The relation between methyl aminolevulinate concentration and inflammation after photodynamic therapy in healthy volunteers†
Susanne
Fabricius
a,
Catharina Margrethe
Lerche
*b,
Peter Alshede
Philipsen
b and
Hans Christian
Wulf
b
aDepartment of Dermatology, Odense University Hospital, Denmark
bDepartment of Dermatology, Bispebjerg University Hospital, Denmark. E-mail: cler0001@bbh.regionh.dk; Fax: +45 35316010; Tel: +45 35312778
First published on 26th June 2012
Abstract
Inflammation and pain are well known adverse-effects in photodynamic therapy (PDT). There is currently a tendency towards introducing lower concentrations of the photosensitizer than used in the standard treatment for various indications. The aim of this study was to investigate whether reduced concentrations of methyl aminolevulinate (MAL) can reduce inflammation (erythema) during PDT treatment. We measured the formation of protoporphyrin IX (PpIX) using fluorescence and monitored both erythema and pain during and after PDT treatment with conventional 16% MAL and threee reduced concentrations of 2, 0.75, and 0.25% in twenty-four healthy volunteers. We found that lowering the MAL concentration reduced PpIX fluorescence and erythema after PDT treatment. There was a strong correlation (R2 = 0.70) between the PpIX fluorescence and erythema after treatment. A further increase in erythema after PDT was dependent on pre-treatment skin erythema. PpIX fluorescence could explain 70% of the increase in erythema (P < 0.0005). Pain and post-treatment hyperpigmentation can be reduced but not eliminated by limiting the MAL concentration. An efficacy study of PDT with these three reduced concentrations has not been performed.
Introduction
Photodynamic therapy (PDT) is an established treatment for actinic keratoses (AK), basal cell carcinoma (BCC) and Bowen's disease. It is characterized by good clinical effectiveness and a very good cosmesis.1 PDT with a reduced concentration of photosensitizer has also been used recently for AK,2 acne3 and cosmetic rejuvenation.4,5The effect of PDT is based on pre-cancerous and cancerous cells’ ability to convert aminolaevulinic acids into the photosensitizer protoporphyrin IX (PpIX) by the heme cycle. There is a limited amount of the enzyme ferro-chelatase, and PpIX is primarily accumulated inside the mitochondria, where excess amounts are produced.6 When the skin is subsequently exposed to light of appropriate wavelengths, the activation of PpIX will generate reactive oxygen species (ROS) leading to destruction of cellular components and finally the death of pre-cancerous and cancerous cells.7 The treatment is not without side effects, most importantly pain during illumination, and inflammation (erythema) in the days after treatment.8,9 When excess amounts of PpIX are formed, it can be found in all parts of a cell and in the extracellular environment.10 When heavy damage is inflicted on the cells, they will die by necrosis, which causes inflammation, rather than by apoptosis.7
Few studies have described inflammation following PDT treatment using reduced concentrations of a photosensitizer.2 Wiegell et al. showed no difference in inflammation expressed as erythema between methyl aminolevulinate (MAL) 8% and the standard photosensitizer MAL 16%.2 Ideally, only a selective destruction of cell components should follow PDT, leading to apoptosis of the cells and thereby only a minimum of inflammation without compromising the effect.11 Also, few studies have dealt with pain management and reduced concentrations of the photosensitizer.2,12–15 The study by Wiegell et al. showed no difference in pain score between MAL 8% and 16%. Furthermore, studies with daylight PDT have shown treatment efficacy to be unrelated to pain.16
The conventional PDT regime includes superficial curettage, application of methyl aminolevulinate 16% (MAL) and occlusion for 3 h.1 PpIX is then activated by red light resulting in damage to and death of the diseased cells.1 More knowledge about the association between concentration and inflammation is needed to develop an even better combination of the different variable factors to diminish pain and erythema.
The aim of the present study is to investigate whether a reduction in the concentration of MAL (0.25, 0.75, and 2%) would reduce erythema compared to conventional PDT treatment. Furthermore, we would like to measure whether a reduction in MAL concentration results in pain reduction. We chose these low concentrations of MAL since we already know that MAL 8% and 16% result in the same amount of PpIX fluorescence.2
Methods
Participants
Twenty-four healthy male volunteers above 18 years of age (mean 30, range 20–51) of Scandinavian ancestry were included in the study. Exclusion criteria: allergy to MAL, exposure to sunlight or use of sun bed within the 30 days prior to inclusion.All volunteers received both oral and written information about the purpose, nature and possible risks and benefits of the trial. Written consent was obtained from all volunteers before enrolment in the study. The trial was approved by the local Ethics Committee (H-D-2009-007). The trial was performed in accordance with the Declaration of Helsinki.
Design
The study was designed as an open randomized single blinded trial. A treatment area on the back of each volunteer was identified and divided by a template into 4 treatment fields each with an area of 2 × 5 cm with at least 3 cm between the fields. The borders of the fields were marked with a black non-fluorescent marker (Stabilo, OHPen universal, permanent). All fields were tape-stripped 10 times with an occlusive dressing (Tegaderm™ roll, 3M, Glostrup, Denmark) in order to imitate skin lesions. MAL was applied in four different concentrations: the commercially available cream with 16% MAL and three others of 2, 0.75, and 0.25% MAL respectively. After application, all treatment fields were covered with a light-impermeable, occlusive dressing for 3 h to avoid photobleaching. Any remaining cream was then gently removed before illumination was performed with a narrow band 630 nm red light LED (Aktilite™ 128; Photocure ASA, Oslo, Norway) using a total light dose of 37 J cm−2. Four follow-up visits were performed on days 1, 2, 3, and 8 after treatment.MAL preparations
The 2, 0.75, and 0.25% MAL dilutions were prepared from MAL 16% cream (200 mg g−1 MAL-HCl) (Metvix®, Photocure, Oslo, Norway) by adding Unguentum M (Crooks Healthcare, UK). All creams were stored in a refrigerator and used within 5 days.Erythema and pigmentation
As an indicator of inflammation we measured erythema. The erythema was assessed by an expert evaluator and measured objectively. The expert evaluator used a scale from 0 to 3, where: 0 = no erythema; 1 = mild erythema (just visible, light pink skin); 2 = moderate erythema (clear red skin colour); and 3 = severe erythema (dark red to bluish skin colour).The objective measurements of erythema and pigmentation were performed using a skin reflectance meter (Optimize Scientific 558, Chromo-Light, Espergaerde, Denmark). This instrument measures skin remittance at 558 and 660 nm and calculates the content of melanin and haemoglobin in the skin independently of each other.17,18 These measurements are given in erythema% and pigmentation% and are thus referred to as such in the present article. To make the best estimate, erythema% and pigmentation% were measured three times in each treatment field and the median value was used in the statistical calculations. Erythema% and pigmentation% were measured before treatment, immediately before illumination, immediately after illumination and at the four follow-up visits. Clinical scores for erythema were performed before treatment and at the four follow-up visits.
A small pilot study of how tape-stripping and vehicle affected the measured erythema% in healthy skin on the forearm showed that this procedure did not increase the erythema% significantly.
Pain score
The volunteers scored their pain each minute during the 9 min of illumination, and recorded their pain in a diary every hour after illumination on the treatment day, twice a day the next 3 days and once a day on the following 5 days. Since PDT was performed at different times of day, the number of assessments varied from three to eleven on the first day. Pain was assessed using a numerical scale ranging from 0 to 10, where 0 is no pain and 10 is the most intense pain. To make it easier for the patients to identify the different treated fields, the diary was supplied with numbered drawings of the fields. Pain was recorded for each field separately.PpIX fluorescence
MAL-induced PpIX fluorescence was measured non-invasively using a fluorescence camera (Medeikonos AB, Gothenburg, Sweden). The amount of PpIX fluorescence was calculated from the photographs by the program MatLab® (MatLab®, MathWorks, Natic, US). Fluorescence was measured before tape-stripping and cream application (background), immediately before and after illumination. PpIX fluorescence and photobleaching was measured and calculated as described by Wiegell et al.16Randomizing
A statistical adviser randomized the volunteers. Since the sequence of both MAL concentration and treatment fields was predefined, randomization only determined which of the four treatment fields should be the first.Statistical analysis
Earlier studies found an increase in redness of 17.6% and a SD of 10.3%.2 If we assess the minimal clinical relevant difference to be 8.8% (50% of 17.6) and choose an intensity of 0.80 and a significance level of 0.05, we would have to include twenty-two healthy volunteers.19Data analysis was performed using SPSS statistics 19.0 (SPSS inc, Chicago, US). To identify differences in pain score, erythema%, erythema clinical scores, and pigmentation% between the treatment fields we used the Wilcoxon signed rank test, since all results were paired. To assess correlations we used Spearman's rank correlation. To determine which parameters were of importance for the development of erythema and inflammation, multiple regression analysis with backward elimination was performed. For all calculations, a P value <0.05 was considered statistically significant.
Results
Erythema
No significant difference was found between the erythema% of the fields before MAL application, but there was a MAL concentration dose-dependent increase in erythema% immediately before illumination. Fig. 1 shows the mean increase in erythema% over time for all four MAL concentrations. Comparisons of the post-treatment erythema% with the pre-treatment erythema% and comparisons of the post-treatment erythema clinical scores with the pre-treatment clinical scores are presented in Table 1. Comparisons of the increase in erythema%, fluorescence, pigmentation% and pain between the different concentrations are given in Table 2. Erythema% was significantly increased 3 days after treatment with 16% and 2% MAL whereas 0.75% MAL only increased erythema% significantly just after treatment, and no significant increase in erythema% was found at any time after 0.25% MAL. The correlation between MAL concentration and erythema% was significant at all points (P = 0.005–0.0005). Backward linear regression analysis with the increase in erythema% one day after treatment, photobleaching, and MAL concentration showed that photobleaching was the only important parameter with an R2 = 0.70. This means that the photobleaching can explain approximately 70% of the increase in erythema%. The same results were found for other control days, but with a decreasing R2 value for every passing control day. There was a clear correlation between increase in erythema% and expert-assessed erythema (P < 0.0005). The lowest measurement of erythema%, which was also judged to be erythema by the expert, was 21.5%. This measurement is lower than some of the pre-treatment measurements of other volunteers as the mean pre-treatment erythema% of all fields was 28.7% (15.4–41.9%).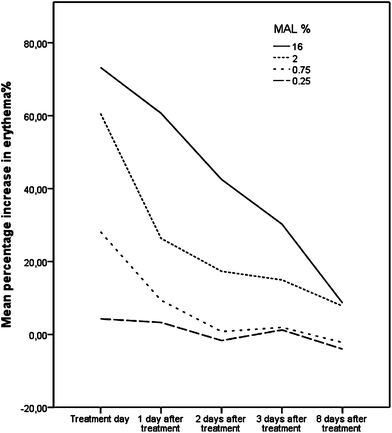 | ||
| Fig. 1 Mean increase in erythema% for four different MAL concentrations. | ||
| Treatment | 16% MAL standard treatment | 2% MAL | 0.75% MAL | 0.25% MAL | ||||
|---|---|---|---|---|---|---|---|---|
| Erythema% | Mean (SD) | P-value | Mean (SD) | P-value | Mean (SD) | P-value | Mean (SD) | P-value |
| Pre-treatment | 29.0 (5.3) | — | 27.4 (5.3) | — | 29.9 (6.1) | — | 28.7 (5.6) | — |
| Immediately before illumination | 35.2 (8.9) | 0.001 | 29.6 (7.1) | 0.077 | 28.8 (7.0) | 0.131 | 27.4 (6.5) | 0.168 |
| Immediately after treatment | 49.7 (10.3) | <0.0005 | 43.8 (10.6) | <0.0005 | 38.5 (13.0) | 0.001 | 29.5 (9.1) | 0.732 |
| 1 Day after treatment | 46.6 (13.7) | <0.0005 | 34.4 (9.2) | <0.0005 | 32.8 (10.5) | 0.094 | 29.2 (6.4) | 0.855 |
| 2 Days after treatment | 42.2 (14.3) | <0.0005 | 32.1 (7.9) | 0.002 | 30.4 (8.2) | 0.986 | 28.0 (5.2) | 0.305 |
| 3 Days after treatment | 37.9 (11.3) | <0.0005 | 31.1 (7.0) | 0.009 | 30.6 (7.1) | 0.670 | 28.3 (5.2) | 0.715 |
| 8 Days after treatment | 31.1 (6.9) | 0.137 | 29.1 (4.9) | 0.046 | 28.8 (5.7) | 0.391 | 27.2 (4.9) | 0.103 |
| Erythema clinical scores (0–3) | Mean (SD) | P-value | Mean (SD) | P-value | Mean (SD) | P-value | Mean (SD) | P-value |
|---|---|---|---|---|---|---|---|---|
| Pre-treatment | 0 | — | 0 | — | 0 | — | 0 | — |
| 1 Day after treatment | 1.9 (0.7) | <0.0005 | 1.1 (0.6) | <0.0005 | 0.7 (0.7) | <0.0005 | 0.3 (0.4) | .014 |
| 2 Days after treatment | 1.6 (0.8) | <0.0005 | 0.8 (0.7) | <0.0005 | 0.4 (0.6) | 0.004 | 0.2 (0.4) | .046 |
| 3 Days after treatment | 1.4 (0.8) | <0.0005 | 0.7 (0.6) | <0.0005 | 0.4 (0.5) | 0.003 | 0 | 1.000 |
| 8 Days after treatment | 1.0 (0.8) | <0.0005 | 0.5 (0.5) | 0.001 | 0.2 (0.4) | 0.046 | 0 | 1.000 |
| Treatment | 16% MAL Standard treatment | 2% MAL | 0.75% MAL | 0.25% MAL | ||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | P-value | Mean (SD) | P-value | Mean (SD) | P-value | ||
| Erythema% increase | Pre-treatment | 29.0 (5.3) | 27.4 (5.3) | 0.170 | 29.9 (6.1) | 0.230 | 28.7 (5.6) | 0.775 |
| Immediately after treatment | 20.7 (8.2) | 16.4 (8.2) | 0.002 | 8.6 (10.1) | <0.0005 | 0.9 (8.5) | <0.0005 | |
| 1 Day after treatment | 17.5 (11.1) | 7.0 (7.3) | <0.0005 | 2.9 (7.2) | <0.0005 | 0.5 (6.1) | <0.0005 | |
| 2 Days after treatment | 12.8 (11.0) | 4.63 (5.7) | 0.001 | 0.04(5.6) | <0.0005 | −0.9 (5.0) | <0.0005 | |
| 3 Days after treatment | 8.8 (9.0) | 3.7 (6.1) | 0.005 | 0.4 (4.6) | <0.0005 | −0.2 (6.0) | 0.001 | |
| 8 Days after treatment | 2.1 (5.8) | 1.7 (4.5) | 0.886 | −1.1 (4.6) | 0.049 | −1.5 (3.9) | 0.001 | |
| Fluorescence (AU) | Before illumination | 4983 (3212) | 1890 (1713) | <0.0005 | 791 (1677) | <0.0005 | 163(284) | <0.0005 |
| After illumination | 62 (233) | 8.5 (27.9) | 0.058 | 8.4 (18.0) | 0.478 | 9.9 (17.0) | 0.421 | |
| Photobleaching | 4881 (3220) | 1847 (1747) | 0.0001 | 794 (1720) | 0.0001 | 160 (285) | 0.0001 | |
| Pigmentation (%) | Before treatment | 14.41 (4.84) | 14.74 (5.35) | 0.627 | 13.53 (5.24) | 0.012 | 14.36 (5.54) | 0.587 |
| 8 Days after treatment | 17.81 (5.17) | 15.03 (4.79) | 0.010 | 14.34 (5.45) | 0.001 | 13.99 (5.52) | 0.001 | |
| Pain (scale 1–10) | During treatment | 1.7 (1.0) | 0.7 (0.7) | <0.0005 | 1.0 (1.2) | 0.017 | 0.7 (0.9) | 0.001 |
| Hours after treatment | 0.4 (0.7) | 0.1 (0.3) | 0.025 | 0.1 (0.2) | 0.022 | 0.1 (0.2) | 0.022 | |
| 1 Day after treatment | 0.2 (0.4) | 0.1 (0.2) | 0.102 | 0.0 (0.2) | 0.063 | 0.0 (0.2) | 0.063 | |
Pain
Fig. 2 and Table 2 give an overview of the pain scores. Pain scores during illumination peaked after 5 min for all four MAL concentrations. There was no significant difference between pain scores during illumination in the fields with the three reduced concentrations but all three were significantly less painful (both mean and maximum pain) than conventional treatment. The maximum duration of pain was 3 days, but on the 3rd day the pain was assessed at 1 on the scale from 0 to 10. Pain during illumination was strongly correlated to the increase in erythema% (P < 0.0005).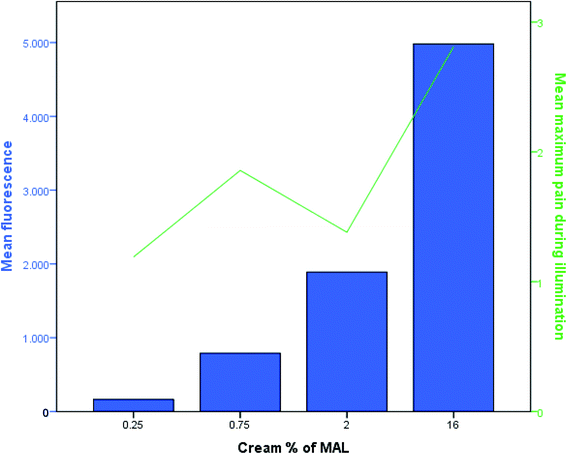 | ||
| Fig. 2 Mean PpIX fluorescence (AU) before illumination (left y-axis) and mean maximum pain during illumination (right y-axis) for the four different MAL concentrations. | ||
PpIX fluorescence
The calculated fluorescence and photobleaching values are, as expected, very similar and are given in Table 2. The mean fluorescence before illumination is shown in Fig. 2 for the four different MAL concentrations.Correlations between the increase in erythema% and photobleaching are highly significant (P = 0.016–0.0005). In order to describe the factors that influence the build-up of fluorescence in the skin, a backward linear regression analysis for MAL concentration, erythema% before treatment, pigmentation%, age and pre-illumination fluorescence was performed. Of these parameters only the MAL concentration and the pre-illumination erythema% were of influence (R2 = 0.50) explaining 50% of the differences in the fluorescence build-up (P < 0.0005).
Pigmentation
Measurements of pigmentation% before treatment and 8 days after treatment are given in Table 2. There was no difference in the measured pigmentation% in the various fields before treatment, however, a significantly higher level in the 16% MAL field than in the fields treated with the three reduced concentrations (P < 0.01–0.001) was observed. The field with MAL 16% was the only field with a significant increase in pigmentation% from pre-treatment to the last control day.Due to technical difficulties, erythema% and pigmentation% measurements are lacking for 1 volunteer on day 1, 3 volunteers on day 2, and 1 volunteer on day 3. Also fluorescence images are missing for 4 volunteers.
Discussion
We know that PDT, as it is performed today, is followed by inflammation and erythema of the skin. The aim of this study was to investigate whether a reduction in the concentration of MAL could reduce erythema as compared to conventional PDT treatment. This is becoming relevant since the production of new formulations containing lower concentrations of photosensitizer for acne treatment 3 and cosmetic rejuvenation is currently a hot research topic.4,5Our study shows that reduced MAL concentrations resulted in reduced erythema. Furthermore, the results of this study suggest that this reduction in erythema and inflammation is mostly due to reduced formation of PpIX and, as a consequence, less photobleaching. The reduced inflammation might be a result of cell death mainly by apoptosis instead of necrosis as a result of the reduced amount of PpIX.
The ideal pathway of PDT is via formation of ROS by light activation of photosensitizers solely in the mitochondria. When the mitochondria are destroyed, the cell will undergo apoptosis, which is normally not followed by inflammation.7 Since active ROS have a very small radius of action,20 we should theoretically—before they react with the cell structures—be able to limit cell death to being a result of apoptosis. In addition, we may avoid necrosis if we are able to control where the ROS are activated in the cells. The ordinary use of MAL ensures a very high level of PpIX9,21 resulting in PpIX leaking to other cellular components.10 Thus, ROS are generated not only in the mitochondria but also in the cytoplasm and in lysosomes.
There was a slightly increased pre-illumination erythema in the MAL 16% treatment field. This finding is consistent with the stinging sensation some patients described immediately after MAL 16% was applied to their skin. Though significant, the pre-treatment increase in erythema% is not high enough to affect the post-illumination erythema. Since we do not know how long this pre-illumination erythema persists, we chose not to correct for it.
There was a strong correlation between the expert assessment of erythema and measured erythema%. Objectively measured erythema by Optimize Scientific is thus an effective, operator-independent and safe method for assessing increases in erythema.
When an expert assesses erythema, the colour of the treatment area is compared to the adjacent normal skin; however, when erythema% is measured, it is not compared to the adjacent skin colour and is thus not relative. The erythema% differs in normal skin in different locations and in different individuals, and the objective measurements of the same locations pre- and post-treatment and in the same individuals correct for this difference.
Pain was formerly thought to be an unavoidable adverse-effect of PDT treatment, however, almost painless and yet, still effective PDT can be performed by continuous daylight illumination.2,22 Our study shows that a reduction of pain by lowering the MAL concentration is possible, but elimination of pain during illumination is difficult, since a reduction from 2% MAL to 0.75 and 0.25% was not followed by a further pain reduction.
Most of the adverse-effects in our study were dependent on the amount of PpIX fluorescence generated. The calculated PpIX fluorescence from this study corresponds with a previous study which showed low concentrations of MAL (0.2 and 2%) induced considerably less fluorescence than MAL 20%.23 In our study there is a large difference in MAL concentration (2 vs. 16%), however, the corresponding difference in PpIX fluorescence is not that large (1890 vs. 4983). This observation corresponds well with the findings in the study by Wiegell et al., which showed no difference in fluorescence between MAL 8% and 16%.2 It seems like there is a concentration limit where higher concentrations of MAL does not give more fluorescence.
We know that the effect of PDT is dependent on the formation of PpIX, however, we have no knowledge as to whether the effect depends on a high level of PpIX, and this question should be investigated. Investigations of unspecific inflammation markers have found increased levels of histamine present after PDT,24 and a murine study showed up-regulation of genes coding for C-reactive protein,25 indicating that uncontrolled damage at the cellular level is part of the response to PDT, with the leakage of toxic cellular components into the surrounding tissue. This leakage may lead to substantial damage to surrounding cells and vessels, oxygen depletion of tissue and finally necrosis. Some authors still believe that this inflammatory response is necessary for long-term tumour control.26–28 We wish to eliminate the inflammatory response and increase the control of PDT solely for apoptosis. Therefore, it is important to limit the damage to the mitochondria and thus spare other cell components.29
Our finding that pre-treatment erythema is of importance for PpIX fluorescence has not previously been reported. The difference in pre-treatment erythema can be caused by many factors e.g. thinner epidermis making the vessels more visible, and warmer skin due to dilated vessels. A thinner epidermis could make the uptake of MAL cream easier and warm skin with dilated vessels might facilitate the enzymatic reactions and increase the supply of oxygen needed for the photodynamic reaction.
According to the product summary of MAL, hyperpigmentation is an uncommon adverse-effect. We did not make a visual assessment of pigmentation, but measured the pigmentation%. The mean increase in pigmentation for MAL 16% was 3.4%, and previous tests have shown that we are visually able to detect a difference in pigmentation% of 2%.30 This means that the increase in measured pigmentation would be just visible. We did not examine whether any long-term hyperpigmentation occurred.
Conclusions
MAL concentrations below 1% can prevent the development of erythema and hyperpigmentation in the days after PDT, and thereby reduce the adverse-effects following conventional treatment. Pain suffered by patients may also be reduced.The next step toward a more specific PDT is to test the effect of PDT performed with a reduced concentration, e.g. 1% MAL. This dose will give an acute reaction with increased erythema% just after treatment, but will allow no prolonged erythema in patients. We do not know whether such a low concentration will be sufficiently effective, however we know from another study that, MAL 8% was as effective as MAL 16%.2 The test with MAL 1% could be performed on patients with grade 1 actinic keratoses. This should be accompanied by close monitoring of adverse-effects and long-term cure rates.
Acknowledgements
We would like to thank Stine Wiegell for helping with the use of the fluorescence camera and the method of calculation for fluorescence. We additionally thank clinical photographer Nis Kentorp for helping with the photo material.References
- L. R. Braathen, R. M. Szeimies, N. Basset-Seguin, R. Bissonnette, P. Foley, D. Pariser, R. Roelandts, A. M. Wennberg and C. A. Morton, Guidelines on the use of photodynamic therapy for nonmelanoma skin cancer: an international consensus. International Society for Photodynamic Therapy in Dermatology, 2005, J. Am. Acad. Dermatol., 2007, 56, 125–143 CrossRef.
- S. R. Wiegell, M. Haedersdal, P. Eriksen and H. C. Wulf, Photodynamic therapy of actinic keratoses with 8% and 16% methyl aminolaevulinate and home-based daylight exposure: a double-blinded randomized clinical trial, Br. J. Dermatol., 2009, 160, 1308–1314 CrossRef CAS.
- R. Bissonnette, C. Maari, S. Nigen, N. Provost and C. Bolduc, Photodynamic therapy with methylaminolevulinate 80 mg g−1 without occlusion improves acne vulgaris, J. Drugs Dermatol., 2010, 9, 1347–1352 Search PubMed.
- P. Bjerring, K. Christiansen, A. Troilius, P. Bekhor and L. J. de, Skin fluorescence controlled photodynamic photorejuvenation (wrinkle reduction), Lasers Surg. Med., 2009, 41, 327–336 CrossRef.
- K. Christiansen, P. Bjerring and A. Troilius, 5-ALA for photodynamic photorejuvenation–optimization of treatment regime based on normal-skin fluorescence measurements, Lasers Surg. Med., 2007, 39, 302–310 CrossRef.
- K. Berg, Photodynamic therapy with 5-ala and ester derivates, in Photo Dynamic Therapy: Basic Principles and Clinical Experience with Metvix PDT, ed. L. R. BraathenPhotocure, Oslo, 2001, pp. 15–21 Search PubMed.
- A. P. Castano, P. Mroz and M. R. Hamblin, Photodynamic therapy and anti-tumour immunity, Nat. Rev. Cancer, 2006, 6, 535–545 CrossRef CAS.
- A. Stritt, H. F. Merk, L. R. Braathen and V. von Felbert, Photodynamic therapy in the treatment of actinic keratosis, Photochem. Photobiol., 2008, 84, 388–398 CrossRef CAS.
- S. R. Wiegell, I. M. Stender, R. Na and H. C. Wulf, Pain associated with photodynamic therapy using 5-aminolevulinic acid or 5-aminolevulinic acid methylester on tape-stripped normal skin, Arch. Dermatol., 2003, 139, 1173–1177 CAS.
- J. M. Gaullier, M. Geze, R. Santus, T. Sa e. Melo, J. C. Maziere, M. Bazin, P. Morliere and L. Dubertret, Subcellular localization of and photosensitization by protoporphyrin IX human keratinocytes and fibroblasts cultivated with 5-aminolevulinic acid, Photochem. Photobiol., 1995, 62, 114–122 CrossRef CAS.
- A. P. Castano, T. N. Demidova and M. R. Hamblin, Mechanisms in photodynamic therapy: part one—photosensitizers and cellular localization, Photodiagn. Photodyn. Ther., 2004, 4, 279–293 CrossRef.
- C. Sandberg, B. Stenquist, I. Rosdahl, A. M. Ros, I. Synnerstad, M. Karlsson, F. Gudmundson, M. B. Ericson, O. Larko and A. M. Wennberg, Important factors for pain during photodynamic therapy for actinic keratosis, Acta Derm.-Venereol., 2006, 86, 404–408 CrossRef.
- J. Skiveren, M. Haedersdal, P. A. Philipsen, S. R. Wiegell and H. C. Wulf, Morphine gel 0.3% does not relieve pain during topical photodynamic therapy: a randomized, double-blind, placebo-controlled study, Acta Derm.-Venereol., 2006, 86, 409–411 CrossRef.
- S. R. Wiegell, M. Haedersdal and H. C. Wulf, Cold water and pauses in illumination reduces pain during photodynamic therapy: a randomized clinical study, Acta Derm. Venereol., 2009, 89, 145–149.
- I. M. Miller, J. S. Nielsen, S. Lophaven and G. B. Jemec, Factors related to pain during routine photodynamic therapy: a descriptive study of 301 patients, J. Eur. Acad. Dermatol. Venereol., 2011, 25, 1275–1281 CrossRef CAS.
- S. R. Wiegell, M. Haedersdal, P. A. Philipsen, P. Eriksen, C. D. Enk and H. C. Wulf, Continuous activation of PpIX by daylight is as effective as and less painful than conventional photodynamic therapy for actinic keratoses; a randomized, controlled, single-blinded study, Br. J. Dermatol., 2008, 158, 740–746 CrossRef CAS.
- R. Na, I. M. Stender, M. Henriksen and H. C. Wulf, Autofluorescence of human skin is age-related after correction for skin pigmentation and redness, J. Invest. Dermatol., 2001, 116, 536–540 CrossRef CAS.
- H. C. Wulf, Methods and an apparatus for determining an individual's ability to stand ultraviolet radiation, US Pat., 4882598, 1986 Search PubMed.
- B. R. Kirkwood, Essentials of Medical Statistics, Blackwell Science Ltd, Malden, Mass, 2003 Search PubMed.
- J. Moan and K. Berg, The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen, Photochem. Photobiol., 1991, 53, 549–553 CrossRef CAS.
- E. Angell-Petersen, R. Sorensen, T. Warloe, A. M. Soler, J. Moan, Q. Peng and K. E. Giercksky, Porphyrin formation in actinic keratosis and basal cell carcinoma after topical application of methyl 5-aminolevulinate, J. Invest. Dermatol., 2006, 126, 265–271 CrossRef CAS.
- S. R. Wiegell, H. C. Wulf, R. M. Szeimies, N. Basset-Seguin, R. Bissonnette, M. J. Gerritsen, Y. Gilaberte, P. Calzavara-Pinton, C. A. Morton, A. Sidoroff and L. R. Braathen, Daylight photodynamic therapy for actinic keratosis: an international consensus: International Society for Photodynamic Therapy in Dermatology, J. Eur. Acad. Dermatol. Venereol., 2012, 26, 673–679 CrossRef.
- A. Juzeniene, P. Juzenas, L. W. Ma, V. Iani and J. Moan, Topical application of 5-aminolaevulinic acid, methyl 5-aminolaevulinate and hexyl 5-aminolaevulinate on normal human skin, Br. J. Dermatol., 2006, 155, 791–799 CrossRef CAS.
- R. C. Brooke, A. Sinha, M. K. Sidhu, R. E. Watson, M. K. Church, P. S. Friedmann, G. F. Clough and L. E. Rhodes, Histamine is released following aminolevulinic acid-photodynamic therapy of human skin and mediates an aminolevulinic acid dose-related immediate inflammatory response, J. Invest. Dermatol., 2006, 126, 2296–2301 CrossRef CAS.
- M. Korbelik, I. Cecic, S. Merchant and J. Sun, Acute phase response induction by cancer treatment with photodynamic therapy, Int. J. Cancer, 2008, 122, 1411–1417 CAS.
- I. Cecic, B. Stott and M. Korbelik, Acute phase response-associated systemic neutrophil mobilization in mice bearing tumors treated by photodynamic therapy, Int. Immunopharmacol., 2006, 6, 1259–1266 CrossRef CAS.
- M. Korbelik, PDT-associated host response and its role in the therapy outcome, Lasers Surg. Med., 2006, 38, 500–508 CrossRef.
- P. C. Kousis, B. W. Henderson, P. G. Maier and S. O. Gollnick, Photodynamic therapy enhancement of antitumor immunity is regulated by neutrophils, Cancer Res., 2007, 67, 10501–10510 CrossRef CAS.
- Z. Ji, G. Yang, V. Vasovic, B. Cunderlikova, Z. Suo, J. M. Nesland and Q. Peng, Subcellular localization pattern of protoporphyrin IX is an important determinant for its photodynamic efficiency of human carcinoma and normal cell lines, J. Photochem. Photobiol., B, 2006, 84, 213–220 CrossRef CAS.
- M. H. Ravnbak and H. C. Wulf, Pigmentation after single and multiple UV-exposures depending on UV-spectrum, Arch. Dermatol. Res., 2007, 299, 25–32 CrossRef CAS.
Footnote |
| † This article is published as part of a themed issue on current topics in photodermatology. |
| This journal is © The Royal Society of Chemistry and Owner Societies 2013 |
