Abstract
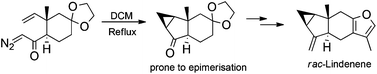
The first synthesis of the sesquiterpene Lindenene is described. A novel non-catalysed intramolecular cyclopropanation reaction between a diazoketone and an unactivated alkene was utilised to construct the relatively labile ketone precursor with complete stereocontrol. This ketone was transformed in three steps into Lindenene.

 Please wait while we load your content...
Please wait while we load your content...