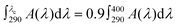The evolution of sunscreen products in the United States – a 12-year cross sectional study†
Steven Q.
Wang‡
*a,
Paul R.
Tanner
b,
Henry W.
Lim
c and
J. F.
Nash
b
aMemorial Sloan-Kettering Cancer Center, New York, NY, USA. E-mail: wangs@mskcc.org; Tel: +1 908-542-3400
bThe Procter & Gamble Company, Sharon Woods Technical Center, Cincinnati, OH, USA
cDepartment of Dermatology, Henry Ford Hospital, Detroit, MI, USA
First published on 21st September 2012
Abstract
Excessive exposure from ultraviolet (UV) radiation contributes to the development of skin cancers and photoaging. Topical sunscreen products remain one of the most widely used forms of protection for the majority of the public. The objective of this analysis was to examine photoprotection trends (e.g., SPF value) and the degree of UVA I protection from 1997 to 2009 in the United States. Sunscreen products purchased and evaluated in 1997 (N = 59), 2003 (N = 188) and again in 2009 (N = 330), totaling 577, were included in this analysis. Information regarding (1) the SPF value, (2) name and concentration of the active ingredients, (3) type of products (i.e., daily vs. recreational/beach), and (4) claims of UVA protection was recorded and analyzed. In addition, the critical wavelength (CW) of 330 products from 2009 was determined. The results showed an increase in the SPF values of products from 1997 to 2009. The percentage of low SPF products (SPF 4–14) decreased from 27% in 1997 to 6% in 2009. The number of products containing a known UVA-I filter (i.e., avobenzone or zinc oxide) increased from 5% in 1997 to 70% in 2009. Lastly, approximately, 225 (68%) of the products tested in 2009 attained CW > 370 nm. In the past decade, sunscreen products have undergone fundamental improvements, the most significant of which is the breadth of protection against UVA I.
Introduction
Ultraviolet (UV) radiation from the sun and artificial radiation sources plays a major role in the development of skin cancers, acceleration of photoaging, and exacerbation of photodermatoses. Photoprotection strategies to prevent excessive solar UV exposure include seeking shade, wearing protective clothing, hats and sunglasses, and applying sunscreens. However, in the United States and Europe, the application of sunscreen is the predominant form of photoprotective behavior practiced by the majority of the public.1Modern-day sunscreens have been shown to reduce the development of actinic keratosis,2 decrease the number of nevi in children,3,4 prevent the development of squamous cell carcinoma5,6 and melanoma,7 and reduce signs of photoaging.8,9 Historically, the first generation of sunscreens had a very simple purpose. These low Sun Protection Factor or SPF products (<SPF 8) were developed to prevent sunburn. Through the ensuing decades of collaboration among the medical professionals, chemists, and formulators, each subsequent generation of sunscreens has delivered higher photoprotection largely weighted in UVB (290–320 nm)/UVA II (320–340 nm) leading to increased SPF values of products. Protection against UVA I (340–400 nm) started to be addressed with the availability of dibenzoylmethane derivates in 1979, titanium dioxide in 1989, and zinc oxide in 1992.10 However, the degree of UVA protection varied significantly among products in the market, often based on the methods used to assess efficacy, or the definition of such protection. Many products claimed to have broad spectrum coverage but offered minimal UVA I protection.11,12 In June 2011, the FDA addressed this issue and published the much anticipated final rule on testing and labeling sunscreen products.8,13
In this study, we analyzed a total of 577 sunscreens marketed in the United States from 1997 to 2009, prior to the release of the 2011 FDA regulation regarding sunscreen product ingredients and labeling.8 We also examined the trend of photoprotection (i.e., SPF value), efficacy claims and the degree of UVA I protection over this 12-year period.
Methods
Sunscreen survey
Commercial sunscreens were purchased from retail stores in Cincinnati, Ohio in 1997, 2003, and 2009. At the different times of purchase (i.e., 1997, 2003 and 2009), a similar approach was used in selecting sunscreen products. The sampling was done in the spring of each year from “mass” (i.e., drugstore, supermarket, and wholesale clubs) and “prestige” (i.e., department store) retail stores. The sampling was designed to cover the breadth of product types in daily and recreation/beach sunscreen categories. Daily sunscreens are defined as products (e.g., moisturizers, color cosmetics, foundations, eye creams, and lip balms, etc.) that are intended to protect consumers from incidental sun exposure that occurs in typical daily activity (e.g., going to work, shopping, etc.) that results in exposure to incidental, low intensity UV radiation. Recreational/beach sunscreens are products designed to protect consumers from intentional, high intensity sun exposure during recreational activities. For each product, the following information was recorded: (1) the SPF value, (2) name and concentration of the active ingredients, (3) type of products (i.e., daily vs. recreational/beach), and (4) claims of UVA protection.In vitro assessment of UVA protection and calculation of the critical wavelength
Only sunscreens purchased in 2009 were tested for UVA protection based on the modified in vitro test method recently agreed by the International Organization for Standardization (ISO) based on the Cosmetics Europe method.14 The critical wavelength (CW) statistic was calculated from the absorption curves generated from this method. Products were evaluated within one year of purchase and before their expiration date.Each sunscreen product was applied uniformly to the roughened side of a 50 × 50 mm HELIOPLATE© HD 6, i.e., polymethylmethacrylate or PMMA plates (HelioScreen Labs, France) with a pre-saturated finger cot. The amount of sunscreen product applied was 1.3 mg cm−2. Following application to the PMMA plate, the product film was allowed to dry under ambient conditions (22 ± 2 °C) for 15 minutes.
The UV transmittance of each product was measured using a Labsphere UV-2000S UV Transmittance Analyzer (Labsphere Inc., North Sutton, NH). Reference measurements were performed using the Helioplate© HD6 substrate coated with mineral oil. Mineral oil was applied to reduce scattering and hence the variability associated with transmission measurements of the “roughened” substrate. UV transmittance of the product film was measured at six different sites on the HD6 substrate. For each sample, three independent replicate product films were prepared and evaluated. The measurement performed on the product sample was corrected for the untreated reference.
The average (i.e., 3 replicates) UV transmittance values for each product were taken for the analysis. The CW was calculated using the following equation:
where A is absorption and λ wavelength. For each absorption spectrum, the integral, which represents the area-under-the curve, was estimated using trapezoidal integration.
In general, the in vitro method conducted was in the “spirit” of the method recommended in the 2011 FDA guideline.8 The key differences were: (1) the in vitro measurement was determined without pre-irradiation in this current study, and (2) the sunscreen application dose (1.3 mg cm−2), consistent with the ISO standard, was applied to the PMMA plates, while the 2011 FDA final rule stipulated an application dose to be 0.75 mg cm−2.
Results
Sunscreen survey analysis
A total of 59 sunscreen products were evaluated in 1997, 188 in 2003 and 330 in 2009. In the 1997 survey, there were 11 (19%) daily and 48 (81%) recreational products. In 2003, there were 70 (37%) daily and 118 (63%) recreational products. In 2009, there were 189 (57%) daily and 141 (43%) recreational products.The SPF values of the surveyed products for all three years are shown in Table 1. In general, the most significant change over the 12-year study period is the apparent reduction in the number of “low” SPF products (i.e., SPF 4–14) and the addition of products with SPF > 50. In this sampling survey, the percentage of low SPF products decreased from 27% in 1997 to 6% in 2009. The SPF values of the products are almost equally distributed between SPF 15–29 and 30–50, with slightly more products in the SPF 15–29 range.
| Percentage of total number of products surveyed | |||
|---|---|---|---|
| SPF range | 1997 | 2003 | 2009 |
| 4 to 14 | 27% | 10% | 6% |
| 15 to 29 | 37% | 50% | 44% |
| 30 to 50 | 36% | 40% | 40% |
| 50+ | 10% | ||
The number of products claiming UVA protection and those containing a UVA I (340–400 nm) filter are shown in Table 2. More than 80% of the products claim to have UVA protection (e.g., broad spectrum, UVA/UVB), and this percentage has remained constant over the 12-year study period. However, the number of products actually containing a known UVA I filter (i.e., avobenzone or zinc oxide) has increased from 5% in 1997 to 70% in 2009.
| Percentage of total number of products surveyed | |||
|---|---|---|---|
| 1997 | 2003 | 2009 | |
| a Zinc oxide or avobenzone. | |||
| UVA claim | 81% | 82% | 80% |
| Contain UVA-I filtersa | 5% | 56% | 70% |
Detailed analysis showing the degree of UVA I protection is presented in Table 3. There was a steady rise in the percentage of products containing the two UVA I filters (i.e., avobenzone and ZnO). From 1997 to 2009, the percentage of products containing ZnO and avobenzone increased from 3% to 16%, and 2% to 54%, respectively. In addition, the percentage of products containing both avobenzone and octocrylene, a combination that is known to enhance the UVA photostability, increased from 0 to 36% over the 12 year period. However, nearly 20% of the products obtained in 2003 and 2009 contained avobenzone and octinoxiate (also known as octylmethoxy cinnamate), a combination that accelerates the photodegradation of both UV filters.
| 1997 Survey | 2003 Survey | 2009 Survey | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Daily | Recreational | Total | Daily | Recreational | Total | Daily | Recreational | Total | ||
| Products | Number | 11 | 48 | 59 | 70 | 118 | 188 | 189 | 141 | 330 |
| Percent | 19% | 81% | 100% | 37% | 63% | 100% | 57% | 43% | 100% | |
| UVA active use (frequency) | Zinc oxide | 18% | 0% | 3% | 14% | 11% | 12% | 16% | 16% | 16% |
| Avobenzone | 0% | 2% | 2% | 31% | 27% | 29% | 43% | 67% | 54% | |
| UV active combinations (frequency) | Avobenzone and octocrylene | 0% | 0% | 0% | 7% | 12% | 10% | 23% | 54% | 36% |
| Avobenzone and octinoxate | 0% | 2% | 2% | 24% | 17% | 20% | 23% | 13% | 19% | |
In vitro assessment of UVA protection: critical wavelength (CW)
A total of 330 sunscreen products purchased in 2009 were analyzed to determine the CW. The results are shown in Table 4. Approximately, 225 (68%) of the products attained CW > 370 nm, and 105 (32%) had CW < 370 nm. All products with CW > 370 nm contained either nano ZnO or avobenzone. Products with CW < 370 nm either did not have these filters or their concentration was not enough for the SPF of the product. In the FDA monograph published in 2011,8 the threshold for meeting the minimal UVA requirement was set at products with CW > 370 nm, as highlighted in Table 4.| Critical wavelength | Percentage of products surveyed in 2009 |
|---|---|
| <325 nm | 0% |
| 325 to 334 nm | 1% |
| 335 to 349 nm | 8% |
| 350 to 369 nm | 23% |
| ≥370 nm | 68% |
Discussion
Sunscreen application has long been recognized as an important tool in photoprotection, mainly because it is the most common strategy used by US consumers to reduce excessive solar UV exposure. Protection against both UVB and UVA is critical, as both UV spectra contribute to the development of skin cancers and acceleration of skin aging. Furthermore, the SPF value and the broad spectrum status are two critical elements that influence consumers when choosing sunscreen products.15 In the present analysis, results from informal surveys of commercial sunscreen products in the United States conducted between 1997 and 2009 were evaluated with attention to the trend of SPF label values and the inclusion of ingredients that provide long wavelength UVA I protection. In addition, the CW of 330 products in 2009 was determined to quantify the percentage of “broad spectrum” products in this sample.In terms of SPF values, there were two major trends observed in this 12-year study period. First, there was a decrease in the number of low SPF (i.e., SPF 4–14) products from 1997 to 2009. Second, as of 2009, more products with SPF > 50 appeared on the market. While there is controversy and data are lacking, this shift is considered by some as a trend that can ensure improved UV protection for the public. It is hypothesized that low SPF products may not deliver adequate protection, especially considering that studies have reported individuals use less than half of the optimal amount of such products.16 As a result of improper application, the in-use SPF of some products is approximately 1/3 of the labeled SPF.17 Furthermore, some behavior studies have suggested that individuals tend to stay in the sun and receive more UV exposure when they use sunscreens.18 Therefore, if inadequate product is applied and people stay longer in sunling, it follow that wearing low SPF products may not provide adequate UV protection.
The generalization that sunscreen products are not properly applied may be a convenient oversimplification. For any topically applied product, dose is determined by amount applied and frequency of application. Most studies with sunscreen application have only focused on amount of product applied. It is further complicated by the fact that compliance, i.e., the amount of product applied and frequency of application, is dependent on the aesthetics of the product.19 For recreational/beach sunscreen products, made to withstand water and other environmental challenges, the formulations are, by necessity, often less than aesthetically pleasing; this results in a tendency for consumers to apply inadequate amount, and/or re-apply only infrequently. Such challenges in product aesthetics are amplified when organic UV filters are added at high concentrations, i.e., >20% w/v, to achieve SPFs beyond 50, 70 or 100.
Instead of producing sunscreens with exceedingly high SPF values that may be less aesthetically pleasing, sunscreen manufacturers may consider an alternative direction. Specifically, manufacturers may wish to consider developing products with SPF 15–30 and appealing sensory properties. These products have the potential to attract more consumers who apply larger amounts of the product more frequently. Moreover, the population health benefits associated with daily application of an SPF 16 sunscreen is well documented by Green and colleagues.5–7 It is noteworthy to mention that the sunscreens used in the studies of Green et al. contained 2% avobenzone and 8% octinoxiate, an unstable combination that leads to photodegradation. Given these data are the most convincing and compelling evidence of the benefit of regular sunscreen application in prevention of skin cancers, it stands to reason that some reassessment of the trend toward promoting higher and higher SPF products be given practical thought. This may also be part of the rationale for the FDA to use SPF 15 as the dividing point at which sunscreen products that are broad spectrum (i.e., critical wavelength ≥ 370 nm) may put on the label a statement that its use, along with other photoprotective measures, would “decrease the risk of skin cancer and early skin aging caused by the sun”.8
As of 2009, there were only 10% of the surveyed products with SPF 50+. Currently, the FDA has proposed to limit the maximum labeled SPF to “50+,”13 which has the potential to discourage manufacturers from producing products with exceedingly high SPF values, i.e., SPF > 60. The ruling to “cap” SPF has not been finalized, and the FDA has encouraged interested parties to submit data to substantiate the benefit of having such high SPF products.
Since the early 1990s, there has been a greater understanding of the biological harm produced by exposure to UVA. It is generally agreed that UVA penetrates deeper into the skin, passes through many types of glass windows and interacts with endogenous and exogenous photosensitizers to generate reactive oxygen species (ROS). These ROS damage DNA bases, lipid membranes and cellular proteins. Clinically, UVA exposure contributes to photocarcinogenesis and aged appearance20 by increasing wrinkles, fragility and dyspigmentations.9
The recognition of the importance of UVA protection is observed as early as 1997, as reflected in the survey data showing 81% of sunscreens in that year claimed to provide UVA protection. The percentage of products with UVA claims did not change over the 12-year studied period. Our study highlighted the discrepancy between UVA claims and actual protection over the UVA II (320–340 nm) and UVA I (340–400 nm) spectra. This difference is especially pronounced in 1997 when only 5% of the products contained either ZnO or avobenzone, two filters known to have protection in UVA I range. In our analysis, titanium dioxide and oxybenzone were excluded as long wavelength UVA filters, because both absorb predominantly in the short UVA range. Oxybenzone is a UVB and short UVA II filter. Titanium dioxide, used in sunscreens as “micronized” or “nano” particles, has resulted in a dual effect: (1) increasing the attenuation of short wavelengths of UV, i.e., UVB, thereby improving its contribution to SPF, and (2) reducing attenuation, i.e., absorption, reflection and scattering, in longer UVA and visible (blue) light which has improved the consumer acceptance of products containing TiO2,21 but limited its UVA I efficacy.
The discrepancy between UVA claims and the presence of UVA I filters may be attributed to the limited availability of such filters and the absence of criteria for claiming UVA protection. Avobenzone and ZnO were approved by FDA in late 1997. In the ensuing years, more sunscreen products have been formulated with these two UVA I filters, thereby closing the gap between UVA claims and full or broad spectrum UVA protection. In 2009, 80% of products claimed UVA protection, and 70% contained UVA I filter(s). This change was largely due to market pressure instead of regulatory enforcement, because there was no FDA final rule on testing and labeling of sunscreens at that time. Thereafter, the regulatory action approving the use of avobenzone22 and ZnO23 had a pronounced impact on sunscreen products even without a mandated method or label.
The FDA resolved the lack of UVA measurement regulations in June 2011 by publishing the final ruling on testing and labeling of sunscreens.8 It adopted a pass/fail test using the in vitro critical wavelength as the only method in assessing UVA protection. The FDA specified that only products with CW > 370 nm can be labeled as “broad spectrum.” Furthermore, products that pass the CW test and have SPF > 15 can display the claim “if used as directed with other sun protection measures, (sunscreen) decrease the risk of skin cancer and early skin aging caused by the sun.” This new claim assertion should also motivate companies to develop and market products that meet the “broad spectrum” criteria (i.e., CW > 370 nm). At the time of this writing, implementation date for the final rule is December 2012.
Based on results of this study, we noted that 255 (68%) of the sunscreens analyzed in 2009 had CW > 370 nm, a criterion set forth in the FDA 2011 final rule. These products would be allowed to claim “broad spectrum” protection. However, it is noteworthy that, in our analysis, the sunscreen samples were not pre-irradiated to assess photostability. Therefore, there is reason to suspect that the actual number of products that meet this CW > 370 nm would be lower. With the full enforcement of the FDA regulation on product testing and labeling to take effect in December 2012, it is very likely that the percentage of products with CW > 370 nm will increase.
There are several limitations of the present analysis that are important. First, this was not a prospective study. Second, the sampling procedure used to purchase sunscreen products, while similar for the three years in which products were obtained, was not established statistically which explains the absence of direct comparisons. Finally, the number of sunscreen products purchased in 1997, 2003 and 2009 was different. This is not a true reflection of product availability but rather a difference in sampling. The selection of products was, in principle, similar but obviously not identical among 1997, 2003 and 2007. Likewise the absolute number of “daily” and “beach/recreation” products is not a true reflection in the ratio of these product forms. Therefore the trends identified in this analysis, e.g., reduction in the number of low SPF products, should be considered with these constraints in mind.
Conclusions
The cross-sectional analysis of a sample of sunscreens purchased in the US from 1997 to 2009 demonstrated an overall improvement in the efficacy of UV protection. Current sunscreens offer higher SPF values, and a significant portion of products offer broad spectrum coverage that would meet the FDA final testing ruling. Advancements seen sunscreens are closely linked to the FDA regulation. Specifically, in the case of UVA protection, we observed that UVA protection increased significantly in many sunscreen products after the approval of zinc oxide and avobenzone as UV filters by the FDA. It is expected that the 2011 FDA ruling on testing and labeling of sunscreens will further elevate the overall efficacy of UV protection. However, there are still many unresolved issues (e.g., SPF cap, dosage forms, and approval of novel UV filters) waiting for the final decision from the FDA. The impact of these changes will certainly alter the landscape of sunscreens in the US.References
- I. Nicol, C. Gaudy, J. Gouvernet, M. A. Richard and J. J. Grob, Skin protection by sunscreens is improved by explicit labeling and providing free sunscreen, J. Invest. Dermatol., 2007, 127, 41–48 CrossRef CAS.
- S. Darlington, G. Williams, R. Neale, C. Frost and A. Green, A randomized controlled trial to assess sunscreen application and beta carotene supplementation in the prevention of solar keratoses, Arch. Dermatol., 2003, 139, 451–455 Search PubMed.
- R. P. Gallagher, J. K. Rivers, T. K. Lee, C. D. Bajdik, D. I. McLean and A. J. Coldman, Broad-spectrum sunscreen use and the development of new nevi in white children: a randomized controlled trial, JAMA, 2000, 283, 2955–2960 CrossRef CAS.
- T. K. Lee, J. K. Rivers and R. P. Gallagher, Site-specific protective effect of broad-spectrum sunscreen on nevus development among white schoolchildren in a randomized trial, J. Am. Acad. Dermatol., 2005, 52, 786–792 CrossRef.
- A. Green, G. Williams, R. Neale, V. Hart, D. Leslie and P. Parsons, et al., Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial, Lancet, 1999, 354, 723–729 CrossRef CAS.
- J. C. van der Pols, G. M. Williams, N. Pandeya, V. Logan and A. C. Green, Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use, Cancer Epidemiol. Biomarkers Prev., 2006, 15, 2546–2548 Search PubMed.
- A. C. Green, G. M. Williams, V. Logan and G. M. Strutton, Reduced melanoma after regular sunscreen use: randomized trial follow-up, J. Clin. Oncol., 2011, 29, 257–263 CrossRef CAS.
- Food and Drug Administration, Labeling and effectiveness testing, Sunscreen drug products for over-the-counter human use http://www.gpo.gov/fdsys/pkg/FR-2011-06-17/pdf/2011-14766.pdf, June 17, 2011.
- J. H. Rabe, A. J. Mamelak, P. J. McElgunn, W. L. Morison and D. N. Sauder, Photoaging: mechanisms and repair, J. Am. Acad. Dermatol., 2006, 55, 1–19 CrossRef.
- R. Roelandts, History of photoprotection, in Clinical Guide to Sunscreens and Photoprotection, ed. H. Lim and Z. D. Draelos, Informa Healthcare, 2009, p. 1–11 Search PubMed.
- S. Q. Wang, J. W. Stanfield and U. Osterwalder, In vitro assessments of UVA protection by popular sunscreens available in the United States, J. Am. Acad. Dermatol., 2008, 59, 934–942 CrossRef.
- S. Q. Wang, J. M. Goulart and H. W. Lim, Lack of UV-A protection in daily moisturizing creams, Arch. Dermatol., 2011, 147, 618–620 Search PubMed.
- S. Q. Wang and H. W. Lim, Current status of the sunscreen regulation in the United States: 2011 Food and Drug Administration's final rule on labeling and effectiveness testing, J. Am. Acad. Dermatol., 2011, 65, 863–869 CrossRef.
- P. J. Matts, V. Alard, M. W. Brown, L. Ferrero, H. Gers-Barlag and N. Issachar, et al., The COLIPA in vitro UVA method: a standard and reproducible measure of sunscreen UVA protection, Int. J. Cosmet. Sci., 2010, 32, 35–46 CrossRef CAS.
- S. Q. Wang and S. W. Dusza, Assessment of sunscreen knowledge: a pilot survey, Br. J. Dermatol., 2009, 161(Suppl 3), 28–32 CrossRef.
- P. Autier, M. Boniol, G. Severi and J. F. Dore, Quantity of sunscreen used by European students, Br. J. Dermatol., 2001, 144, 288–291 CrossRef CAS.
- A. Faurschou and H. C. Wulf, The relation between sun protection factor and amount of sunscreen applied in vivo, Br. J. Dermatol., 2007, 156, 716–719 CrossRef CAS.
- E. Thieden, P. A. Philipsen, J. Sandby-Moller and H. C. Wulf, Sunscreen use related to UV exposure, age, sex, and occupation based on personal dosimeter readings and sun-exposure behavior diaries, Arch. Dermatol., 2005, 141, 967–973 Search PubMed.
- G. Watkins, The importance of incorporating aesthetics into topical formulations, Drug Delivery Technol., 2010, 10, 51–54 CAS.
- J. R. Gordon and J. C. Brieva, Images in clinical medicine. Unilateral dermatoheliosis, New England J. Med., 2012, 366, e25 CrossRef.
- M. Anderson, J. Hewitt and S. Spruce, Broad-spectrum physical sunscreens: titanium dioxide and zinc oxide, in Sunscreens: Development, Evaluation, and Regulatory Aspects, ed. N. Lowe, N. Shaath and M. Pathak, Marcel Dekker Inc, New York, 1997, pp. 353–399 Search PubMed.
- Food and Drug Administration, Sunscreen drug products for over-the-counter human use, Marketing Status of Products Containing Avobenzone, Enforcement Policy, http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/Over-the-CounterOTCDrugs/StatusofOTCRulemakings/ucm090435.pdf, April 30, 1997.
- Food and Drug Administration, Health and Human Services. Sunscreen drug products for over-the-counter human use, final monograph, Final rule, Fed Regist, http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/Over-the-CounterOTCDrugs/StatusofOTCRulemakings/ucm090244.pdf, May 21, 1999.
Footnotes |
| † This article is published as part of a themed issue on current topics in photodermatology. |
| ‡ Present address: Dermatology Service, Memorial Sloan-Kettering Cancer Center, 136 Mountain View Blvd, Basking Ridge, NJ 07920. |
| This journal is © The Royal Society of Chemistry and Owner Societies 2013 |