Fluorescent labeling and tracking of nanoclay†‡
Carlos A.
Diaz§¶
a,
Yining
Xia¶
a,
Maria
Rubino¶
*a,
Rafael
Auras¶
*a,
Krishnamurthy
Jayaraman
b and
Joseph
Hotchkiss
a
aSchool of Packaging, Michigan State University, East Lansing, Michigan 48824, USA
bChemical Engineering and Materials Science, Michigan State University, East Lansing, Michigan 48824, USA. E-mail: mariar@msu.edu; aurasraf@msu.edu
First published on 8th November 2012
Abstract
We report a methodology developed to detect and track stable fluorescent-labeled nanoclay, in polymer–clay nanocomposite films, and in a contact solvent after migration testing. Fluorescein-5-maleimide (fluorescein) or tetramethylrhodamine-5-maleimide (rhodamine) was covalently bonded to organically modified montmorillonite (o-MMT). Fluorescein- and rhodamine-labeled nanoclay showed good thermal stability up to 220 °C and the rhodamine-labeled nanoclay remained stable at 250 °C. Confocal laser scanning microscopy was used to confirm the tagging and to detect the fluorescent-labeled nanoclays in various systems.
Engineered nanomaterial (ENM) production has expanded significantly in the last decade, with sales increasing from $0.4 billion (U.S.) in 2005 to $1.4 billion (U.S.) in 2010.1 Sales of nanocomposites, produced by the addition of ENMs to polymeric matrices, are estimated to reach $2.4 billion (U.S.) by 2016.2 As applications for ENMs continue to expand, there is increasing concern about the potential health and environmental risks associated with exposure to nanoparticles from ENMs. The nanoparticles, due to their small size, high surface area and surface reactivity, have the potential to induce cytotoxic effects3 as well as genotoxic effects, inflammation and even cancer.4 Currently, there is a lack of information to quantify exposure to ENMs and the associated concerns. The basic transport and fate of nanoparticles from nanocomposites when exposed to different conditions are not well understood, nor are their effects on biological systems and the environment.5–7 A research strategy report recently issued by the National Research Council in the U.S. stresses the need to assess the risk associated with exposure to ENMs, including modeling of the fate and transport of nanoparticles.5
Nanoclays, such as organically modified montmorillonite (o-MMT), are most widely used for nanocomposite applications in the packaging and automotive parts industries because of their natural abundance, high mechanical strength, and high aspect ratio.8,9 The good efficiency–cost balance of o-MMT as a nanofiller accounts for its use in about half of the entire nanocomposite market (approximately 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons in 2011).2 When o-MMT is compounded with polymers and exposed to moderate temperatures, these nanoparticles can move within the polymer matrix towards the surface and migrate to the surroundings. Non-diffusive mechanisms for nanoclay particle migration have been proposed to explain increases in the o-MMT content of nanocomposite surfaces during heating of polypropylene (PP)/o-MMT and nylon-6/o-MMT.10–14 The movement of nanoclay particles could also be modified by other factors such as interaction with different solvents and radiation. A better understanding of migration in nanocomposites is extremely important for determining the exposure dose, and this requires knowledge of the basic mass transport parameters of the nanoparticles.
000 metric tons in 2011).2 When o-MMT is compounded with polymers and exposed to moderate temperatures, these nanoparticles can move within the polymer matrix towards the surface and migrate to the surroundings. Non-diffusive mechanisms for nanoclay particle migration have been proposed to explain increases in the o-MMT content of nanocomposite surfaces during heating of polypropylene (PP)/o-MMT and nylon-6/o-MMT.10–14 The movement of nanoclay particles could also be modified by other factors such as interaction with different solvents and radiation. A better understanding of migration in nanocomposites is extremely important for determining the exposure dose, and this requires knowledge of the basic mass transport parameters of the nanoparticles.
Challenges in evaluating the transport and fate of ENMs from nanocomposites include the lack of tools and methodologies available to adequately track their movement and position.5,15 The current approaches for tracking and detecting nanoclays involve elemental analysis via atomic absorption spectrometry (AAS) or inductively coupled plasma mass spectroscopy (ICP-MS) to detect trace amounts of a specific element.16–18 However, these methods lack the ability to track single or clustered nanoclay particles and their positions, which hampers monitoring them in time, a key aspect in modeling the transport processes.
Fluorescent labeling is a promising approach for particle tracking due to its simplicity and inherently low detection limits.19 In nanocomposites, fluorescent labels have been used to monitor nanofiller homogeneity and to characterize colloidal stability in liquids and transport.20 Direct incorporation of a fluorescent organic dye into layered silicates like MMT can be accomplished by ionic exchange. This approach has been used to monitor the mixing and exfoliation processes during extrusion of polymer clay nanocomposites.21 However, the fluorescent component is not covalently bonded to the clay substrate and could be easily dislodged from the substrate during the extrusion process. We proposed that covalent attachment of the fluorescent tag to the nanoclay might provide stability to the bond between the fluorescent tag and the clay.
We report here a new methodology to detect and track stable fluorescent-labeled o-MMT in a polymer–clay nanocomposite after film manufacture and preliminary mass transport–migration testing. First, two fluorescent tags were selected that could form covalent bonds with o-MMT upon the functionalization of the nanoclay substrate. Second, the thermal stability of the fluorescent-labeled o-MMT was studied at high temperatures to simulate the melt-processing conditions used in polymer film manufacture. Third, the fluorescent-labeled o-MMT was incorporated into a model polymer (polypropylene) matrix; nanocomposite films were manufactured and the fluorescence of the films was detected. Finally, a preliminary mass transport–migration test was carried out by exposing the nanocomposite films to ethanol at 80 °C and evaluating the solvent for trace amounts of labeled o-MMT.
Labeling of the o-MMT was carried out in a two-step process as depicted in Fig. 1. In the silylation step (using an established procedure22,23) the hydroxyls on the edges of the nanoclay platelets were converted into thiol moieties in the presence of a mercaptosilane; the reaction was carried out in an aqueous methanol solution. In the conjugation step a thiol-reactive dye, either fluorescein-5-maleimide (fluorescein) or tetramethylrhodamine-5-maleimide (rhodamine), was reacted with the silane-treated nanoclay. The selection of the fluorescent tag was based on label photostability, high extinction coefficients and high fluorescence quantum yield. The thiol-reactive group (i.e., maleimide) allowed the reaction to be carried out at neutral pH with high selectivity and promoted the development of a covalent bond.
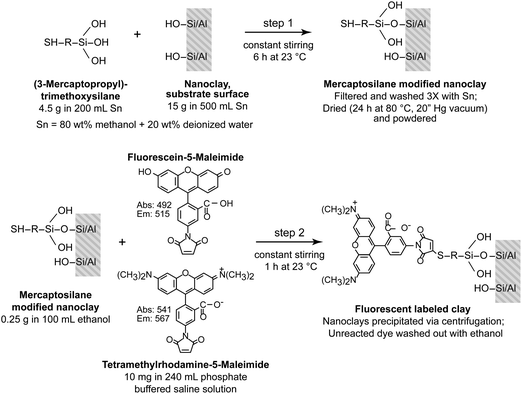 | ||
| Fig. 1 Schematic of the fluorescent-labeling procedure. Step 1: silane treatment of nanoclay to convert hydroxyl groups into thiol moieties. Step 2: fluorescent dye conjugation with silane-modified nanoclay. The nanoclay was labeled with either fluorescein-5-maleimide (fluorescein) or tetramethylrhodamine-5-maleimide (rhodamine) following the same procedure. | ||
Confocal laser scanning microscopy (CLSM) was used for the detection of fluorescent-labeled nanoclays because this technique has the ability to detect spatially resolved emission intensities and the capability to analyze emission intensity as a function of the z-position.24 Confocal images can yield quantitative information with an optical resolution approaching 200 nm25,26 and are well suited for detection of fluorescent particles and also to track movement in different media. New single-particle tracking (SPT) techniques that have been developed in the last decade may help detect nanoparticles having an aspect ratio smaller than that of o-MMT.26 Confocal microscopy-aided spectrophotometric analysis was used to characterize the emission spectra of the labeled samples. In Fig. 2, CLSM images of the rhodamine- and fluorescein-labeled nanoclays and unlabeled counterparts show a clear distinction between the labeled and unlabeled nanoclays. The fluorescence signals in red (Fig. 2a) and green (Fig. 2b) confirm the presence of rhodamine and fluorescein, respectively, attached to the nanoclay.
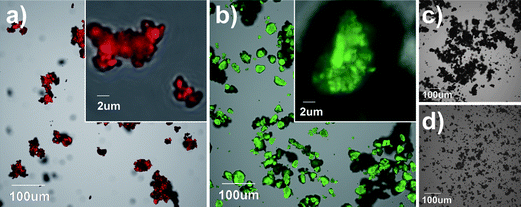 | ||
| Fig. 2 Confocal micrographs showing nanoclay and clustered nanoclays with fluorescent tags, (a) rhodamine and (b) fluorescein, and without fluorescent tags, (c) and (d), respectively. Micrographs (a and c) and (b and d) were taken using the same parameters. The images were acquired with an Olympus FluoView FV1000 confocal laser scanning microscope configured on a fully automated inverted IX81 microscope. The fluorescence was excited using 543 nm (a and c) and 488 nm (b and d) lasers. The red fluorescence signal was captured using a 560 long pass emission filter, and the green fluorescence signal was captured using a 505–525 nm band pass emission filter. The transmitted light image was generated in a brightfield mode. | ||
Before attempting to track the fluorescent-labeled nanoclays in polymer nanocomposites, it was important to understand the effect of polymer processing temperatures on the fluorescence intensity and stability. Processing of polymer systems involves melt mixing and forming at melting temperatures of 150–250 °C. High temperatures may prompt clay–dye and dye–dye interactions as well as changes in the microenvironment, which can result in modulations of the absorption and emission properties as well as fluorescence quenching.24 We exposed the labeled nanoclay powders to 220 and 250 °C for 15 min under a continuous purge of nitrogen (60 mL min−1). After heating, the samples were mounted on glass microscope slides for confocal analysis to determine the stability of the fluorescent labels as a function of temperature. Further studies are being carried out to determine the stability of the labeled clays under other conditions that may affect their fluorescence (i.e., air, oxygen and shear conditions).
Fig. 3 shows the emission spectra of the two labeled nanoclays before and after the exposure at 220 and 250 °C. The heated and unheated rhodamine-labeled nanoclay had similar emission patterns. For the rhodamine-labeled samples, exposure to 220 and 250 °C resulted in only a slight shift of the emission peaks towards a higher wavelength, with minimum changes in fluorescence intensity; the relative integral fluorescence emission (RIFE) was not significantly different at either exposure temperature. On the other hand, the emission patterns of the fluorescein-labeled nanoclay showed a significant increase in fluorescence intensity for those samples exposed to 220 °C and a complete drop at 250 °C. Raising the temperature likely affected the chemical structure of the fluorophore, causing fluorescence enhancement at 220 °C and quenching at 250 °C. This effect was quantified by the RIFE parameter, which increased from 1 to 3.6 at 220 °C and decreased to 0.34 at 250 °C. The results indicated that the fluorescein tag is more heat sensitive than the rhodamine tag. Both labels showed good thermal stability at 220 °C, but only the rhodamine label remained stable at 250 °C.
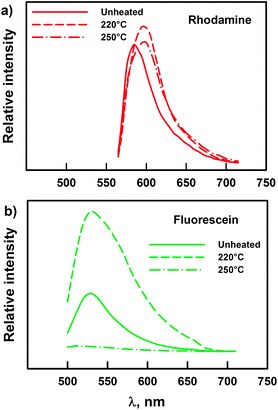 | ||
| Fig. 3 Fluorescence emission spectra of (a) rhodamine-labeled and (b) fluorescein-labeled nanoclay before and after exposure to 220 and 250 °C for 15 min. The excitation wavelengths were 543 nm (a) and 488 nm (b). Nanoclay samples were heated in a TGA furnace from room temperature and isothermally maintained at the set temperature for 15 min. Changes in emission spectra were quantified using the relative integral fluorescence emission (RIFE) parameter. For rhodamine: RIFEno heat = 1.00 ± 0.14A, RIFE220°C = 1.31 ± 0.35A, RIFE250°C = 1.35 ± 0.17A. For fluorescein: RIFEno heat = 1.00 ± 0.08A, RIFE220°C = 3.60 ± 0.58B, RIFE250°C = 0.34 ± 0.10C. The mean values with different uppercase superscripts are significantly different (p < 0.05) according to Tukey's HSD test. | ||
To prepare the polymer–clay nanocomposite, the fluorescent-labeled o-MMT was introduced in a polypropylene (PP) matrix through melt blending in an internal mixer. Films were made via compression molding from preblended nanocomposite granulates. PP and nanoclay are a good nanocomposite model system because both components are extensively used in consumer and non-consumer goods and packaging applications. This preparation technique can be applied to other common polymers such as nylon, poly(ethylene terephthalate) and poly(lactic acid). The PP was blended with 3 wt% nanoclay and 12 wt% compatibilizer (maleic anhydride modified polypropylene) to enhance dispersion of the nanoclay. Fig. 4 shows the morphology of the nanocomposite as observed by X-ray diffraction and transmission electron microscopy. Labeled and unlabeled nanocomposite films were prepared: the labeled nanocomposite films included 15 wt% of either fluorescein- or rhodamine-labeled o-MMT based on the total amount of clay (3 wt%).
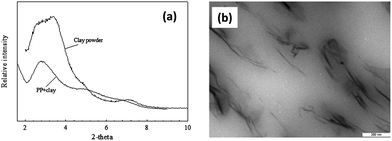 | ||
| Fig. 4 PP-clay nanocomposite characterization. (a) X-ray diffraction patterns for nanoclay and nanocomposite. (b) Transmission electron micrograph of nanocomposite showing intercalated and exfoliated structures. | ||
Confocal micrographs showing evidence of both fluorescent tags in the labeled nanoclays in the nanocomposite films are provided in Fig. 5 (see also Video S1 and S2‡). The emission intensity depends on the concentration of fluorophores; thus the bright particles indicated a cluster of fluorescent dye molecules attached to the nanoclay. If the dye molecules were to disassociate from the nanoclay, the dye would be more diluted and thus the emission intensity would be dimmer. The detection limit depends on the specific fluorophore (i.e., extinction coefficient and quantum yield) and the parameters in the confocal apparatus. A typical limit of detection of fluorescence in solution is in the order of 10−12 mol cm−2.24,27
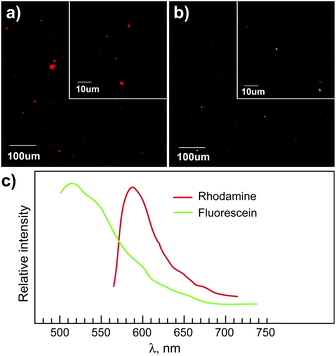 | ||
| Fig. 5 Confocal micrographs of (a) rhodamine-labeled and (b) fluorescein-labeled nanocomposite films (85 wt% PP, 3 wt% nanoclay, 12 wt% MAPP). Films of ∼100 μm thickness were prepared from melt-mixed pellets via compression molding (175 °C, 10 tons) using a Teflon mold. The mixing was performed on an internal mixer heated at 180 °C for 6 min at 80 rpm under nitrogen atmosphere. (c) Emission spectra of the bright particles in (a) and (b). | ||
The ability to track nanoparticles is critical for investigating the potential of the particles to migrate and/or transfer from polymer matrices into biological systems and the environment. We carried out a preliminary migration test to assess the ability to track the rhodamine- and fluorescein-labeled o-MMT from the polymer matrix of a nanocomposite film into a solvent, and the rhodamine tracking results are reported here. Before starting the migration test the films were thoroughly rinsed with ethanol (100%) to remove loose surface particles. Using a migration cell (ASTM D4754), the rhodamine-labeled nanocomposite films were then exposed to 100% ethanol at 80 °C for 4 h. Ethanol (100%) is alternatively used as a food simulant for high-alcoholic beverages or fatty foods, as recommended by the U.S. Food and Drug Administration.28 A temperature of 80 °C was chosen to represent a hot fill process or an accelerated shelf-life testing temperature that these film materials could be exposed to. After exposure, the ethanol was placed in a cuvette and allowed to evaporate to isolate any migrated precipitate for CLSM analysis. Fig. 6a compares sample cuvettes after evaporation of the solvent taken from the migration cell before the exposure test (Fig. 6a, left) and after the test (Fig. 6a, right). Residue was observed only in the latter cuvette and was attributed to nanocomposite components that migrated into the solvent. Confocal microscopy of the residue (Fig. 6b) showed well defined bright particles, which confirmed the migration of labeled o-MMT into the solvent. In addition, the emission spectrum of the particles (Fig. 6c) matched the characteristic spectrum from the rhodamine-labeled clay (Fig. 5c). These results demonstrated the ability of the methodology to track labeled o-MMT movement from one medium to another (from the nanocomposite into the simulant or solvent). Additional studies are being pursued to evaluate the free surface energy and the surface properties of the labeled nanoclays, and to determine whether modification of the clay due to the presence of the label has an impact on the diffusion behavior of that clay.
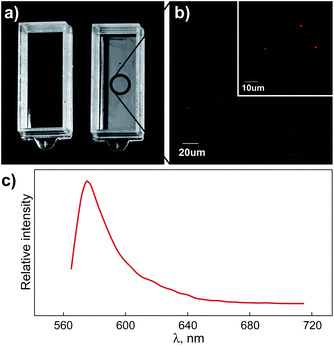 | ||
| Fig. 6 Detection of labeled nanoclay after the migration test. (a) The solvent (i.e., 100% ethanol) was placed in a cuvette before (a, left) and after (a, right) the migration test (80 °C, 4 h) and allowed to evaporate. (b) Confocal micrograph of the residue in the cuvette (a, right). (c) Emission spectrum of the bright particles in (b). | ||
In summary, a new method for the fluorescent labeling of nanoclays was developed that covalently attached fluorescein-5-maleimide (fluorescein) or tetramethylrhodamine-5-maleimide (rhodamine) to silane-treated o-MMT. The tagging was confirmed via CLSM. Both fluorescent labels showed good thermal stability up to 220 °C and the rhodamine label withstood 250 °C. After the labeled o-MMT was incorporated into a polypropylene matrix and nanocomposite films were extruded, the fluorescent labels were again detectable with CLSM. Preliminary migration testing with rhodamine-labeled o-MMT showed that some nanoclay migrated from the polymer matrix into the solvent (i.e., ethanol). The proposed methodology has the potential to track o-MMT and other nanoclays in various polymer nanocomposite systems and detect nanoparticle migration into solvents or possibly other surrounding environments like biological systems.
Abbreviations
| ENM | Engineering nanomaterial; |
| o-MMT | Organomodified montmorillonite; |
| CLSM | Confocal laser scanning microscopy; |
| PP | Polypropylene; |
| RIFE | Relative integral fluorescence emission. |
Acknowledgements
This work was financially supported by the Center for Packaging Innovation and Sustainability, School of Packaging, Michigan State University. The authors thank the staff at the Center for Advanced Microscopy, Michigan State University, for their assistance in SEM, TEM and CLSM characterization, and especially the support from Dr Melinda Frame with the fluorescence spectroscopy, imaging and probes.References
- Frost & Sullivan, Nanomaterials–Strategic Portfolio Management (Technical Insights), 2010 Search PubMed.
- BCC Research NAN021E – Global Markets for Nanocomposites, Nanoparticles, Nanoclays, and Nanotubes, http://www.bccresearch.com/report/nanocomposites-global-markets-nan021e.html, accessed 13 June 2012.
- A. Magrez, S. Kasas, V. Salicio, N. Pasquier, J. W. Seo, M. Celio, S. Catsicas, B. Schwaller and L. Forró, Nano Lett., 2006, 6, 1121–1125 CrossRef CAS.
- K. Savolainen, H. Alenius, H. Norppa, L. Pylkkänen, T. Tuomi and G. Kasper, Toxicology, 2010, 269, 92–104 CrossRef CAS.
- NRC (National Research Council), A Research Strategy for Environmental, Health, and Safety Aspects of Engineered Nanomaterials, National Academy Press, Washington, DC, 2012 Search PubMed.
- EFSA (European Food Safety Authority), EFSA J., 2009, 958, 1–39 Search PubMed.
- J. M. Johnston, M. Lowry, S. Beaulieu and E. Bowles, State-of-the-Science Report on Predictive Models and Modeling Approaches for Characterizing and Evaluating Exposure to Nanomaterials, U.S. Environmental Protection Agency, Washington, DC, 2010, EPA/600/R-10/129 (NTIS PB2011–105273), http://www.epa.gov/athens/publications/reports/Johnston_EPA600R10129_State_of_Science_Predictive_Models.pdf, accessed 23 July 2012 Search PubMed.
- D. M. Marquis, É. Guillaume and C. Chivas-Joly, in Nanocomposites and Polymers with Analytical Methods, ed. J. Cuppoletti, InTech, 2005, pp. 261–284 Search PubMed.
- T. Jiang, Y. Wang, J. Yeh and Z. Fan, Eur. Polym. J., 2005, 41, 459–466 CrossRef CAS.
- M. Lewin, Fire Mater., 2003, 27, 1–7 CrossRef CAS.
- M. Zammarano, J. W. Gilman, M. Nyden, E. M. Pearce and M. Lewin, Macromol. Rapid Commun., 2006, 27, 693–696 CrossRef CAS.
- Y. Tang, M. Lewin and E. M. Pearce, Macromol. Rapid Commun., 2006, 27, 1545–1549 CrossRef CAS.
- Y. Tang and M. Lewin, Polym. Degrad. Stab., 2007, 92, 53–60 CrossRef CAS.
- M. Lewin and Y. Tang, Macromolecules, 2008, 41, 13–17 CrossRef CAS.
- EFSA (European Food Safety Authority), EFSA J., 2011, 9, 2140 Search PubMed.
- M. Avella, J. J. De Vlieger, M. E. Errico, S. Fischer, P. Vacca and M. G. Volpe, Food Chem., 2005, 93, 467–474 CrossRef CAS.
- B. Schmidt, J. H. Petersen, C. Bender Koch, D. Plackett, N. R. Johansen, V. Katiyar and E. H. Larsen, Food Addit. Contam., Part A, 2009, 26, 1619–1627 CrossRef CAS.
- B. Schmidt, V. Katiyar, D. Plackett, E. H. Larsen, N. Gerds, C. B. Koch and J. H. Petersen, Food Addit. Contam., Part A, 2011, 28, 956–966 CrossRef CAS.
- M. Dahan, P. Alivisatos and W. J. Parak, in Single Particle Tracking and Single Molecule Energy Transfer, ed. C. C. Bräuchle, D. C. Lamb and J. Michaelis, Wiley-VCH Mannheim, 2009, pp. 67–96 Search PubMed.
- O. Raccurt, J. Samuel, O. Poncelet, S. Szenknect and F. Tardif, in NSTI-Nanotech, 2008, pp. 704–707 Search PubMed.
- P. H. Maupin, J. W. Gilman, R. H. Harris, S. Bellayer, A. J. Bur, S. C. Roth, M. Murariu, A. B. Morgan and J. D. Harris, Macromol. Rapid Commun., 2004, 25, 788–792 CrossRef CAS.
- A. K. Chaudhary, Rheology Modification and Foaming of Polypropylene – Clay Nanocomposites with Coupling Agents, PhD dissertation, Michigan State University, 2010.
- K. Jayaraman, T. J. Pathak and A. K. Chaudhary, Novel Nanocomposites and Nanocomposite Foams and Methods and Products Related to Same US 2010/0310802 A1(Patent Application), 2010 Search PubMed.
- K. Hoffmann, R. Mix, J. F. Friedrich and U. Resch-Genger, in Reviews in Fluorescence 2008, ed. C. D. Geddes, Springer New York, New York, NY, 2010, pp. 139–160 Search PubMed.
- A. H. Nashat, M. Moronne and M. Ferrari, Biotechnol. Bioeng., 1998, 60, 137–146 CrossRef CAS.
- G. Enrico and V. Levi in Single Particle Tracking and Single Molecule Energy Transfer, ed. C. Bräuchle, D. C. Lamb and J. Michaelis, Wiley-VCH, Weinheim, 2010, pp. 1–23 Search PubMed.
- V. Ivanov, J. Behnisch, A. Hollander, F. Mehdorn and H. Zimmermann, Surf. Interface Anal., 1996, 24, 257–262 CrossRef CAS.
- FDA (U.S. Food and Drug Administration) Guidance for Industry, Preparation of Premarket Submissions for Food Contact Substances, Chemistry Recommendations, http://www.fda.gov/Food/GuidanceComplianceRegulatoryInformation/GuidanceDocuments/FoodIngredientsandPackaging/ucm081818.htm#iid1c, accessed 14 June 2012.
Footnotes |
| † The authors declare no competing financial interest. |
| ‡ Electronic supplementary information (ESI) available: Additional information regarding production of nanocomposites, the silylation procedure, labeling of the nanoclays, characterization of the labels, thermal stability of the labels, and the migration test. See DOI: 10.1039/c2nr32978f |
| § Present Address: Manufacturing & Mechanical Engineering Technology & Packaging Science, Rochester Institute of Technology, 78 Lomb Memorial Drive, Rochester, NY 14623, USA. |
| ¶ These authors contributed equally to this work. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. |
| This journal is © The Royal Society of Chemistry 2013 |
