 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Spectral effects of UV on psoriasis†
Sophie C.
Weatherhead
*ab,
Peter M.
Farr
ab and
Nicholas J.
Reynolds
ab
aDepartment of Dermatology, Royal Victoria Infirmary, Newcastle-upon-Tyne, UK
bDermatological Sciences, Newcastle University, Newcastle-upon-Tyne, UK. E-mail: sophie.weatherhead@ncl.ac.uk
First published on 18th September 2012
Abstract
Ultraviolet B (UVB) is a highly effective, relatively safe, affordable and widely used therapeutic option for moderate psoriasis. Several types of UVB lamp are available to treat psoriasis, both broadband and narrowband, allowing a choice of spectral emission. However despite years of clinical use, the mechanism of action of UVB in clearing psoriasis remained incompletely understood. Moreover, there has been little insight into how the relative effectiveness of different UVB wavelengths linked to the mechanism of action, although it is known that the action spectrum for clearance of psoriasis differs from the action spectrum of erythema. This paper examines the existing literature from which our current treatments have evolved, and offers new insight into the use of keratinocyte apoptosis as a biomarker which may help to optimise UV treatment in the future. When combined with a systems biology approach, this potential biomarker may provide insight into which wavelengths of UV are the most effective in clearing psoriasis, allowing a more rational and potentially an individually tailored approach to optimising phototherapy for psoriasis.
Introduction
The beneficial effects of sunlight on the skin have been known for thousands of years, with records of heliotherapy being practiced in Ancient Greece, Egypt and Rome. In more modern times, Niels Finsen was the first to use artificial UV sources to treat skin disease, and in particular lupus vulgaris, for which he was awarded a Nobel prize in 1903. The ultraviolet radiation spectrum is divided somewhat arbitrarily into 3 main wavebands; UVC (100–290 nm), UVB (290–320) and UVA (320–400 nm), although the exact spectral definitions differ slightly between America and Europe. UVC is almost completely filtered by the atmosphere, with insignificant quantities reaching the Earth's surface. UVB penetrates into the epidermis and is maximally effective in terms of erythema production with the minimal erythema dose (MED) being up to 1000 times less than that for UVA. The erythemal response of uninvolved skin is an important consideration when treating psoriasis as it is this that limits the dose that can be used for treatment; plaques of psoriasis being relatively resistant to becoming more erythematous with UV exposure.1 Both UVB and psoralen-UVA (PUVA, in which the patient takes oral Psoralen and is then exposed to UVA) are used to effectively treat psoriasis. UVA alone is not thought to be effective in treating psoriasis, at least with the doses that are currently able to be delivered. Similarly, “sunbed” treatment, using UVA fluorescent lamps (which frequently emit small amounts of UVB) are only minimally effective,2 although indoor sunbeds with higher UVB emissions are more effective than ‘standard’ sunbeds (when equal exposure times are given).3 This difference is negated when exposure times for standard sunbeds are prolonged, to give an equal erythemal dose.3 PUVA is highly effective in clearing psoriasis, and has been commercially available since 1976, but its use is limited by the established risk of skin cancer after a cumulative treatment dose of approximately 1000 J cm−2 (and resulting in a clinical decision to often not exceed around 5 courses of PUVA over the patient's life-time).4 UVB is a relatively safe treatment modality which can successfully clear psoriasis in 60–70% patients.5–7 The first UVB lamps used to treat psoriasis had a broad spectral emission (“broadband”), often including some wavelengths <290 nm (UVC), but in the late 1980s narrowband UVB lamps (311 nm +/−2) were introduced with potentially greater efficacy in some patients. More recently the 308 nm excimer laser and lamp have been shown to have equal efficacy to narrowband UVB in clinical studies.8–11The action spectrum for clearance of psoriasis can be defined as the relative effectiveness of different wavelengths in achieving clearance of plaques. To optimise the efficacy of phototherapy for psoriasis it is important to know which wavelengths are the most effective in plaque clearance. However, our current phototherapy treatments are based on the action spectrum derived from studying just four patients with psoriasis. In 1981, Parrish and Jaenicke published their landmark study examining four male Caucasian patients with psoriasis.12 Seven plaques were chosen from each subject and irradiated daily in localised areas with wavelengths of UV ranging from 254 nm to 320 nm and the clinical response recorded. The authors showed that wavelengths over 300 nm and less that 320 nm were effective in clearing plaques at doses equal to or less than the MED, but those less than 300 nm were ineffective even at doses of up to 28 times the MED. Around this time, a phosphor coating on low-pressure mercury bulbs was developed that resulted in an emission of UVB peaking at 311 nm +/−2 nm, and therefore these lamps were developed commercially to treat psoriasis (narrowband UVB). Whether the optimal therapeutic wavelength shows inter-individual variation remains to be determined. However, by gaining insight into the mechanism of plaque clearance,13 we hypothesise that characteristics of psoriatic plaques may contribute to variation in therapeutic responses to UVB. Together, these factors suggest that further treatment benefit could be obtained by optimising wavelengths of UV radiation delivered to individual patients. Further research in this area is warranted.
Defined action spectra in the skin
Action spectra within the skin will differ according to the absorption spectra of the chromophores they target. If action spectra are similar for different variables, e.g. erythema and DNA damage, this would imply common chromophores. Erythema induced by UVB and UVC is, in part, mediated by release of prostaglandins from the epidermis, with erythema peaking approximately 12–15 h post irradiation.14 The degree of erythema induced by UV is affected by individual susceptibility, site of irradiation, skin thickness, photoadaptation of the skin due to previous UV exposure and dose of UV administered.15 Several erythema action spectra have been published, but only one has been endorsed as internationally recognised by the CIE (Commission Internationale de l’Éclairage); this was originally described by McKinlay and Diffey in 1987,16 and later updated17 (for a review of the erythema action spectra see Webb et al., 2011).18 This action spectrum is also used to calculate the UV index for public health information in weather forecasting, and to calibrate instruments to measure erythemally weighted UV which in turn will determine the accuracy of the dose of UV administered to patients. As shown in Fig. 1, the erythema action spectrum peaks at 290–300 nm, falls off rapidly between 300 and 325 nm, then declines steadily thereafter. However, it is unclear which layers of the epidermis are responsible for the release of erythema-inducing cytokines, although shorter wavelengths of UV (e.g. 290 nm) can clearly penetrate the epidermis sufficiently deeply to induce these biological changes. However, although wavelengths between 250 nm and 300 nm have a similar erythemal response at low doses, there appears to be very different erythemal effect at higher doses, with wavelengths around 300 nm having a much steeper erythemal response than wavelengths of 254 and 280 nm,19 suggesting that different cytokine mediators may be involved.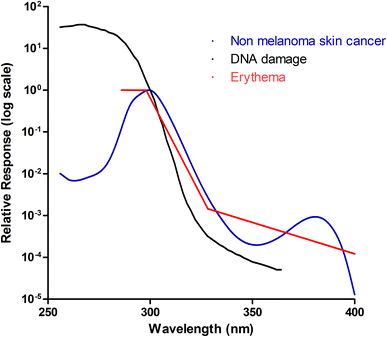 | ||
| Fig. 1 Known action spectra within the skin. Three action spectra are shown: non-melanoma skin cancer (blue), DNA damage (black) and erythema (red). These follow a similar pattern, suggesting that they may have a common chromophore. They peak within the UVC/UVB range and decline as the wavelength increases. It should be noted however, that the action spectrum for DNA damage is calculated using data from cultured cell and adjusted for transmission through the human epidermis. This makes it difficult to directly compare with the other 2 in vivo spectra as the optics of the epidermis will affect transmission and some chromophores (e.g. DNA) can act as “sunscreens” to UVR. | ||
The DNA damage action spectrum was defined by Setlow in 1974 to identify which wavelengths are most carcinogenic to the skin (Fig. 1).20 Affected epidermal cells are predominantly basal, however, the action spectrum parallels that of the erythema action spectrum for wavelengths greater than 300 nm, suggesting a possible common chromophore. This is supported by studies looking at the action spectrum of UVB (290–320 nm) for the production of the proinflammatory cytokine TNFα, within human epidermis in vivo.21,22 Young et al. showed that DNA is likely to be a major chromophore for erythema in the UVB range.21 Walker and Young later showed that the action spectrum of soluble TNFα production, which is thought to play a role in UV-induced skin cancer, closely paralleled the action spectrum of erythema and DNA damage in the basal layer.22 It has maximum efficacy at 300 nm, suggesting that cyclobutane pyrimidine dimers trigger TNFα production and release within human epidermis. The action spectrum for mammalian non melanoma skin cancer, which was defined by de Gruijl and van der Leun in 1994,23 lies between the erythemal and DNA-damage action spectra and the erythema action spectrum within the UVB range, but show a sine-like effect at longer wavelengths, between 340–400 nm (see Fig. 1). This spectrum was calculated using mice studies and human epidermis ex vivo, although it was noted that the action spectra can vary between individuals.
Other action spectra in the skin include the effects of UV on immunosuppression, which shows a peak at 300 nm and a further peak at 370 nm; although the UVA peak is likely to be the greatest contributor to immunosuppression due to the far greater amount of UVA contributing to total daily UV exposure.24 UV has also been shown to activate the transcription factor Nuclear Factor of Activated T cells (NFAT),25 which regulates COX-2 production and may thereby contribute to UV-induced skin cancer formation.26 The action spectrum for NFAT activation has only been derived in cultured keratinocytes where it was shown to be inversely related to wavelength. It is distinct to the DNA damage/erythema action spectra,26 although it is not possible to directly compare in vivo and in vitro systems as the absorption spectrum of single cells in vitro will not be complicated by the differing optics of the skin, and hence penetration into the epidermis.
Because UVB exerts a multitude of effects, it cannot be assumed that the action spectrum for psoriasis will depend on basal layer DNA damage and follow a similar plot to those shown in Fig. 1, as this is dependent on the depth of UV penetration required for maximal biological activity. Therefore, only if the biological target for psoriasis clearance by UV is within the basal layer (as for DNA damage and skin cancer induction), would the action spectrum be expected to be similar.
Clinical studies
Clearance of psoriatic plaques following UVR varies both between and within patients, and is dependent on a number of factors. Inter-patient response is influenced by skin type/colour,27,28 concurrent illness (e.g. streptococcal throat infection), stress and use of concomitant medication. Site, thickness and size of plaques will also affect response, with clinical observation suggesting that thinner smaller plaques generally clear faster.Monochromatic excimer lasers (308 nm) can be effective as a treatment for localised psoriasis8,10,11,29 and have been demonstrated to be as effective as 311 nm UVB in clearing psoriasis.11 Over recent years, the 308 nm excimer laser has been used to effectively clear individual plaques using doses of 3–6 MEDs.30 One study showed that irradiation of matched psoriatic plaques in 15 patients with the 308 nm excimer laser, 308 nm UVB lamp and 311 nm UVB lamp resulted in similar efficacy for all three irradiation sources over a 10 week period, with a mean of 24 treatments required for clearance.11 More recently, an open, single-blinded right/left comparison trial compared the 308 nm excimer laser and 311 nm UV lamp in 16 patients and again found similar efficacy overall although nine patients responded better to the 308 nm excimer laser and four to the 311 nm lamp.31 Therefore, although these treatments are highly effective in clearing psoriasis, they clear psoriasis to varying extents in different patients, are not effective in all patients and show inter-individual variation in efficacy.
Designing experiments to investigate which wavelengths most effectively clear psoriasis and the factors regulating response may include expanding the original studies of Parrish and Jaenicke using a large sample size. Plaques would need to be matched within patients for size, thickness and location, across a range of age groups in men and women. However, such experiments are very time consuming for both patient and investigator and require the use of a calibrated monochromator, which are only available in a few specialised centres. It is unlikely that such an experiment would be undertaken today, particularly in view of alternative therapeutic modalities available. Moreover, initial doses used in treatment are usually based on the patients’ MED for a given wavelength, and would therefore need to be measured for each wavelength prior to irradiation. Whether or not relating response to a patient's MED is the best way to compare doses will remain unclear until the exact mechanism of plaque clearance is defined. Only if the biological effect of clearance occurs in the same location (depth) of the epidermis as inducing factors for erythema, can the use of MED as a way of equating doses be justified. Our recent work has shown that keratinocyte apoptosis is an important mechanism of psoriatic plaque remodelling in response to UVB, and that this occurs predominantly in the basal and suprabasal epidermis of psoriatic plaques.13 Moreover, by adopting a systems biology approach we created a mathematical model of psoriatic epidermis and its response to UVB that allowed us to make predictions about effects of specific input variables on the defined outcomes.13 This approach will facilitate the design of specific intervention studies in humans to address some remaining unanswered questions.
The erythema action spectrum is similar to that of the DNA damage and skin cancer action spectra which affect the basal layer, suggesting that MED is likely to be a good way of determining equal dose between different wavelengths. UV-induced keratinocyte apoptosis may therefore be a good biomarker to predict plaque clearance.
The effects of penetration
The efficacy of UVB in clearing plaques may be related to depth of penetration, and it is likely that UV needs to penetrate at least to the basal layer to remodel plaques back to normal.13 Longer wavelengths (UVA) penetrate deeper into the skin, and may cause effects within the dermis, whereas shorter wavelengths do not penetrate as deeply. The average in vivo transmission of UV through skin on the lower back follows an exponential curve, with maximal change between 275 nm and 290 nm. In normal forearm skin, opticoacoustic studies have demonstrated that wavelengths of 314 nm penetrate into the lower epidermis approximately 14 times more than 290 nm UVB,32 therefore a large difference in penetration occurs within a small range of wavelengths.In normal skin, UV-induced damage can be limited by apoptosis, and both UVB and UVC have been shown to induce apoptosis of normal human keratinocytes in vitro, although UVC appears to cause significantly more (6–4) photoproducts and cyclobutane pyrimidine dimers than UVB in cultured keratinocytes.33 When equal levels of keratinocyte apoptosis are generated with UVC and UVB, greater release of mitochondrial apoptotic pathway factors (such as cytochrome c) has been shown following UVC, with UVB inducing more apoptosis via the extrinsic pathway.33 This suggests that effects of UV on keratinocytes are at least in part, wavelength specific, irrespective of differences in penetration.
Apoptosis as a biomarker of psoriatic clearance
We have recently investigated the biological effects between 2 h and 48 h after in vivo irradiation of plaques of psoriasis from 53 patients with equi-erythemogenic doses of two wavelengths of UVB.13 Our results showed significant keratinocyte apoptosis in those patients treated with the “effective” wavelength of UVB (311 nm) but not following an equally erythemogenic dose (3 MEDs) of a clinically “ineffective” wavelength (290 nm). If the chromophore for apoptosis is in the lower epidermis, then reduced penetration of 290 nm UV may partially explain this result, however, there was no direct relationship between epidermal thickness and apoptosis (as discussed below). A dynamic, interactive mathematical model was used to predict how many apoptotic cells would be expected to occur at a single time-point 18–24 h post UVB irradiation (i.e. measureable by an end-point assay such as immunohistochemistry). The stochastic model was developed using carefully measured data (e.g. the time-course and dose–response of UVB-induced keratinocyte apoptosis), histological (e.g. morphological pattern of psoriasis, pattern of stem cell distribution etc.), and historic data (e.g. previously calculated birth rate of cells in psoriasis, turn-over time of stem cells/transit amplifying cells and transit time of differentiating cells) (Fig. 2) and is available for use at http://research.ncl.ac.uk/psoriasis. This model is fully described in the ESI† of our previously published work.13 The model predicted that both stem and transit amplifying cells would need to undergo apoptosis in response to therapeutic UVB in psoriasis, and that approximately seven exposures would be required for doses of 3 MEDs compared to 25–30 exposures for sub-erythemogenic doses of UVB (311 nm). These predictions fit with clinical observations and suggest that the quantity of apoptosis seen in our patients’ lesional skin was sufficient to remodel psoriatic epidermis back to phenotypically normal skin.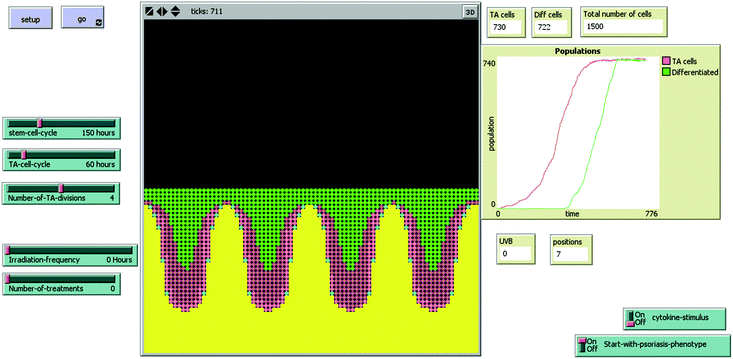 | ||
| Fig. 2 Interactive model of psoriatic epidermis, which can be adapted to incorporate different action spectra The model interface consists of buttons, sliders, and monitors to allow easy adjustment of parameters by the user, and observation of the result of these adjustments. Each ‘tick’ represents one hour, and is displayed at the top of the interface. ‘Start-with-psoriasis-phenotype on/off’ allows the user to choose whether to start the model as normal or psoriatic phenotype, but does not affect the running of the model in any way. The positions monitor goes from ‘1’ (normal) to ‘7’ (maximal psoriatic phenotype for this model), and will change according to the number of cells and proliferation rate within the model. ‘Cytokine-stimulus’ allows the user to choose to ‘switch on a stimulus’ to increase the proportion of actively proliferating stem and TA cells. Note that it takes approximately 600 h for the model to start to reach equilibrium (as shown in the overview display window), which is then self-sustaining until irradiated with UVB. Yellow patches represent the basement membrane, dermis and below. Stem cells are shown as blue, TA cells as pink (light pink when actively dividing and darker pink when resting), and green cells are differentiated. | ||
An important observation was that keratinocyte apoptosis does not occur in psoriatic plaques in vivo in response to doses of up to 3 MEDs of 290 nm UVB at time-points between 4 and 48 h (n = 26).13 Extending this observation further, the biological effects of five different wavelengths of UVB (296 nm, 301 nm, 306 nm, 311 nm and 320 nm) were examined in matched psoriatic plaques in vivo from 18 patients (Weatherhead et al., unpublished data). This study showed that keratinocyte apoptosis occurs in psoriatic plaques following irradiation with wavelengths between 300 and 320 nm only, fitting well with Parrish and Jaenicke's original study which showed that only these wavelengths are clinically effective in clearing psoriasis.12 Interestingly, this study showed wide inter-patient variation, with peak keratinocyte apoptotic response in 50% of the patients following 311 nm, 17% of patients following 301 nm and 22% of patients had a peak following 306 nm UVB. Apoptotic counts in plaques following irradiation with these 3 wavelengths were all significantly greater than following irradiation with 296 nm or 320 nm UV (p = 0.0314). Longer wavelengths of UV penetrate deeper into the epidermis, and as apoptosis is seen in the lower epidermis, it could be argued that the optimal wavelength for apoptosis induction will be dependent on epidermal thickness within the plaque. However, although a positive correlation was shown between epidermal thickness and optimal wavelength for apoptosis induction, this was not statistically significant amongst this relatively small sample. The apoptotic response of the 5 wavelengths studied was also independent of patients’ skin type and the administered irradiation dose. It is therefore unclear from this work why some patients have a differential apoptotic response to wavelengths which induce keratinocyte apoptosis in other subjects. One possible explanation is that this arises due to sample error and chance observation of transient events (apoptosis; which can be completed within 20 min),13 with larger numbers of patients being required to reduce this potential bias.
If results of this study are representative of the general psoriatic population, it follows that UVB lamps with emission in the 301–311 nm range (and no significant emission of highly erythemal wavelengths < 301 nm) would appear the most efficient in inducing epidermal keratinocyte apoptosis, and therefore potentially would result in the best clinical outcome for patients if apoptosis is an important mechanism of psoriasis clearance. Broadband UVB lamps used to treat psoriasis may be “selective” (with little emission at 300 nm or shorter wavelengths, e.g. UV6), or “conventional” (with significant emission < 300 nm, e.g. TL12, FS40). It would therefore follow that narrowband UVB (TL01; 311 nm +/−2 nm)/selective broadband UVB should be more effective for a given erythemal dose than conventional broadband UVB, as a greater proportion of the output is within the apoptosis-inducing spectrum. Several small half-body studies have shown a significantly greater efficacy for narrowband rather than conventional broadband UVB,5,34–36 and a further study found no significant difference in psoriasis clearance between TL01 and UV6 in a large randomised study of 100 patients.7 There has not been any demonstrated difference in skin cancer risk between narrowband and broadband UVB in humans,37 but mouse studies suggest a 2–3 times greater risk of SCC per MED with TL01 compared to TL12 lamps.38
The above data assume that peak erythemal response occurs 18–24 h (peak is around 12–15 h but we measure MED at 24 h for convenience) following UVB irradiation in non-lesional psoriatic epidermis, as in normal skin the peak in erythemal time-course has been demonstrated to be similar for UVA, UVB and UVC.39,40 Ideally an erythema time-course should be obtained for each wavelength to exclude a different time-course in psoriatic skin, however, when patients were reviewed at 48 h following initial MED irradiations there was no suggestion of a delayed erythemal response for any wavelength tested.
Overall, these results suggest that keratinocyte apoptosis is important in UVB-induced clearance of psoriasis, and this may be useful as a biomarker to further investigate which wavelengths of UVB are most effective in clearing psoriasis and to investigate factors that contribute to inter-individual variation in response. An intriguing possibility is to develop biomarkers of response that allow assessment of wavelength specificity in individual patients with the ultimate aim of individualising therapeutic delivery of UVB.
Conclusions
Understanding which wavelengths of UV are most effective in clearing psoriasis will help advance our understanding of how UV works to clear psoriasis and to optimise our current phototherapy options for patients. Giving the optimal wavelength potentially reduces the overall dose required for treatment, thereby making treatments safer for patients, allowing patients to be treated with less visits to hospital and over a shorter period of time. Furthermore, increasing our understanding of how UV works to clear psoriasis may open new avenues for optimisation of UV treatment by synergistic use of another agent, allowing greater efficacy.The thickness of psoriasis plaques will differ significantly between body sites and individuals, and clinical observation shows that UV penetration through hyperkeratotic plaques will be limited. However, although penetration of UV is important in determining which wavelengths can have biological effects on the lower epidermis, it is likely that other wavelength specific factors (e.g. activation of the intrinsic versus extrinsic pathways) will be important in influencing clearance.
Several action spectra have been well defined within the normal epidermis, but only one study has attempted to examine the action spectrum for UV-induced clearance of psoriasis.12 This landmark study helped define the range of UVB wavelengths which can clear psoriasis, and showed that the action spectrum for UV-induced clearance of psoriasis is clearly distinct from the erythema action spectrum, but did not distinguish the optimal wavelength for clearance. Defining this more precisely would be laborious and difficult to perform clinically, but may be possible if directed using a biomarker. In this paper, we have described UV-induced keratinocyte apoptosis occurring following irradiation of psoriatic plaques in vivo and suggest that this may be a useful biomarker of clinical response. Early data indicate that this could potentially be useful to help define the action spectrum for plaque clearance, which combined with a systems biology approach would allow a more targeted and feasible clinical study to be undertaken. A further interesting study would be to compare the efficacy of these narrowband UVB sources compared to broadband UVB (in particular filtering out wavelengths below 300 nm, which induce erythema but are ineffective in clearing psoriasis), which may allow tolerance of greater UV doses while allowing for individual peak responses at wavelengths between 300 and 320 nm.
References
- E. L. Speight and P. M. Farr, Erythemal and therapeutic response of psoriasis to PUVA using high-dose UVA, Br. J. Dermatol., 1994, 131(5), 667–672 CrossRef CAS.
- R. J. Turner, D. Walshaw, B. L. Diffey and P. M. Farr, A controlled study of ultraviolet A sunbed treatment of psoriasis, Br. J. Dermatol., 2000, 143(5), 957–963 CrossRef CAS.
- S. Das, J. J. Lloyd, D. Walshaw, B. L. Diffey and P. M. Farr, Response of psoriasis to sunbed treatment: comparison of conventional ultraviolet a lamps with new higher ultraviolet B-emitting lamps, Br. J. Dermatol., 2002, 147(5), 966–972 CrossRef CAS.
- R. S. Stern and E. J. Lunder, Risk of squamous cell carcinoma and methoxsalen (psoralen) and UV-A radiation (PUVA). A meta-analysis, Arch. Dermatol., 1998, 134(12), 1582–1585 CAS.
- T. R. Coven, L. H. Burack, R. Gilleaudeau, M. Keogh, M. Ozawa and J. G. Krueger, Narrowband UV-B produces superior clinical and histopathological resolution of moderate-to-severe psoriasis in patients compared with broadband UV-B, Arch. Dermatol., 1997, 133(12), 1514–1522 CAS.
- P. M. Gordon, B. L. Diffey, J. N. Matthews and P. M. Farr, A randomized comparison of narrow-band TL-01 phototherapy and PUVA photochemotherapy for psoriasis, J. Am. Acad. Dermatol., 1999, 41(5), 728–732 CrossRef CAS.
- S. M. Kirke, S. Lowder, J. J. Lloyd, B. L. Diffey, J. N. Matthews and P. M. Farr, A randomized comparison of selective broadband UVB and narrowband UVB in the treatment of psoriasis, J. Invest. Dermatol., 2007, 127(7), 1641–1646 CAS.
- P. Asawanonda, R. R. Anderson, Y. Chang and C. R. Taylor, 308 nm excimer laser for the treatment of psoriasis: a dose-response study, Arch. Dermatol., 2000, 136(5), 619–624 CAS.
- S. R. Feldman, B. G. Mellen, T. S. Housman, R. E. Fitzpatrick, R. G. Geronemus and P. M. Friedman, et al., Efficacy of the 308 nm excimer laser for treatment of psoriasis: results of a multicenter study, J. Am. Acad. Dermatol., 2002, 46(6), 900–906 CrossRef.
- W. Gerber, B. Arheilger, T. A. Ha, J. Hermann and H. M. Ockenfels, Ultraviolet B 308 nm excimer laser treatment of psoriasis: a new phototherapeutic approach, Br. J. Dermatol., 2003, 149(6), 1250–1258 CrossRef CAS.
- K. Kollner, M. B. Wimmershoff, C. Hintz, M. Landthaler and U. Hohenleutner, Comparison of the 308 nm excimer laser and a 308 nm excimer lamp with 311 nm narrowband ultraviolet B in the treatment of psoriasis, Br. J. Dermatol., 2005, 152(4), 750–754 CrossRef CAS.
- J. A. Parrish and K. F. Jaenicke, Action spectrum for phototherapy of psoriasis, J. Invest. Dermatol., 1981, 76(5), 359–362 CrossRef CAS.
- S. C. Weatherhead, P. M. Farr, D. Jamieson, J. S. Hallinan, J. J. Lloyd and A. Wipat, et al., Keratinocyte apoptosis in epidermal remodeling and clearance of psoriasis induced by UV radiation, J. Invest. Dermatol., 2011, 131(9), 1916–1926 CrossRef CAS.
- I. Man, R. S. Dawe, J. Ferguson and S. H. Ibbotson, An intraindividual study of the characteristics of erythema induced by bath and oral methoxsalen photochemotherapy and narrowband ultraviolet B, Photochem. Photobiol., 2003, 78(1), 55–60 CrossRef CAS.
- P. M. Gordon, P. J. Saunders, B. L. Diffey and P. M. Farr, Phototesting prior to narrowband (TL-01) ultraviolet B phototherapy, Br. J. Dermatol., 1998, 139(5), 811–814 CrossRef CAS.
- A. F. McKinlay and B. L. Diffey, A reference action spectrum for ultra-violet induced erythema in human skin. in Human Exposure to Ultraviolet Radiation: Risks and Regulations, 1987, pp. 83–87 Search PubMed.
- B. L. Diffey, Observed and predicted minimal erythema doses: a comparative study, Photochem. Photobiol., 1994, 60(4), 380–382 CrossRef CAS.
- A. R. Webb, H. Slaper, P. Koepke and A. W. Schmalwieser, Know your standard: clarifying the CIE erythema action spectrum, Photochem. Photobiol., 2011, 87(2), 483–486 CrossRef CAS.
- P. M. Farr and B. L. Diffey, The erythemal response of human skin to ultraviolet radiation, Br. J. Dermatol., 1985, 113(1), 65–76 CrossRef CAS.
- R. B. Setlow, The wavelengths in sunlight effective in producing skin cancer: a theoretical analysis, Proc. Natl. Acad. Sci. U. S. A., 1974, 71(9), 3363–3366 CrossRef CAS.
- A. R. Young, C. A. Chadwick, G. I. Harrison, O. Nikaido, J. Ramsden and C. S. Potten, The similarity of action spectra for thymine dimers in human epidermis and erythema suggests that DNA is the chromophore for erythema, J. Invest. Dermatol., 1998, 111(6), 982–988 CrossRef CAS.
- S. L. Walker and A. R. Young, An action spectrum (290–320 nm) for TNFalpha protein in human skin in vivo suggests that basal-layer epidermal DNA is the chromophore, Proc. Natl. Acad. Sci. U. S. A., 2007, 104(48), 19051–19054 CrossRef CAS.
- F. R. de Gruijl and J. C. van der Leun, Estimate of the wavelength dependency of ultraviolet carcinogenesis in humans and its relevance to the risk assessment of a stratospheric ozone depletion, Health Phys., 1994, 67(4), 319–325 CrossRef CAS.
- D. L. Damian, Y. J. Matthews, T. A. Phan and G. M. Halliday, An action spectrum for ultraviolet radiation-induced immunosuppression in humans, Br. J. Dermatol., 2011, 164(3), 657–659 CAS.
- L. D. Sun, W. Li, S. Yang, X. Fan, K. L. Yan and Y. H. Liang, et al., Evidence for a novel psoriasis susceptibility locus at 9q33–9q34 in Chinese Hans, J. Invest. Dermatol., 2007, 127(5), 1140–1144 CrossRef CAS.
- R. J. Flockhart, B. L. Diffey, P. M. Farr, J. Lloyd and N. J. Reynolds, NFAT regulates induction of COX-2 and apoptosis of keratinocytes in response to ultraviolet radiation exposure, FASEB J., 2008, 22(12), 4218–4227 CrossRef CAS.
- J. K. Wagner, E. J. Parra, L. Norton H, C. Jovel and M. D. Shriver, Skin responses to ultraviolet radiation: effects of constitutive pigmentation, sex, and ancestry, Pigm. Cell Res., 2002, 15(5), 385–390 CrossRef.
- T. Ha, H. Javedan, K. Waterston, L. Naysmith and J. L. Rees, The relationship between constitutive pigmentation and sensitivity to ultraviolet radiation induced erythema is dose-dependent, Pigm. Cell Res., 2003, 16(5), 477–479 CrossRef.
- B. Bianchi, P. Campolmi, L. Mavilia, A. Danesi, R. Rossi and P. Cappugi, Monochromatic excimer light (308 nm): an immunohistochemical study of cutaneous T cells and apoptosis-related molecules in psoriasis, J. Eur. Acad. Dermatol. Venereol., 2003, 17(4), 408–413 CrossRef CAS.
- A. Menter, N. J. Korman, C. A. Elmets, S. R. Feldman, J. M. Gelfand and K. B. Gordon, et al., Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy, J. Am. Acad. Dermatol., 2010, 62(1), 114–135 CrossRef.
- S. M. Goldinger, R. Dummer, P. Schmid, M. Prinz Vavricka, G. Burg and S. Lauchli, Excimer laser versus narrow-band UVB (311 nm) in the treatment of psoriasis vulgaris, Dermatology, 2006, 213(2), 134–139 CrossRef.
- M. Meinhardt, R. Krebs, A. Anders, U. Heinrich and H. Tronnier, Absorption spectra of human skin in vivo in the ultraviolet wavelength range measured by optoacoustics, Photochem. Photobiol., 2009, 85(1), 70–77 CrossRef CAS.
- R. Takasawa, H. Nakamura, T. Mori and S. Tanuma, Differential apoptotic pathways in human keratinocyte HaCaT cells exposed to UVB and UVC, Apoptosis, 2005, 10(5), 1121–1130 CrossRef CAS.
- K. Storbeck, E. Holzle, N. Schurer, P. Lehmann and G. Plewig, Narrow-band UVB (311 nm) versus conventional broad-band UVB with and without dithranol in phototherapy for psoriasis, J. Am. Acad. Dermatol., 1993, 28(2), 227–231 CrossRef CAS.
- H. van Weelden, E. Young and J. C. van der Leun, Therapy of psoriasis: comparison of photochemotherapy and several variants of phototherapy, Br. J. Dermatol., 1980, 103(1), 1–9 CrossRef CAS.
- I. B. Walters, L. H. Burack, T. R. Coven, P. Gilleaudeau and J. G. Krueger, Suberythemogenic narrow-band UVB is markedly more effective than conventional UVB in treatment of psoriasis vulgaris, J. Am. Acad. Dermatol., 1999, 40(6), 893–900 CrossRef CAS.
- M. Weischer, A. Blum, F. Eberhard, M. Rocken and M. Berneburg, No evidence for increased skin cancer risk in psoriasis patients treated with broadband or narrowband UVB phototherapy: a first retrospective study, Acta Derm.-Venereol., 2004, 84(5), 370–374 CrossRef.
- A. Young, Carcinogenicity of UVB phototherapy assessed, Lancet, 1995, 345(8962), 1431–1432 CrossRef.
- P. M. Farr, J. E. Besag and B. L. Diffey, The time course of UVB and UVC erythema, J. Invest. Dermatol., 1988, 91(5), 454–457 CrossRef CAS.
- B. L. Diffey, P. M. Farr and A. M. Oakley, Quantitative studies on UVA-induced erythema in human skin, Br. J. Dermatol., 1987, 117(1), 57–66 CrossRef CAS.
Footnote |
| † This article is published as part of a themed issue on current topics in photodermatology. |
| This journal is © The Royal Society of Chemistry and Owner Societies 2013 |
