europeans and East Asians, Mol. Biol. Evol., 2007, 24, 710–722 Search PubMed.
- R. L. Lamason, M. A. Mohideen, J. R. Mest, A. C. Wong, H. L. Norton and M. C. Aros, et al., SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans, Science, 2005, 310, 1782–1786 CrossRef CAS.
- R. A. Sturm, A golden age of human pigmentation genetics, Trends Genet., 2006, 22, 464–468 Search PubMed.
- J. Ancans, D. J. Tobin, M. J. Hoogduijn, N. P. Smit, K. Wakamatsu and A. J. Thody, Melanosomal pH controls rate of melanogenesis, eumelanin/phaeomelanin ratio and melanosome maturation in melanocytes and melanoma cells, Exp. Cell Res., 2001, 268, 26–35 CrossRef CAS.
- J. P. Ortonne, Photoprotective properties of skin melanin, Br. J. Dermatol., 2002, 146(s61), 7–10 Search PubMed.
- N. Kollias, R. M. Sayre, L. Zeise and M. R. Chedekel, Photoprotection by melanin, J. Photochem. Photobiol., B, 1991, 9, 135–160 CrossRef CAS.
- R. M. Halder and K. M. Bang, Skin cancer in blacks in the United States, Dermatol. Clin., 1988, 6, 397–405 Search PubMed.
- H. M. Gloster Jr. and K. Neal, Skin cancer in skin of color, J. Am. Acad. Dermatol., 2006, 55, 741–760 Search PubMed.
- S. de Winter, A. A. Vink, L. Roza and S. Pavel, Solar-simulated skin adaptation and its effect on subsequent UV-induced epidermal DNA damage, J. Invest. Dermatol., 2001, 117, 678–682 Search PubMed.
- P. Meredith and P. Sarna, The physical and chemical properties of eumelanin, Pigm. Cell Res., 2006, 19, 572–594 Search PubMed.
- B. L. Diffey, Human exposure to ultraviolet radiation, in Photodermatology, ed. H. W. Lim, H. Hoenigsman and J. L. M. Hawk, Informa Health care, New York, 2007, pp. 17–27 Search PubMed.
- R. R. Anderson and J. A. Parrish, Optical properties of human skin, in The Science of Photomedicine, ed. J. D. Regan and J. A. Parrish, Plenum Press, New York, 1982, pp. 147–194 Search PubMed.
- L. C. Harber, D. R. BickersI. Kochevar, et al., The photochemistry of cutaneous molecules, in Photosensitivity Diseases: Principles of Diagnosis and Treatment, ed. L. C. Harber and D. R. Bickers, BC Decker, Philadelphia, 2nd edn, 1989, pp. 36–45 Search PubMed.
- K. H. Kaidbey, P. P. Agin, R. M. Sayre and A. M. Kligman, Photoprotection by melanin-a comparison of black and Caucasian skin, J. Am. Acad. Dermatol., 1979, 27, 787–788 Search PubMed.
- S. K. Kiprono, J. E. Masenga, B. M. Chaula and B. Naafs, Skin flora: differences between people affected by Albinism and those with normally pigmented skin in Northern Tanzania – cross sectional study, BMC Dermatol., 2012, 12, 12 Search PubMed.
- D. Deng, Y. Hang, H. Chen and H. Li, Prevalence of photodermatosis in four regions at different altitudes in Yunnan province, China, J. Dermatol., 2006, 33, 537–540 Search PubMed.
- R. S. Dawe, Prevalences of chronic photodermatoses in Scotland, Photodermatol., Photoimmunol. Photomed., 2009, 25, 59–60 Search PubMed.
- D. Shibeshi, Pattern of skin diseases at the University teaching hospital, Addis Ababa, Ethiopia, Int. J. Dermatol., 2000, 39, 822–825 Search PubMed.
- H. A. Kerr and H. W. Lim, Photodermatoses in African Americans: a retrospective analysis of 135 patients over a 7-year period, J. Am. Acad. Dermatol., 2007, 57, 638–643 Search PubMed.
- P. C. Adams, D. M. Reboussin, J. C. Barton, C. E. McLaren, J. H. Eckfeldt and G. D. McLaren, et al., Hemochromatosis and iron-overload screening in a racially diverse population, N. Engl. J. Med., 2005, 352, 1769–1778 Search PubMed.
- Y. M. Olumide, Photodermatoses in Lagos, Int. J. Dermatol., 1987, 26, 295–299 Search PubMed.
- S. N. Wong and L. S. Khoo, Analysis of photodermatoses seen in a predominantly Asian population at a photodermatology clinic in Singapore, Photodermatol., Photoimmunol. Photomed., 2005, 21, 40–44 Search PubMed.
- S. W. Khoo, Y. K. Tay and S. N. Tham, Photodermatoses in a Singapore skin referral centre, Clin. Exp. Dermatol., 1996, 21, 263–268 Search PubMed.
- T. Meola, M. Sanchez, H. W. Lim, M. R. Buchness and N. A. Soter, Chronic actinic dermatitis associated with human immunodeficiency virus infection, Br. J. Dermatol., 1997, 137, 431–436 Search PubMed.
- A. R. Wadhwani, A study of the clinical spectrum of photodermatoses with a focus on chronic actinic dermatitis, MD Dermatology thesis, All India Institute of Medical Sciences, New Delhi, December 2010 Search PubMed.
- A. J. Stratigos, C. Antoniou, E. Papathanakou, M. Daboudi, K. Tranaka, K. Tsara and A. D. Katsambas, Spectrum of idiopathic photodermatoses in a Mediterranean country, Int. J. Dermatol., 2003, 42, 449–454 CrossRef.
- R. B. Crouch, P. A. Foley and C. S. Baker, Analysis of patients with suspected photosensitivity referred for investigation to an Australian photodermatology clinic, J. Am. Acad. Dermatol., 2003, 48, 714–720 Search PubMed.
- W. D. Tutrone, C. T. Spann, N. Scheinfeld and V. A. Deleo, Polymorphic light eruption, Dermatol. Ther., 2003, 16, 28–39 Search PubMed.
- J. L. M. Hawk and P. G. Norris, Abnormal responses to ultraviolet radiation: idiopathic, in Fitzpatrick's Dermatology in General Medicine, 5th edn, McGraw-Hill, New York, 1999, pp. 1573–1589 Search PubMed.
- T. R. Bedi, Summertime actinic lichenoid eruption, Dermatologica, 1978, 157, 115–125 Search PubMed.
- T. Horio, K. Danno and F. Furukawa, et al., Micropapular light eruption, Nippon Hifuka Gakkai Zashi, 1986, 96, 519–522 Search PubMed.
- A. P. Kontos, C. A. Cusack, M. Chaffins and H. W. Lim, Polymorphous light eruption in African Americans: pinpoint papular variant, Photodermatol., Photoimmunol. Photomed., 2002, 18, 303–306 Search PubMed.
- L. Y. Chiam and W. S. Chong, Pinpoint papular polymorphous light eruption in Asian skin: a variant in darker-skinned individuals, Photodermatol., Photoimmunol. Photomed., 2009, 25, 71–74 Search PubMed.
- I. Bansal, H. Kerr, J. J. Janiga, H. S. Qureshi, M. Chaffins, H. W. Lim and A. Ormsby, Pinpoint papular variant of polymorphous light eruption: clinical and pathological correlation, J. Eur. Acad. Dermatol. Venereol., 2006, 20, 406–410 Search PubMed.
- N. Shah, A study of clinicopathological features of photosensitive lichenoid eruption, MD Dermatology thesis, All India Institute of Medical Sciences, New Delhi, November 2007 Search PubMed.
- M. Guarrera, P. Cardo, A. E. Rebora, D. Schena, P. Calzavara-Pinton and M. Venturini, et al., Polymorphous light eruption and benign summer light eruption in Italy, Photodermatol., Photoimmunol. Photomed., 2011, 27, 35–39 Search PubMed.
- L. Sharma and A. Basnet, A clinicoepidemiological study of polymorphic light eruption, Indian J. Dermatol., Venereol. Leprol., 2008, 74, 15–17 Search PubMed.
- J. L. Hawk, Chronic actinic dermatitis, Photodermatol., Photoimmunol. Photomed., 2004, 20, 312–314 CrossRef CAS.
- J. L. Hawk and I. A. Magnus, Chronic actinic dermatitis – an idiopathic photosensitivity syndrome including actinic reticuloid and photosensitive eczema [proceedings], Br. J. Dermatol., 1979, 101(Suppl 17), 24 Search PubMed.
- V. K. Somani, Chronic actinic dermatitis—a study of clinical features, Indian J. Dermatol., Venereol. Leprol., 2005, 71, 409–413 Search PubMed.
- H. W. Lim, W. L. Morison, R. Kamide, M. R. Buchness, R. Harris and N. A. Soter, Chronic actinic dermatitis. An analysis of 51 patients evaluated in the United States and Japan, Arch. Dermatol., 1994, 130, 1284–1289 Search PubMed.
- H. Menagé, J. S. Ross, P. G. Norris, J. L. Hawk and I. R. White, Contact and photocontact sensitization in chronic actinic dermatitis: sesquiterpene lactone mix is an important allergen, Br. J. Dermatol., 1995, 132, 543–547 CAS.
- C. R. Srinivas, C. S. Sekar and R. Jayashree, Photodermatoses in India, Indian J. Dermatol., Venereol. Leprol., 2012, 78, 1–8 Search PubMed.
- H. K. Kar, S. Langar, T. C. Arora, P. Sharma, A. Raina and M. Bhardwaj, Occurrence of plant sensitivity among patients of photodermatoses: a control-matched study of 156 cases from New Delhi, Indian J. Dermatol., Venereol. Leprol., 2009, 75, 483–487 Search PubMed.
- V. K. Sharma, G. Sethuraman and R. Bhat, Evolution of clinical pattern of parthenium dermatitis: a study of 74 cases, Contact Dermatitis, 2005, 53, 84–88 Search PubMed.
- M. T. Hojyo-Tomoka, M. E. Vega-Memije, R. Cortes-Franco and L. Domínguez-Soto, Diagnosis and treatment of actinic prurigo, Dermatol. Ther., 2003, 16, 40–44 Search PubMed.
- A. Suárez, M. C. Valbuena, M. Rey and L. de Porras Quintana, Association of HLA subtype DRB10407 in Colombian patients with actinic prurigo, Photodermatol., Photoimmunol. Photomed., 2006, 22, 55–58 CrossRef CAS.
- R. Akaraphanth, J. Sindhavananda and P. Gritiyarangsan, Adult-onset actinic prurigo in Thailand, Photodermatol., Photoimmunol. Photomed., 2007, 23, 234–237 Search PubMed.
- S. Ryckaert and R. Roelandts, Solar urticaria – a report of 25 cases and difficulties in phototesting, Arch. Dermatol., 1998, 134, 71–74 Search PubMed.
- V. K. Sharma and U. Kumar, Urticaria: AIIMS approach and experience, in Handbook of Urticaria, ed. V. K. Sharma, Massey Art Press, New Delhi, 1st edn, 2008, pp. 82–87 Search PubMed.
- W. K. Jacyk and Y. Moosa, Crusts and vacciniform scars on sun-exposed skin, Clin. Exp. Dermatol., 2010, 35, 97–98 Search PubMed.
- I. Katzenellenbogen, Lichen planus actinicus (Lichen planus in subtropical countries), Dermatologica, 1962, 124, 10 Search PubMed.
- A. Dostrovsky and F. Sagher, Lichen planus in subtropical countries, Arch. Dermatol., 1949, 59, 308 Search PubMed.
- G. O. Alabi and J. B. Akinsanya, Lichen planus in tropical Africa, Trop. Geogr. Med., 1981, 33, 143–147 Search PubMed.
- D. Kachhawa, V. Kachhawa, G. Kalla and L. P. Gupta, A clinico-aetiological profile of 375 cases of lichen planus, Indian J. Dermatol. Venereol. Leprol., 1995, 61, 276–279 Search PubMed.
- L. Domínguez-Soto, M. T. Hojyo-Tomoka, E. Vega-Memije, R. Cortés-Franco, L. Waxtein and E. Guevara, Photodermatoses in tropical countries, Clin. Dermatol., 1999, 17, 237–243 Search PubMed.
- K. Karthikeyan and D. M. Thappa, Pellagra and skin, Int. J. Dermatol., 2002, 41, 476–481 CrossRef.
- P. Wan, S. Moat and A. Anstey, Pellagra: a review with emphasis on photosensitivity, Br. J. Dermatol., 2011, 164(6), 1188–1200 Search PubMed.
- A. K. Bajaj, S. Rastogi, A. Misra, K. Misra and S. Bajaj, Occupational and systemic contact dermatitis with photosensitivity due to vitamin B6, Contact Dermatitis, 2001, 44(3), 184 Search PubMed.
- K. Morimoto, A. Kawada, M. Hiruma and A. Ishibashi, Photosensitivity from pyridoxine hydrochloride (vitamin B6), J. Am. Acad. Dermatol., 1996, 35(2), 304–305 Search PubMed.
- Y. Kubo, D. Fukumoto, T. Ishigami, Y. Hida and S. Arase, Diltiazem-associated photodistributed hyperpigmentation: report of two Japanese cases and published work review, J. Dermatol., 2010, 37, 807–811 Search PubMed.
- E. Masuoka, T. Bito, H. Shimizu and C. Nishigori, Dysfunction of melanocytes in photoleukomelanoderma following photosensitivity caused by hydrochlorothiazide, Photodermatol., Photoimmunol. Photomed., 2011, 27, 328–330 Search PubMed.
- N. Desai, A. F. Alexis and V. A. DeLeo, Facial hyperpigmentation caused by diltiazem hydrochloride, Cutis, 2010, 86, 82–84 Search PubMed.
- D. Bilu, A. J. Mamelak, R. H. Nguyen, P. C. Queiroz, J. Kowalski and W. L. Morison, et al., Clinical and epidemiologic characterization of photosensitivity in HIV-positive individuals, Photodermatol., Photoimmunol. Photomed., 2004, 20(4), 175–183 CrossRef.
- N. Gregory and V. A. Deleo, Clinical manifestations of photosensitivity in patients with human immunodeficiency virus infection, Arch. Dermatol., 1994, 130, 630–633 Search PubMed.
- H. Singh, P. Singh, P. Tiwari, V. Dey, N. Dulhani and A. Singh, Dermatological manifestations in HIV-infected patients at a tertiary care hospital in a tribal (Bastar) region of Chhattisgarh, India, Indian J. Dermatol., 2009, 54, 338–341 Search PubMed.
- N. Kumarasamy, S. Solomon, P. Madhivanan, B. Ravikumar, S. P. Thyagarajan and P. Yesudian, Dermatologic manifestations among human immunodeficiency virus patients in south India, Int. J. Dermatol., 2000, 39, 192–195 Search PubMed.
- J. I. Youn, J. K. Oh, B. K. Kim, D. H. Suh, J. H. Chung, S. J. Oh, J. J. Kim and S. H. Kang, Relationship between skin phototype and MED in Korean, brown skin, Photodermatol., Photoimmunol. Photomed., 1997, 13, 208–211 Search PubMed.
- J. I. Youn, J. Y. Park, S. J. Jo, J. H. Rim and Y. B. Choe, Assessment of the usefulness of skin phototype and skin color as the parameter of cutaneous narrow band UVB sensitivity in psoriasis patients, Photodermatol., Photoimmunol. Photomed., 2003, 19(5), 261–264 Search PubMed.
- M. N. Venkataram and A. A. Haitham, Correlating skin phototype and minimum erythema dose in Arab skin, Int. J. Dermatol., 2003, 42, 191–192 Search PubMed.
- P. M. Farr and R. S. Dawe, Phototesting, in Photodermatology, ed. H. W. Lim, H. Hoenigsman and J. L. M. Hawk, Informa Health care, New York, 2007, pp. 433–440 Search PubMed.
- A. Faurschou and H. C. Wulf, European Dermatology Guideline for the photodermatoses, Phototesting, http://www.euroderm.org/images/stories/guidelines/guideline_-phototesting.pdf.
- R. V. Mehta, S. D. Shenoi, C. Balachandran and S. Pai, Minimal erythema response (MED) to solar simulated irradiation in normal Indian skin, Indian J. Dermatol. Venereol Leprol., 2004, 70, 277–279 Search PubMed.
- P. Bejoy, C. R. Srinivas and S. D. Shenoi, Phototesting in the idiopathic photodermatoses among Indians, Indian J. Dermatol., 1998, 43, 1–3 Search PubMed.
Footnote |
| † This article is published as part of a themed issue on current topics in photodermatology. |
| This journal is © The Royal Society of Chemistry and Owner Societies 2013 |
