Di- and triheteroarylalkanes via self-condensation and intramolecular Friedel–Crafts type reaction of heteroaryl alcohols†
Abstract
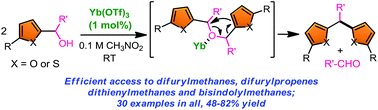
An efficient synthetic approach to diheteroarylmethanes and 1,3-diheteroarylpropenes has been developed via Yb(III)-catalyzed sequential self-condensation of 2-furfuryl (or 2-thienyl or 3-indolyl) alcohols followed by intramolecular Friedel–Crafts type reaction and elimination of an aldehyde. This method offers a powerful entry and a potential alternative to the traditional synthesis of diheteroarylalkanes, which are precursors to the synthesis of several intriguing heteroaryls and more significantly, to the synthesis of biofuels.

 Please wait while we load your content...
Please wait while we load your content...