CIS–ZnS quantum dots for self-aligned liquid crystal molecules with superior electro-optic properties†
Won-Kyu Lee‡
b,
Seung Jun Hwang‡c,
Min-Jae Choa,
Hong-Gyu Parka,
Jin-Woo Hand,
Seogjeong Songb,
Jong Hyun Jangc and
Dae-Shik Seo*a
aInformation Display Device Laboratory, Department of Electrical and Electronic Engineering, Yonsei University, 262 Seongsanno, Seodaemun-gu, Seoul 120-749, Republic of Korea. E-mail: dsseo@yonsei.ac.kr; Fax: +82 2 3147 1351; Tel: +82 2 2123 4617
bElectronic Materials Research Institute, Kolon Central Research Park, Kolon Industries, Inc., Yongin 446-797, Republic of Korea
cFuel Cell Research Center, Korea Institute of Science and Technology, 39-1 Hawolgok-dong, Seoul 136-791, Republic of Korea
dElectronic Material Lab., New Business R&D center, Samsung Corning Precision Materials Co.,Ltd. 644-1, Jinpyeong-dong, Gumi-Si, Gyeongsangbuk-Do, Republic of Korea
First published on 23rd October 2012
Abstract
We demonstrate self-aligned and high-performance liquid crystal (LC) systems doped with 1-dimensional (1D) chain-like clusters of CuInS2 (CIS)–ZnS core–shell quantum dots (QDs). By changing the cell fabrication method of the LC–QD composites, we can selectively control the orientation of the LC molecules between the homogeneous and homeotropic states without conventional LC alignment layers. The homeotropic alignment of LCs was achieved by random dropcasting and the homogeneous alignment was performed using a capillary injection of LC–QDs due to the random or linear diffusion of QD clusters into ITO defects. The electrically compensated bend (ECB)- and vertically aligned (VA) mode LC displays (LCDs) containing our LC–QD composite both showed superior electro-optic (EO) properties. A 37.1% reduction in the threshold voltage (Vth) and a 36.6% decrease in the response time were observed for ECB mode LCDs, and a 47.0% reduction in the Vth and a 38.3% decrease in the response time were observed for VA mode LCDs, meaning that the proposed LC–QD composites have a great potential for the production of advanced flexible LCDs.
1. Introduction
The uniform alignment of liquid crystalline (LC) molecules in a preferred orientation is one of the most important technical challenges in the fabrication of high-quality liquid crystal displays (LCDs). Commercially, mechanical rubbing of spin-coated polyimide (PI) layers is employed to provide topographical microgrooves that give rise to the uniaxial homogeneous and homeotropic alignment of LCs.1 However, this process has some critical drawbacks, including the introduction of dust particles and the creation of electrostatic charges due to the friction between the rubbing roller and the substrate.2 Consequently, noncontact methods for LC alignment have been developed to resolve the shortcomings of rubbing, including the following: ion-beam irradiation,3–5 photoalignment,6 self-assembled monolayers,7 and oblique evaporation of silicon mono-oxides.8 Nevertheless, from an industrial point of view, these alignment methods are difficult to adopt on a commercial scale due to the required surface treatments of the substrates or the use of expensive vacuum equipment.The addition of nanomaterials to LCDs has attracted significant attention because the resulting material has potential as an alternative to the conventional rubbing process and also may exhibit unique electro-optic properties for novel display applications.9 In an LC cell composed of nanoparticle (NP) suspensions in an LC matrix, the dispersed NPs generate defects in the LC phase and break up the continuous rotational symmetry. In comparison with conventional chemical synthetic methods, it is much easier to modify the properties of LCs by doping them with NPs, and this method has led to many new electro-optical (EO) characteristics.10–14 Specifically, Nakamura from Sharp invented a self-aligned LC–fullerene composite, wherein the surface can be vertically aligned without LC alignment layers.15 LCDs prepared without using conventional alignment layers are especially suitable for flexible LC displays because they can be fabricated under ambient conditions without a post-annealing process, such as the heat treatment necessary for the imidization of PI layers at 200 °C. Similarly, a series of NP–LC mixtures that can self-align without an LC alignment process have been proposed, including LCs combined with polyhedral oligomeric silsesquioxanes (POSS),16 gold NPs,17 carbon nanotubes,18 and nickel NPs containing different morphologies.19 However, the resulting LC device switching (both low threshold voltage and fast response time) was inferior compared with that of LCDs containing PI alignment layers. Therefore, there is a need to develop new LC composites that can not only be aligned without an alignment process but also exhibit superior EO characteristics for practical applications.
In recent years, the understanding of the interaction between quantum dots (QDs) and LCs has emerged as a challenging area of intense research that can lead to the controlled self-assembly of QDs and improved electro-optic characteristics of LCs.20 Hirst et al. studied the organization mechanism of QDs by using an anisotropic LC medium and investigated the possibility of fabricating multifunctional switchable devices.21 More recently, the effects of QDs on the properties of LC materials have been reported, including changes to the memory effect, dielectric anisotropy (Δε), elastic constant (K) and threshold voltage (Vth).22–26 Moreover, it was shown that agglomerated QDs with a specific size and concentration have the potential to affect the degree of homogeneous and homeotropic alignment of LCs.22,24–26
Herein, we suggest a strikingly simple method of doping CuInS2(CIS)–ZnS-QDs into LCs with both positive and negative dielectric anisotropies to achieve self-alignment based on QDs with 1-dimensional (1D) chain-like clusters that can diffuse into the microdefects on the surface of ITO. By changing the fabrication method of the LC–QD composites, we can selectively control the orientation of the LC molecules between the homogeneous and homeotropic states without using the conventional LC alignment process. Moreover, our CIS-QD doped LC devices exhibited superior EO characteristics compared with those containing PI alignment layers. By increasing the doping concentration to 0.02 mol L−1, a 37.1% reduction in the Vth and a 36.6% decrease in the response time were observed for electrically compensated bend (ECB) mode LCDs, and a 47.0% reduction in the Vth and a 38.3% decrease in the response time were observed for vertically aligned (VA) mode LCDs.
2. Experimental section
2.1 Materials
Copper(I) iodide (99.999%), indium acetate (99.99%), 1-dodecanethiol (98%), 1-octadecene (90%), Zn acetate dihydrate (reagent grade), Zn stearate (10–12% Zn basis), potassium ethylxanthogenate (96%), dimethylformamide (99.8%), toluene (99.8%), and all other solvents were purchased from Sigma-Aldrich and used as received. Commercial nematic LCs (5CB) with positive and negative dielectric anisotropies were purchased from Merck (ESI, S1†).2.2 Synthesis of CuInS2 (CIS) quantum dots (QDs)
For the synthesis of CIS–ZnS QDs, we carefully followed the Yang group's report:27 a three-neck round bottom flask was charged with copper(I) iodide (CuI, 0.05 mM), indium acetate (In(OAc)3, 0.1 mM), 1-dodecanethiol (4.174 mM) and 1-octadecene (8 mL). The solution was degassed and stirred at 80 °C for 1 h. The temperature was then increased to 220 °C under an Ar atmosphere, and the reaction mixture was stirred for an additional 15 min.2.3 Preparation of the ZnS shell stock solution
The ZnS shell stock solution, composed of Zn and S precursors, was prepared by dissolving zinc acetate (Zn(OAc)2, 15 mM) and potassium ethylxanthogenate (30 mM) in 60 mL of deionized water to afford a Zn ethylxanthate precipitate, which was then washed and dried at 80 °C. Subsequently, 1 mL of zinc ethylxanthate solution was rehomogenized with zinc stearate (8 mL), DMF (1 mL), and toluene (10 mL) in a 1-octadecene solution (60 mL) by using an ultrasonic bath for 12 h.2.4 Preparation of CuInS2–ZnS core–shell QDs
For the in situ fabrication of CIS–ZnS core–shell QDs, a solution of CIS core was cooled to ambient temperature, and then 7.5 mL of the prepared ZnS shell stock solution and 14 mL of 1-octadecene were added drop-wise to the CIS core solution using a syringe pump. After the reaction mixture was maintained at 200 °C for 12 h, the solution was cooled to room temperature, and the CIS–ZnS particles were purified by precipitation and centrifugation with the addition of chloroform and ethanol.2.5 Liquid crystal cell fabrication
Synthesized QDs were dispersed in LCs with either positive or negative dielectric anisotropies via ultrasonication for 2 hours. The concentration of QD–LC composites was varied from 0.01 to 0.02 mol L−1. Subsequently, the LC–QD composites were injected using a capillary mechanism into a sandwiched ITO glass with cell gaps of 60 and 5 μm. Alternatively, a solution of LC–QDs was dropcast on the bottom of the ITO surface (spacers were already sprayed on the ITO glass) and then carefully covered with an upper ITO glass. These LC cells were fabricated at room temperature. The cells with 60 μm gaps were used for POM analysis, whereas VA and ECB cells (cell gaps of 5 μm) were employed for the characterization of EO properties of LC–QD composites.2.6 Characterization
Particle size, size distribution, and dispersion were confirmed by high-resolution and scanning transmission electron microscopy (HRTEM, STEM, FEI, 200 keV). A nickel holey carbon support grid was dipped in a chloroform solution to deposit the QDs onto the film. The composition and elemental mapping of QDs embedded in the LC matrix were analyzed using an electron probe microanalyzer (EPMA, JXA-8500F). EPMA cells were prepared by spin coating LC–QD composites on Si wafers and then drying on a hot plate at 60 °C for 2 days. The LC alignment characteristics were observed using a cross-polarized Optical Microscope (OM) (BXP 51, Olympus). The electro-optical characteristics of the VA- and ECB-LCDs were measured using an LCD evaluation system (LCD-700; Otsuka Electronics).3. Results and discussion
The morphology of the as-synthesized CIS–ZnS-QDs was investigated by using transmission electron microscopy (TEM), and the images are provided in Fig. 1a–d. The scanning TEM (STEM) image in Fig. 1a reveals that the synthesized CIS–ZnS-QDs self-assemble into 1D chain-like clusters, which are localized in a given area.28–30 Interestingly, the nearly monodisperse nanocrystals were observed upon magnification of the self-assembled area (Fig. 1b and c). Based on these observations, we postulated that the local alignment of QDs, which is similar to the 1D self-assembly of magnetite nanoparticles, could serve as a guide for the liquid crystal host.31 According to the EDX composition data, the bright areas in the STEM image are composed of assembled QDs, whereas the dark areas are the nickel grid of the TEM sample (ESI, S2†). The length and width of each cluster varies from 1 to 3 μm and from 500 to 700 nm, respectively. Also, the high-resolution TEM (HRTEM) image and the electron diffraction pattern of the synthesized QDs are provided in Fig. 1c and d. The individual QD is nearly spherical in shape and has an average diameter of 2.2 nm. A HRTEM image of a single QD and the corresponding Fourier-transform (FT) pattern indicate that it was indeed a piece of single crystal (Fig. 2). | ||
| Fig. 1 (a and b) STEM images of synthesized CIS–ZnS QDs with different magnitude. The HRTEM image of individual QDs (c) and the electron diffraction pattern of CIS–ZnS core–shell structured QDs (d). EPMA elemental mappings of QDs in the LC matrix (e–h). | ||
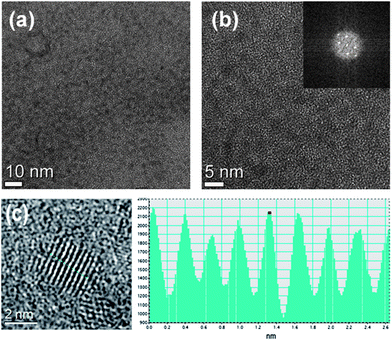 | ||
| Fig. 2 TEM and HRTEM images of synthesized CIS–ZnS core–shell QDs (a and b) and the corresponding FT pattern (inset). The images of a single QD with lattice stripes and its line profile (dotted line) are also shown in (c). | ||
To obtain an elemental map of QDs embedded in the LC matrix, we employed an electron probe microanalyzer (EPMA) to image a spin-coated LC–QD composite film. Fig. 1e–h show that all QD elements (i.e., Cu, In, S, and Zn) are linearly concentrated in the LC matrix, and a relatively small number of elements are dispersed around the core. This localization is possibly observed because the ZnS shell, which is itself a capping layer, confers a strong self-affinity to the QDs and gives rise to their microphase separation in the LC matrix.32 The EPMA analysis demonstrates that the CIS–ZnS QDs in LCs are also concentrated in the form of a 1D chain-like shape, similar to that of pure QDs without the LC medium. The range of concentrated elements in the LC media closely coincides with the size of the QD clusters in Fig. 1a (ESI, S3†). The clusters with this size can create the microgroove effect of rubbed PIs or patterned alignment layers that guide the LC orientation as it linearly diffuses into the microdefects on the surface of the ITO or LC media.33,34 In addition, the photoluminescence (PL) spectra of pure QDs and LC–QD composites indirectly show that the agglomeration of CIS–ZnS-QDs in various media is nearly the same (ESI, S4†).
A possible self-alignment mechanism of LC–CIS QD composites on the surface of ITO is proposed in Fig. 2. It has been shown that the surfaces of substrates, such as glass, oxides, and metals, exhibit the ability to vertically align the liquid crystal when certain cleaning processes are employed.31 However, the reproducibility and uniformity of this homeotropic alignment is not sufficient to produce a reliable LC device. This problem can be solved by QD clusters, which enhance the intrinsic capability of the ITO substrates for homeotropic LC alignment. The homeotropic LC alignment on the surface of the oxide is explained by the competition between the van der Waals interactions of alignment layers and LC molecules and the molecular interactions between LC molecules.5 The strong van der Waals interactions between the surface of ITO and LC molecules, which are more effective than LC molecular interactions, are thought to produce a random LC alignment. When LC–QD composites were deposited on the surface of ITO, the microsized QD clusters might randomly diffuse into the microdefects on the surface of ITO (i.e., grain boundaries or dislocations) such that the surface energy is minimized and the van der Waals interactions are sufficiently decreased. This approach provides a better balance between the van der Waals forces and the LC molecular interactions, allowing for homeotropic alignment (Fig. 2a).35–38 van der Waals interactions are represented by V = −λ/r6 (r: the distance between two objects, λ: a constant depending on the properties of objects), where λ is proportional to the product of the polarizability of the ITO and LC medium. By filling in the microdefects with QDs, the polarizability of the ITO surface is reduced, leading to the homeotropic alignment of LCs.
Fig. 2b demonstrates the effect of the 1D structure on the homogeneous orientation of LC molecules. When the LC cell was filled with a positive LC–QD composite using capillary action, the majority of the 1D chain structures of the QD clusters arrange themselves along the filling direction, which might be the result of the fluid effect.39 The LC molecules near the QD clusters on the surface of ITO are guided by the 1D structure and align parallel to the filling direction. In turn, the aligned molecules orient the neighboring LCs in the same direction. As a result, the LC molecules on the surface of ITO are aligned by the direct or indirect guide of the 1D structure of QDs under capillary forces. The homogeneous alignment of LCs on both the top and bottom ITO surfaces then gradually propagates into the bulk, inducing the complete planar alignment of the LC–QD composite. The 1D structure of QD clusters might act as a quasi-level supporter for the homogeneous alignment of LC molecules when introduced to the surface of ITO using capillary forces.40
To verify the self-alignment capability of LC–QD composites, LC cells with negative and positive dielectric anisotropies were fabricated using different LC–QD filling methods (Fig. 3). Consequently, whereas the LC–QD composite with negative dielectric anisotropy exhibited uniform homeotropic alignment in the cells prepared by dropcasting (Fig. 3e and f), cells fabricated by capillary filling did not exhibit good alignment in the LC–QD composite (Fig. 3a and b). In contrast, LC–QD composite cells with positive anisotropy that were fabricated using capillary filling exhibited uniform homogeneous alignment (Fig. 3c and d), whereas cells fabricated using the dropcasting method show poor alignment (Fig. 3g and h). The polarizing optical microscopy (POM) images shown in Fig. 3a and b reveal the presence of QD clusters in the form of anisotropic light-scattering centers parallel to the filling direction. QD clusters that are linearly injected by capillary forces are likely to perturb the homeotropic LC alignment states. In addition, the presence of a planar texture with oily streaks and chain-like birefringent stripes (Fig. 4c and d) indicates that QD clusters can induce homogeneous LC alignment by using capillary forces.19–21,25,26,34 However, no such anisotropic texture of LC–QD composites is observed for the cells prepared by dropcasting (Fig. 4e–h); instead, a schlieren texture is observed, as shown in Fig. 4g and h (ESI, S5†). These differences are attributed to the aforementioned different mechanisms of homeotropic and homogeneous alignment of LC–QD composites without alignment layers. The same trends are observed irrespective of the QD doping concentration (0.01 and 0.02 mol L−1).
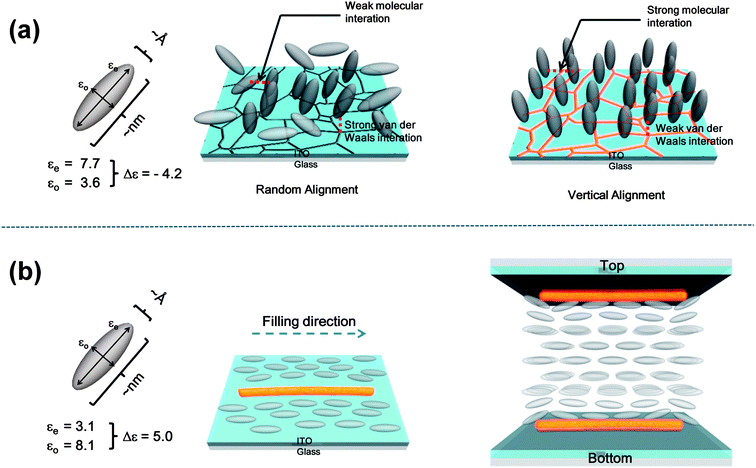 | ||
| Fig. 3 The mechanism for self-alignment of the LC–QD composite on the ITO surface. (a) Dropcasting of QD doped LCs (with negative Δε) induces homeotropic alignment of LCs and (b) capillary injected 1D structure of the QD cluster guides the LCs (with positive Δε) to the filling direction and achieves homogeneous alignment. | ||
 | ||
| Fig. 4 POM images (with crossed polarizers) of the LC–QD composite with different filling methods. The doping concentration varies from 0.01 mol L−1 (a, c, e and g) to 0.02 mol L−1 (b, d, f and h). Negative dielectric anisotropic LC–QDs show good homeotropic alignment with dropcasting (e and f) and random alignment (a and b) with capillary filling. Reversely, positive dielectric anisotropic LC–QDs exhibit uniform homogeneous alignment (c and d) with capillary filling and poor alignment states (g and h) with dropcasting. The inset in each figure shows the same area with parallel (un-crossed) polarizers. Blue arrows show the capillary filling direction. | ||
Superior EO characteristics, including a decreased Vth and fast response times, were achieved in both VA- and ECB-mode LCDs prepared from LC–QD composites, as shown in Fig. 4a and b. Stable voltage–transmittance (V–T) plots of VA- and ECB-mode LCDs confirm that the homeotropic and homogeneously aligned LC–QD composites can successfully switch from OFF to ON in the presence of an electric field above a certain threshold (E > Eth) due to the electric Freedericksz transition. Moreover, we were able to control the Vth of the device by changing the QD doping concentration. In VA mode, the Vth at 10% transmittance was reduced by 47.0% compared with the conventional VA-LCD containing PI alignment layers as the QD doping concentration was increased to 0.02 mol L−1. In addition, a 37.1% decrease in the Vth at 90% transmittance was recorded for ECB-LCDs with LC–QD composites in comparison with the conventional ECB-LCD (Fig. 5). The relatively small amount of QDs monodispersed in the LC medium that were not concentrated at the surface of ITO reduce the elastic constants (K) of the nematic LCs, as the Hegmann group elucidated in a recent study. Consequently, the Vth for the LC molecular reorientation should also be suppressed because the Vth is proportional to π(K/ε0Δε)1/2.
 | ||
| Fig. 5 The EO characteristics of VA and ECB mode LCDs containing the LC–QD composite. (a) V–T curves (left) and fall (right upper) and rise (right lower) times of VA-LCDs with LC–QD composites and a rubbed PI layer. (b) V–T curves (left) of ECB-LCDs with LC–QD composites and a rubbed PI layer. Rise (right upper) and fall (right lower) times are also shown with the LC–QD composite and a PI layer. The doping concentration varies from 0.01 mol L−1 to 0.02 mol L−1. | ||
The transmittance vs. time curves for both VA- and ECB-mode LCDs depict the rise and fall process in Fig. 3a and b (right to the V–T curves), which makes it possible to evaluate the characteristic rise and fall response times of the LC–QD composite at different doping concentrations. It should be noted that LC–QD composites exhibited very fast rise and fall times not only in the VA cells (fall and rise times of 3.4 and 15.0 ms, respectively) but also in the ECB cells (rise and fall times of 3.6 and 2.3 ms, respectively) at the increased doping concentration of 0.02 mol L−1, averaged over more than 10 cells. In comparison with the switching speed of conventional VA- and ECB-mode LCDs with PI alignment layers, the LC–QD composite cells exhibit very promising speeds (fall and rise times of 10.0 and 19.8 ms, respectively, in the VA mode; rise and fall times of 4.6 and 4.7 ms in the ECB mode, respectively, of the rubbed PI samples). In general, when K is decreased, the response time is increased (ESI, S6†). However, although K might be decreased in this study, the response time is also reduced. This result indicates that the response time, including the rise and fall times, is affected by some other factors, such as the viscosity or focused electric field flux around the small amount of monodispersed QDs, rather than K.41
4. Conclusions
In summary, CIS–ZnS QD doped LCs with both positive and negative dielectric anisotropies were successfully self-aligned with homeotropic or homogeneous states using different LC cell fabrication methods. The QDs were self-assembled in an LC matrix into peculiar 1D chain-like clusters, which diffused randomly or linearly into the defects on the surface of ITO. The homeotropic alignment of LCs was achieved by random dropcasting and the homogeneous alignment was performed using a capillary injection of LC–QDs. Furthermore, LC–QD composites show the superior EO characteristics of a reduced Vth and the fast response times for both VA- and ECB-mode LC cells with controllable device switching. The proposed CIS QD doped LC composite material provides a great deal of fabrication freedom for the LCD industries. Expensive alignment layer deposition and troublesome surface treatments and post-annealing processes do not need to be included and neither do additional compensation circuits for controlling the device switching speed, thereby leading to an extremely cost-effective and practical method for the production of advanced flexible LCDs.Notes and references
- D. W. Berreman, Phys. Rev. Lett., 1972, 32, 2345 Search PubMed.
- J. van Haaren, Nature, 2001, 411, 29–30 CrossRef CAS.
- P. Chaudhari, J. Lacey, J. Doyle, E. Galligan, S.-C. A. Lien, A. Callegari, G. Hougham, N. D. Lang, P. S. Andry, R. John, K. H. Yang, M. Lu, C. Cai, J. Speidell, S. Purushothaman, J. Ritsko, M. Samant, J. Stöhr, Y. Nakagawa, Y. Katoh, Y. Saitoh, K. Sakai, H. Satoh, S. Odahara, H. Nakano, J. Nakagaki and Y. Shiota, Nature, 2001, 411, 56 CrossRef CAS.
- J. Stöhr, M. G. Samant, J. Luning, A. C. Callegari, P. Chaudhari, J. P. Doyle, J. A. Lacey, S. A. Lien, S. Purushothaman and J. L. Speidell, Science, 2001, 292, 2299 CrossRef.
- W. K. Lee, B. Y. Oh, J. H. Lim, H. G. Park, B. Y. Kim, H. J. Na and D. S. Seo, Appl. Phys. Lett., 2009, 94, 223507 CrossRef.
- (a) J. Fang, Y. Shi, J. E. Maclennan, N. A. Clark, M. J. Farrow and D. M. Walba, Langmuir, 2010, 26, 17482 CrossRef; (b) D. Y. Zhao, W. Huang, H. Cao, Y. D. Zheng, G. J. Wang, Z. Yang and H. Yang, J. Phys. Chem. B, 2009, 113, 2961 CrossRef CAS.
- P. Prompinit, A. S. Achalkumar and J. P. Bramble, ACS Appl. Mater. Interfaces, 2010, 2, 3686 CAS.
- J. L. Janning, Appl. Phys. Lett., 1972, 21, 173 CrossRef CAS.
- H. Qi and T. Hegmann, J. Mater. Chem., 2008, 18, 3288 RSC.
- S. Kaur, S. P. Singh, A. M. Biradar, A. Choudhary and K. Sreenivas, Appl. Phys. Lett., 2007, 91, 023120 CrossRef.
- B. F. Li, H. Huang, X. K. Ding, W. B. Li, Y. H. Yin, L. P. Wang, H. Cao and H. Yang, Liq. Cryst., 2008, 35, 49 CrossRef CAS.
- Y. Shiraishi and N. Toshima, Appl. Phys. Lett., 2002, 81, 28457 CrossRef.
- W. K. Lee, J. H. Choi, H. J. Na, J. H. Lim, J. M. Han, J. Y. Hwang and D. S. Seo, Opt. Lett., 2009, 34, 3653 CrossRef CAS.
- W. K. Lee, Y. S. Choi, Y. G. Kang, J. Sung, D. S. Seo and C. Park, Adv. Funct. Mater., 2011, 21, 3843–3850 CrossRef CAS.
- M. Nakamura, Y. Hashimoto, T. Shinimiya and S. Mizushima, U. S. Pat., No. 2005/0062927, 2005.
- (a) S. C. Jeng, C. W. Kuo, H. L. Wang and C. C. Liao, Appl. Phys. Lett., 2007, 91, 061112 CrossRef; (b) S. J. Hwang, S. C. Jeng, C. Y. Yang, C. W. Kuo and C. C. Liao, J. Phys. D: Appl. Phys., 2009, 42, 025102 CrossRef; (c) S. C. Jeng, S. J. Hwang and C. Y. Yang, Opt. Lett., 2009, 34, 455 CrossRef CAS.
- H. Qi and T. Hegmann, ACS Appl. Mater. Interfaces, 2009, 1, 1731 CAS.
- (a) S. Y. Lu and L. C. Chien, Opt. Express, 2008, 16, 12777 CrossRef CAS; (b) J. M. Russell, S. Oh, I. LaRue, O. Zhou and E. T. Samulski, Thin Solid Films, 2006, 509, 53 CrossRef CAS; (c) J. Lagerwall, G. Scalia, M. Haluska, U. Dettlaff-Weglikowska, S. Roth and F. Giesselmann, Adv. Mater., 2007, 19, 259–364 CrossRef; (d) M. D. Lynch and D. L. Patrick, Nano Lett., 2002, 2, 1197–1201 CrossRef CAS.
- D. Zhao, W. Zhou, X. Cui, Y. Tian, L. Guo and H. Yang, Adv. Mater., 2011, 23, 5779–5784 CrossRef CAS.
- R. Basu and G. S. Iannacchione, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2009, 010701 CrossRef (R).
- L. S. Hirst, J. Kirchhoff, R. Inman and S. Ghosh, Proc. SPIE, 2010, 7618, 76180F CrossRef.
- B. Kinkead and T. Hegmann, J. Mater. Chem., 2010, 20, 448–458 RSC.
- S. Kumar and L. K. Sagar, Chem. Commun., 2011, 47, 12182–12184 RSC.
- J. Mirzaei, M. Urbanski, K. Yu, H.-S. Kitzerow and T. Hegmann, J. Mater. Chem., 2011, 21, 12710 RSC.
- A. Kumar, J. Prakash, M. T. Khan, S. K. Dhawanm and A. M. Biradar, Appl. Phys. Lett., 2010, 97, 163113 CrossRef.
- A. Kumar, P. Silotia and A. B. Biradar, Appl. Phys. Lett., 2011, 99, 072902 CrossRef.
- D. Nam, W. Song and H. Yang, J. Mater. Chem., 2011, 21, 18220–18226 RSC.
- S. Huang, Z. Dai, F. Qu, L. Zhang and X. Zhu, Nanotechnology, 2002, 13, 691–694 CrossRef CAS.
- C. Martinez-Boubeta, K. Simeonidis, D. Serantes, I. Conde-Leboran, I. Kazakis, G. Stefanou, L. Pena, R. Galceran, L. Balcells, C. Monty, D. Baldomir, M. Mitrakas and M. Angelakeris, Adv. Funct. Mater., 2012, 22, 3737–3744 CrossRef CAS.
- S. Acharya, S. Kundu, J. P. Hill, G. J. Richards and K. Ariga, Adv. Mater., 2009, 21, 989–993 CrossRef CAS.
- J. Vallooran, S. Bolisetty and R. Mezzenga, Adv. Mater., 2011, 23, 3932–3937 CrossRef CAS.
- D. E. Fogg, L. H. Radzilowski, B. O. Dabbousi, R. R. Schrock, E. L. Thomas and M. G. Bawendi, Macromolecules, 1997, 30, 8433–8439 CrossRef CAS.
- D. Abras, G. Pranami and N. L. Abbott, Soft Matter, 2012, 8, 2026 RSC.
- D. Coursault, J. Grand, B. Zappone, H. Ayeb, G. Levi, N. Felidj and E. Lacaze, Adv. Mater., 2012, 24, 1461–1465 CrossRef CAS.
- J. Cognard, Mol. Cryt. Liq. Cryst. Suppl. Ser., 1982, 1, 1 CAS.
- M. Lu, Jpn. J. Appl. Phys., Part 1, 2004, 43, 8156 CrossRef CAS.
- C. Chen, P. J. Bos, J. Kim, Q. Li and J. E. Anderson, J. Appl. Phys., 2006, 99, 123523 CrossRef.
- B. H. Hwang, K. C. Kim, H. J. Ahn, J. B. Kim, D. C. Hyun, and H. K. Baik, Proceedings of the 21st International Liquid Crystal Conference, Keystone. 2006, p. 983 Search PubMed.
- P. Yang, Nature, 2003, 425, 243 CrossRef CAS.
- W. Zhou, L. J. Lin, D. Y. Zhao and L. Guo, J. Am. Chem. Soc., 2011, 133, 8389 CrossRef CAS.
- Y. S. Ha, H. J. Kim, H. G. Park and D. S. Seo, Opt. Express, 2012, 20, 6448–6455 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2nr32458j |
| ‡ Authors with equal contributions. |
| This journal is © The Royal Society of Chemistry 2013 |
