DOI:
10.1039/C2NR32536E
(Paper)
Nanoscale, 2013,
5, 337-341
Direct tri-constituent co-assembly of highly ordered mesoporous carbon counter electrode for dye-sensitized solar cells
Received
31st August 2012
, Accepted 31st October 2012
First published on 5th November 2012
Abstract
Controlling over ordered porosity by self-assembly is challenging in the area of materials science. Materials with highly ordered aperture are favorable candidates in catalysis and energy conversion device. Here we describe a facile process to synthesize highly ordered mesoporous carbon (OMC) by direct tri-constituent co-assembly method, which uses resols as the carbon precursor, tri-block copolymer F127 as the soft template and tetraethoxysilane (TEOS) as the inorganic precursor. The obtained products are characterized by small-angle X-ray diffraction (SAXD), Brunauer–Emmett–Teller (BET) nitrogen sorption–desorption measurement and transmission electron microscope (TEM). The results indicate that the OMC possesses high surface areas of 1209 m2 g−1, homogeneous pore size of 4.6 nm and a large pore volume of 1.65 cm3 g−1. The advantages of high electrochemical active surface area and favorable accessible porosity of OMC benefit the catalysis of I3− to I−. As a result, the OMC counter electrode displays a remarkable property when it was applied in dye-sensitized solar cells (DSSCs). For comparison, carbon black (CB) counter electrode and Pt counter electrode have also been prepared. When these different counter electrodes were applied for dye-sensitized solar cells (DSSCs), the power-conversion efficiency (η) of the DSSCs with CB counter electrode are measured to be 5.10%, whereas the corresponding values is 6.39% for the DSSC with OMC counter electrode, which is comparable to 6.84% of the cell with Pt counter electrode under the same experimental conditions.
Introduction
Dye-sensitized solar cells (DSSCs) have drawn extensive attention over the past two decades for their potential low cost and high energy conversion efficiency.1 The counter electrode plays an important role in DSSCs, which reduces I3− to I− to complete the cycle of electron transfer.2,3 Usually the platinized F-doped SnO2 (FTO) is used as counter electrode in DSSCs, owing to the inherent high catalytic activity and commendable conductivity of platinum. However, Pt is a noble metal and the methods used for preparing Pt counter electrodes are high energy-consuming which hinders its commercial application. Besides, the corrosion of Pt in the electrolyte endangers the long-term stability of DSSCs.4,5 Therefore, many studies have been devoted to developing alternative functional materials to replace Pt as counter electrodes for DSSCs.
Carbon may be a promising candidate for its abundant resource availability, high chemical stability, good electrical conductivity and favorable catalytic efficiency. Consequently, many carbon-based functional materials including carbon black, carbon nanotube, activated carbon, graphite, and graphene have been studied extensively.6–10 Specifically, ordered mesoporous carbon (OMC) was recently reported to reveal faster infiltration of electrolyte and remarkably enhancement of effective catalysis area owing to its large pore volume, accessible porosity and high surface area.11–14 As always, almost all the synthetic strategies of OMC materials involve the use of hard templates (two-step template method) or soft templates (one-step template method). Unfortunately, these methods usually involve multistep processes to obtain the final product,15,16 and sometimes will lead to the loss of pore structure during the high-temperature carbonization, which reduces the ordering and surface area of OMC seriously.17 Thus, development of OMC with high ordering using a simple method is highly desirable.
Herein, we report a facile method to prepare OMC using resols as the carbon precursor, tri-block copolymer F127 as the soft template and tetraethoxysilane (TEOS) as the inorganic precursor. To the best of our knowledge, this is the first time OMC using direct tri-constituent co-assembly method as counter electrodes for DSSCs has been reported. The results show that the obtained product processes high ordering, large pore volume and enormous surface area. As a result, the OMC counter electrodes show a favorable performance that is comparable to Pt counter electrode.
Experimental
Preparation of ordered mesoporous carbon
The OMC is prepared by a direct tri-constituent co-assembly method with resols as carbon precursor, tri-block copolymer F127 as the soft template and tetraethoxysilane (TEOS) as the inorganic precursor according to the procedure reported by the Zhao group.18 Resols preparation: 5 g of phenol was melted at 42 °C, and then 1.06 g of 20 wt% NaOH aqueous solution was added. After stirring for 10 min, 8.85 g of 37 wt% formaldehyde aqueous solution was added, and the mixture was stirred at 70 °C for 1 h. The mixture was neutralized by 2 M HCl solution after naturally cooling down to room temperature. Water was removed under vacuum at 45 °C. Then the mixture was made up of 20 wt% resols ethanolic solution. OMC was prepared as following. 4.8 g of tri-block copolymer F127 was dissolved in 21 g of ethanol at 40 °C, followed by the addition of 3 g of 0.2 M HCl solution. 6.24 g of TEOS was added to the mixture and stirred for 10 min. Then 15 g of 20 wt% resols ethanolic solution was added and stirred for 2 h. The mixture was transferred to a Petri dish and evaporated at room temperature for 8 h, followed by thermal polymerization at 100 °C for 24 h. Then the obtained product was calcined at 900 °C for 2 h under argon atmosphere. The product was grounded and transferred into 10 wt% HF solution, the mixture was stirred for 20 h to remove the silica component. After washing and drying, OMC powder was obtained.
To obtain OMC paste, 0.25 g of OMC powder and 3 g of H2O (containing 0.01 g sodium carboxymethylcellulose) were transferred into a small beaker, followed by stirring for 2 h. The as-prepared paste was coated on FTO-glass by doctor-blading method. Then the OMC electrode was heated at 100 °C for 30 min after naturally drying. For comparison purposes, carbon black (CB) counter electrode and Pt counter electrode were prepared. CB counter electrode was prepared by carbon black (40 nm, Top vender, Beijing, China), and the method was similar to OMC counter electrode. The Pt electrode was prepared by depositing a layer of Pt on FTO by magnetron sputtering.
Preparation of DSSCs
Dye sensitized TiO2 photoanodes were prepared according to the previously reported method.2 The electrolyte, composed of 0.1 M 1-propy-3-methylimidazolium iodide (PMII), 0.03M I2, 0.05 M LiI, 0.1 M GNCS, 0.5 M 4-tert-butylpridine (TBP) in mixed solvent of propylene carbonate (PC) and acetonitrile, was injected into the cell.
Characterizations and measurements
Small angle X-ray diffraction (SAXD) measurement was performed using a D8 diffractometer (Bruker Corp.) with Cu Kα radiation at 40 kV. Transmission electron microscopy (TEM) images were obtained on JEM2010FEF (JEOL, Japan). The specific surface area and pore size of OMC were investigated by Brunauer–Emmett–Teller (BET) nitrogen sorption–desorption measurement (JW-BK, China). Photovoltaic measurements were performed by applying external potential bias to the device under AM1.5 simulated illumination (Newport, 91192) with a power density of 100 mW cm−2. The irradiated area of each cell was kept at 0.25 cm2 by using a light-tight metal mask. Impedance measurements were performed by an electrochemical workstation (CHI660C, CH Instruments) with the frequency ranging from 100 kHz to 0.1 Hz in the illumination. Cyclic voltammetry was also performed on CHI 660C electrochemical workstation with a Pt as auxiliary electrode, an Ag/AgCl electrode as reference electrode, and Pt/FTO, OMC/FTO or CB/FTO as working electrode in an acetonitrile solution containing 10 mM LiI, 1 mM I2, and 0.1 M LiClO4 as supporting electrolyte at a scan rate of 50 mV s−1.
Results and discussion
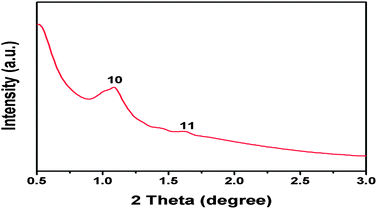
The preparation of OMC can be briefly described by the following process, as shown in Fig. 1, which includes three steps: (1) using resols, tri-block copolymer F127 and tetraethoxysilane (TEOS) as precursors to self-assemble at 40 °C in ethanol solution, (2) removing F127 and carbonizing in argon atmosphere, (3) dislodging silica to form OMC. The small-angle XRD pattern of OMC (Fig. 2) showed an intense diffraction peak and a weak peak at a 2θ range of 1° to 2° that was indexed as (10), (11) indicating the ordered 2D hexagonal mesoporous structure (p6mm).17,19 The intense (10) peak at about 2θ = 1.08° indicated a d-spacing values of 8.17 nm and the cell parameter of 9.43 nm. TEM images (Fig. 3) of OMC further confirmed the ordered 2D hexagonal mesoporous structure.
 |
| | Fig. 1 Schematic representation of the preparation of OMC. | |
 |
| | Fig. 3 TEM image for the ordered mesoporous carbon viewed from (a) perpendicular to the pore channels, and (b) parallel to the pore channels. | |
The N2 sorption isotherm of OMC exhibited a type-IV curve with a distinct capillary condensation step at a relative pressure of 0.4–0.7, which also indicated the ordered mesoporous structure (Fig. 4a). The BET surface area of OMC was 1209 m2 g−1, and the pore volume was 1.65 cm3 g−1. The BJH (Barrett–Joyner–Halenda) pore size distribution curve exhibited a uniform pore size of 4.6 nm. (Fig. 4b).
 |
| | Fig. 4 (a) Nitrogen sorption isotherms of ordered mesoporous carbon. (b) The BJH pore size distribution plot of ordered mesoporous carbon. | |
The photocurrent density versus voltage characteristics of DSSCs based on OMC counter electrode was shown in Fig. 5. For comparison purposes, the photovoltaic performances of DSSCs based on CB counter electrode and Pt counter electrode were also shown in Fig. 5. Details of the photovoltaic performance parameters are listed in Table 1. The DSSCs based on OMC counter electrode exhibited a conversion efficiency (η) of 6.39% which was comparable to Pt counter with the η of 6.84%, whereas the DSSCs based on CB counter electrode showed the η of 5.10%. The DSSCs employing OMC counter electrode gave a short-circuit current density (Jsc) of 14.10 mA cm−2, an open-circuit voltage (Voc) of 0.772 V, and a fill factor (FF) of 0.58. The Jsc, Voc and FF of device employing Pt counter electrode were 13.06 mA cm−2, 0.770 V and 0.68, respectively. The parameters of device employing CB counter electrode were 11.36 mA cm−2, 0.770 V and 0.58, respectively. It should be noticed that the performance of device employing OMC electrode is not only much better than that of device employing CB electrode but also is comparable to that of DSSCs with a Pt electrode.
 |
| | Fig. 5 Photocurrent–voltage characteristics of DSSCs employing Pt, OMC and CB counter electrode measured under one sun illumination (AM 1.5–100 mW cm−2 simulated irradiation) with active area of 0.25 cm2. | |
Table 1 The performance of DSSCs based on Pt, OMC and CB counter electrodes
| Sample |
V
oc (V) |
J
sc (mA cm−2) |
FF (%) |
η (%) |
R
s (Ω) |
R
ct1 (Ω) |
| Pt |
0.770 |
13.06 |
68 |
6.84 |
17.1 |
2.0 |
| OMC |
0.772 |
14.10 |
58 |
6.39 |
25.5 |
4.7 |
| CB |
0.770 |
11.36 |
58 |
5.10 |
25.0 |
4.2 |
As it is well know, the counter electrodes play an important role that serves to catalyze the reduction of I3− to I− in DSSC. Cyclic voltammetry was used to analysis and compare the catalytic activity of the three kinds of counter electrodes under the same condition (Fig. 6). Two pairs of redox peaks were observed on three kinds of counter electrodes, and the left pair was explained to the oxidation and reduction of I−/I3−.20 The peak current density reflected the catalytic activity of the counter electrodes.10,21 The more negative reduction peak of the OMC electrode compared with that of the Pt electrode and the CB electrode suggests the superior catalytic activity of OMC and that OMC electrode was an efficient counter electrode in DSSC. This result should be attributed to the accessible porosity for I− and I3− ions in the OMC electrode and the large electrochemical active surface area for I3− ions reduction. Because of the advantages mentioned above, the Jsc of the device based on OMC electrode was much higher than that of the device based on CB electrode and even slightly higher than that of the device based on Pt electrode.
To further elucidate the correlations of the properties of the counter electrodes and the performances of the devices, electrochemical impedance spectroscopy (EIS) analysis was carried out and the results were shown in the form of a Nyquist plot (Fig. 7). The charge transfer resistance (Rct1) at the counter electrode–electrolyte interface reflected the intrinsic catalytic properties of the counter electrode materials.22 By fitting the experimental data with the inset equivalent circuit, Rct1 at the counter electrode–electrolyte interface was obtained (Table 1). Rct1 of the OMC electrode (4.7 Ω) approximated to that of the CB electrode (4.2 Ω), and Rct1 of the Pt electrode (2.0 Ω) was about half of that of the carbon-based electrode. This is because the intrinsic catalytic activity of Pt is better than that of carbon. In addition, the internal series resistance (Rs) of DSSCs can be obtained from EIS (Table 1). Rs is composed of the electrolyte resistance and the sheet resistance of the electrode.23,24 Obviously, Rs of the device based on the Pt counter electrode was lower than that of the device based on the carbon-based counter electrode, this was due to the lower Rct1 and lower sheet resistance of the Pt electrode compared with the carbon-based counter electrode. As a result, the fill factor (FF) of the device employing Pt electrode was higher than that of the device employing the carbon-based electrode. However, it was encouraging that the high electrochemical active surface area and the favorable accessible porosity of the OMC electrode made up for the relatively poor intrinsic catalytic activity, so the OMC electrode revealed an excellent catalytic performance after all. As a result, the Jsc of the device based on the OMC counter electrode was much higher than that of the device based on the BC counter electrode and even slightly higher than the device based on the OMC counter electrode and the overall conversion efficiency of the device based on the OMC counter electrode was comparable with that of DSSCs based on the Pt counter electrode.
 |
| | Fig. 7 Nyquist plots of DSSCs based on Pt, OMC and CB counter electrodes obtained at open circuit conditions under 100 mW cm−2. | |
Conclusions
In conclusion, OMC synthesized by direct tri-constituent co-assembly was prepared as a counter electrode for DSSCs. The OMC electrode was found to have the advantages of high electrochemical active surface area, large pore volume, accessible porosity and high catalytic activity toward the reduction of I3− to I−. Finally, DSSCs employing this OMC counter electrode achieve conversion efficiency as high as 6.39% which was comparable to that of the device employing Pt counter electrode.
Acknowledgements
We gratefully acknowledge the financial supports of this work by the National Basic Research Program (no. 2011CB933300) of China and the National Science Fund for Talent Training in Basic Science (Grant no J0830310).
Notes and references
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef CAS.
- Q. Tai, B. Chen, F. Guo, S. Xu, H. Hu, B. Sebo and X. Z. Zhao, ACS Nano, 2011, 5(5), 3795 CrossRef CAS.
- W. Sun, X. Sun, T. Peng, Y. Liu, H. Zhu, S. Guo and X. Zhao, J. Power Sources, 2012, 201, 402 CrossRef CAS.
- D. J. Ham, E. Ramasamy, J. Lee and J. S. Lee, Chem. Commun., 2010, 46, 8600 RSC.
- G. Syrrokostas, A. Siokou, G. Leftheriotis and P. Yianoulis, Sol. Energy Mater. Sol. Cells, 2012, 103, 119 CrossRef CAS.
- P. Li, J. Wu, J. Lin, M. Huang, Y. Huang and Q. Li, Sol. Energy, 2009, 83, 845 CrossRef CAS.
- J. Han, H. Kim, D. Y. Kim, S. M. Jo and S. Y. Jang, ACS Nano, 2010, 4, 3503 CrossRef CAS.
- H. Hu, B. L. Chen, C. H. Bu, Q. D. Tai, F. Guo, S. Xu, J. H. Xu and X. Z. Zhao, Electrochim. Acta, 2011, 56, 8463 CrossRef CAS.
- J. Chen, K. Li, Y. Luo, X. Guo, D. Li, M. Deng, S. Huang and Q. Meng, Carbon, 2009, 47, 2704 CrossRef CAS.
- J. D. Roy-Mayhew, D. J. Bozym, C. Punckt and I. A. Aksay, ACS Nano, 2010, 4(10), 6203 CrossRef CAS.
- R. Ryoo, S. H. Joo and S. Jun, J. Phys. Chem. B, 1999, 103, 7743 CrossRef CAS.
- X. Chen, Y. S. Jun, K. Takanabe, K. Maeda, K. Domen, X. Fu, M. Antonietti and X. Wang, Chem. Mater., 2009, 21, 4093 CrossRef CAS.
- J. Ding, K. Y. Chan, J. Ren and F. Xiao, Electrochim. Acta, 2005, 50, 3131 CrossRef CAS.
- D. Yuan, J. Zeng, N. Kristian, Y. Wang and X. Wang, Electrochem. Commun., 2009, 11, 313 CrossRef CAS.
- E. Ramasamy, J. Chun and J. Lee, Carbon, 2010, 48, 4563 CrossRef CAS.
- J. Zhou, J. He, T. Wang, D. Sun, G. Zhao, X. Chen, D. Wang and Z. Di, J. Mater. Chem., 2008, 18, 5776 RSC.
- X. Zhuang, Y. Wan, C. Feng, Y. Shen and D. Zhao, Chem. Mater., 2009, 21, 706 CrossRef CAS.
- R. Liu, Y. Shi, Y. Wan, Y. Meng, F. Zhang, D. Gu, Z. Chen, B. Tu and D. Zhao, J. Am. Chem. Soc., 2006, 128, 11652 CrossRef CAS.
- Y. Meng, D. Gu, F. Zhang, Y. Shi, H. Yang, Z. Li, C. Yu, B. Tu and D. Zhao, Angew. Chem., 2005, 117, 7215 CrossRef.
- K. Imoto, K. Takahashi, T. Yamaguchi, T. Komura, J. Nakamura and K. Murata, Sol.
Energy Mater. Sol. Cells, 2003, 79, 459 CrossRef CAS.
- H. Sun, Y. Luo, Y. Zhang, D. Li, Z. Yu, K. Li and Q. Meng, J. Phys. Chem. C, 2010, 114, 11673 CAS.
- C. Xu, J. Li, X. Wang, J. Wang, L. Wan, Y. Li, M. Zhang, X. Shang and Y. Yang, Mater. Chem. Phys., 2012, 132, 858 CrossRef CAS.
- Q. Jiang, G. Li, F. Wang and X. Gao, Electrochem. Commun., 2010, 12, 924 CrossRef CAS.
- D. Zhang, X. Li, S. Chen, F. Tao, Z. Sun, X. Yin and S. Huang, J. Solid State Electrochem., 2010, 14, 1541 CrossRef CAS.
Footnote |
| † These authors contributed equally to the work. |
|
| This journal is © The Royal Society of Chemistry 2013 |
Click here to see how this site uses Cookies. View our privacy policy here.