Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The chemistry and biological activity of the Hyacinthaceae
Dulcie A. Mulholland
*ab,
Sianne L. Schwikkard
ab and
Neil R. Crouch
bc
aNatural Products Research Group, Department of Chemistry, University of Surrey, Guildford, GU2 7XH, United Kingdom
bSchool of Chemistry and Physics, University of KwaZulu-Natal, 4041 Durban, South Africa
cEthnobotany Unit, South African National Biodiversity Institute, P.O. Box 52099, 4007 Berea Road, South Africa
First published on 29th July 2013
Abstract
Covering: 1914 to 2012
The Hyacinthaceae (sensu APGII), with approximately 900 species in about 70 genera, can be divided into three main subfamilies, the Hyacinthoideae, the Urgineoideae and the Ornithogaloideae, with a small fourth subfamily the Oziroëoideae, restricted to South America. The plants included in this family have long been used in traditional medicine for a wide range of medicinal applications. This, together with some significant toxicity to livestock has led to the chemical composition of many of the species being investigated. The compounds found are, for the most part, subfamily-restricted, with homoisoflavanones and spirocyclic nortriterpenoids characterising the Hyacinthoideae, bufadienolides characterising the Urgineoideae, and cardenolides and steroidal glycosides characterising the Ornithogaloideae. The phytochemical profiles of 38 genera of the Hyacinthaceae will be discussed as well as any biological activity associated with both crude extracts and compounds isolated. The Hyacinthaceae of southern Africa were last reviewed in 2000 (T. S. Pohl, N. R. Crouch and D. A. Mulholland, Curr. Org. Chem., 2000, 4, 1287–1324; ref. 1); the current contribution considers the family at a global level.
 Dulcie A. Mulholland | Dulcie Mulholland leads the Natural Products Research Group and is Head of the Department of Chemistry at the University of Surrey. She undertook her PhD at the University of Natal, Durban, South Africa, under the supervision of David Taylor on the Chemistry of the Meliaceae. For the last 20 years she has collaborated with Neil Crouch investigating the phytochemistry of the Hyacinthaceae of southern Africa. |
 Sianne L. Schwikkard | Sianne Schwikkard obtained her PhD from the University of Kwazulu-Natal, South Africa in 1998 (under the supervision of Professor Dulcie Mulholland). This was followed by post-doctoral studies at Virginia Tech with Professor David Kingston and further post-doctoral studies at the Rand Academic University with Professor Fanie van Heerden. After spending 18 months working for Sasol Ltd, Sianne relocated to the United Kingdom and following a post-doctoral position, under Professor Keith Jones at Kingston University, has continued to teach in a part-time capacity at Kingston University in addition to her position as a part-time research associate at the University of Surrey. |
 Neil R. Crouch | Neil Crouch PhD (Natal) has worked as an ethnobotanist for the South African National Biodiversity Institute (SANBI) for nearly 20 years, largely on Zulu traditional medicine. He networks SANBI in natural products, pharmacological, horticultural, conservation, and Indigenous Knowledge Systems cataloguing programmes and is accordingly involved in state-sponsored bioprospecting consortia that develop new drugs for malaria and tuberculosis. As an Honorary Professor in the School of Chemistry and Physics at the University of KwaZulu-Natal, he explores his strong interest in the chemistry, traditional use and biosystematics of the Hyacinthaceae, a plant family with a major centre of diversity in southern Africa. |
This review reflects a (bracketed)† family and subfamily taxonomic arrangement for the Hyacinthaceae sensu APGII.2 A subsequent arrangement (APGIII)3 based on further molecular analyses is not here adopted, where the Hyacinthaceae was hypothetically included in an expanded circumscription of the Asparagaceae. In that the group of plants here dealt with are monophyletic,4 the systematic approach adopted for delimiting and naming family, subfamily or tribal level classifications ultimately has little bearing in relation to this review. We have adopted largely the phylogenies and generic concepts of Speta,5 Pfosser and Speta,4 and subsequent workers (Martínez-Azorín et al.)6 who have considered molecular, morphological and biogeographic characters in their circumscriptions. Ali Syed et al.7 have provided an excellent overview of alternative and often controversial systematic arrangements, of primary and secondary centres of geographic diversity, and the distribution of subfamilies and tribes at a global level. It should be noted that such is the state of flux of generic circumscription in the Hyacinthaceae that when providing a subfamilial classification for this unit (Asparagaceae subfamily Scilloideae sensu APGIII) Chase et al.8 did not list genera names, which they otherwise offered with most other expanded Asparagalean families they treated. Given the past nomenclatural instability of the Hyacinthaceae, the names of plants under which the original phytochemical and pharmacological work was conducted often differs radically from the names accepted currently. For this reason we here provide both current and reported names, with species authors, to make clear this link. The literally reported (and sometimes incorrectly spelt) taxon names and species authors (if given) are provided in parentheses. The subfamilies are discussed in turn, starting with the Hyacinthoideae as most work has been conducted on this, the largest, subfamily. The Urgineoideae and finally the Ornithogaloideae follow. No reported investigations into the Oziroëoideae appear in the literature. Within each subfamily, those species receiving a substantial amount of attention will be discussed first, followed by the genera grouped by region (Europe and the Mediterranean, Middle and Far East and North Africa, southern Africa and finally, in the case of the Urgineoideae, India). Plants have been considered on a regional basis as in many cases the secondary metabolites found reflect a strong geographic pattern. Any early work on a genus is discussed before later work.
1 The Hyacinthoideae
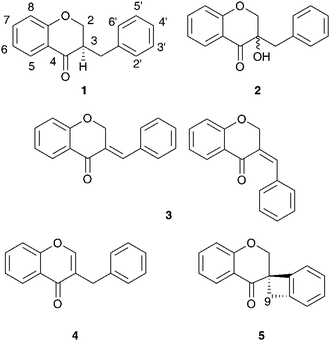
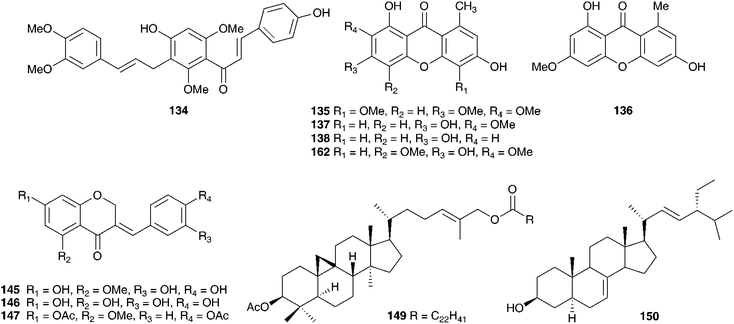
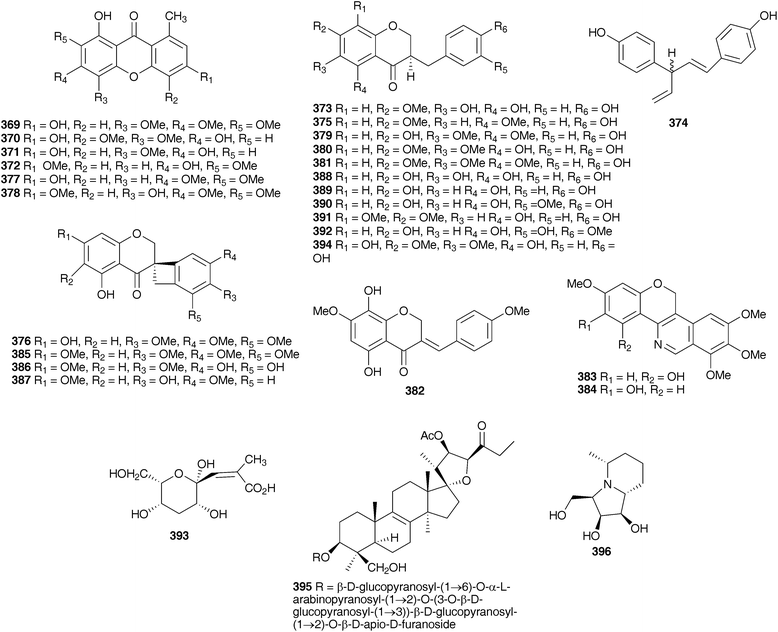
The subfamily Hyacinthoideae comprises more than 400 species in thirty-eight genera.5,7 The Hyacinthoideae are distributed in sub-Saharan Africa, India, Madagascar, eastern Asia, the Mediterranean region and Eurasia. Sub-Saharan Africa appears to be the centre of origin for the subfamily, with a secondary centre of diversification and radiation being the Mediterranean region.7 Twenty genera of the Hyacinthoideae have been investigated, with the bulbs receiving most attention. The exception is Hyacinthus orientalis L., the flowers of which have been extensively examined. Homoisoflavanones and spirocyclic nortriterpenoids are widespread throughout the subfamily with chalcones, xanthones, flavonoids, alkaloids, stilbenoids, chromones, norlignans and benzopyranones also being reported.Abegaz et al.9 and du Toit et al.10 have reviewed the phytochemistry, biological activity, biosynthesis and synthesis of the homoisoflavanones. The homoisoflavanones can be divided into five categories, the 3-benzyl-4-chromanones 1, the 3-hydroxy-3-benzyl-4-chromanones 2, the 3-benzylidene-4-chromanones (E or Z) 3, the 3-benzyl-chrom-2-en-4-ones 4 and the scillascillins 5. The spirocyclic nortriterpenoids are generally of the lanosterol-type, showing a wide range of substitution patterns. The genera receiving the most attention, Eucomis L'Hér., Muscari Mill. and Ledebouria Roth. will be discussed first, followed by the lesser-studied Eurasian and Mediterranean genera Leopoldia Parl., Scilla L., Barnardia Lindl., Othocallis Salisb., Oncostema Raf., Hyacinthus L., Bellevalia Lapeyr., Autonoe (Webb & Berthel.) Speta and Hyacinthoides Medik. Lastly, the southern African genera Drimiopsis Lindl. & Paxton, Resnova van der Merwe, Veltheimia Gled., Merwilla Speta, Lachenalia J.Jacq. ex Murray, Pseudoprospero Speta, Schizocarphus van der Merwe and Spetaea Wetchnig & Pfosser are dealt with.
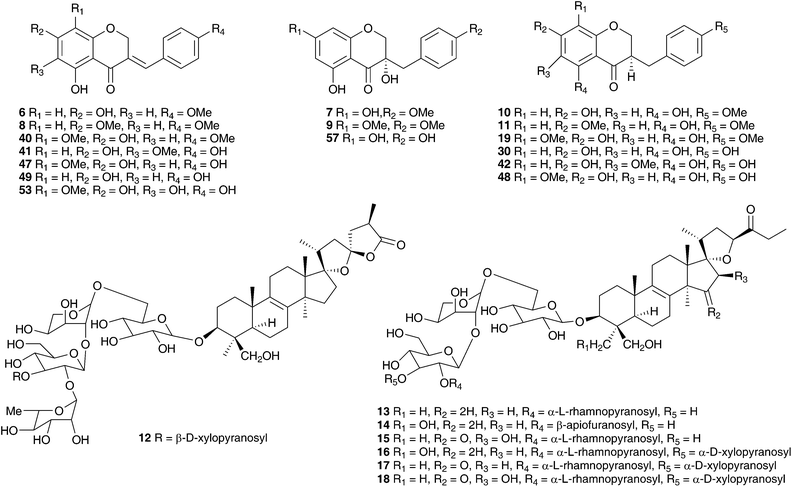
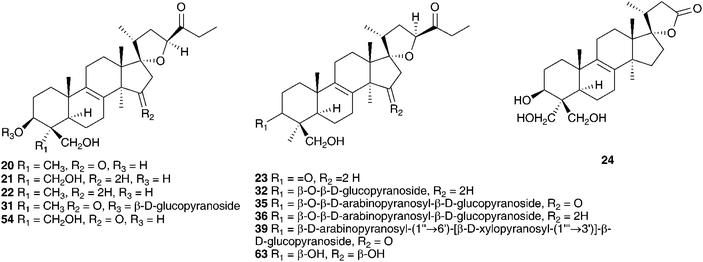
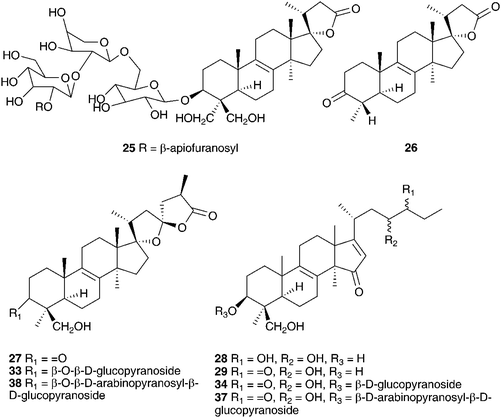
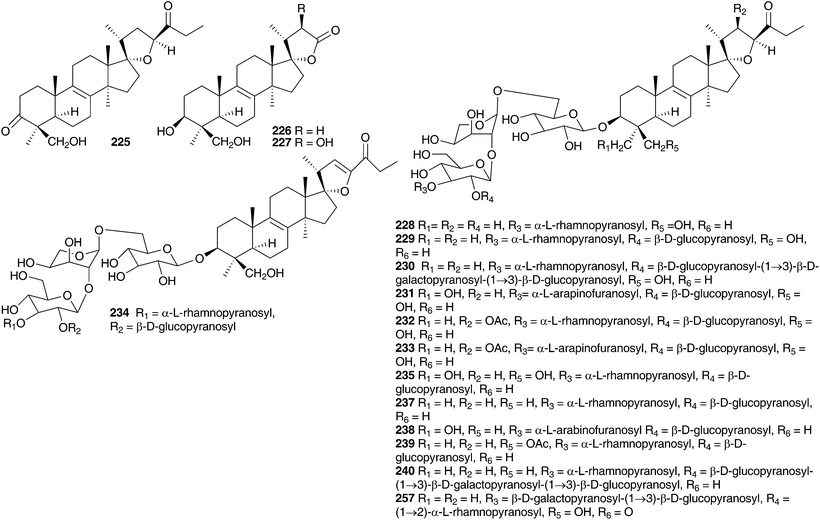
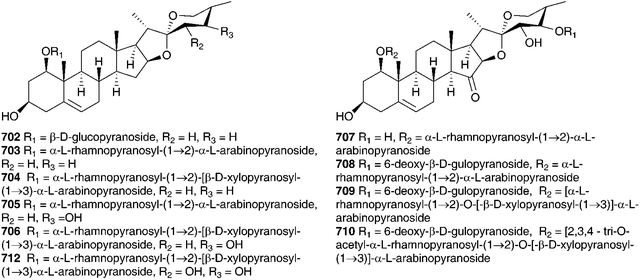
The genus Eucomis is widespread throughout eastern and southern Africa. It is used by traditional healers to treat a wide range of complaints, including rheumatism, as an anti-inflammatory and as a treatment for mental disease. Homoisoflavanones and triterpenoid glycosides have been isolated from the bulbs of many of the species investigated, with the homoisoflavanones usually accumulating in the waxy layer between the storage leaves of the bulb and the terpenoids in the bulb tissue.11 Early work on Eucomis bicolor Baker resulted in the isolation of the yellow crystalline needles of eucomin 6 and the colourless hexagonal plates of eucomol 7.12 The configuration at C-3 of eucomol 7 was determined by CD experiments and confirmed by X-ray analysis of the p-bromophenacyl derivative.13 Four additional homoisoflavonoids have been isolated from the bulbs of E. bicolor, (8–11)14,15 followed a number of years later by the identification of seven triterpenoid oligosaccharides (12–18).16,17 Scillasaponin A 12 has a modified spirocyclic side chain at C-17 and C-23 and has been found to inhibit cyclic AMP phosphodiesterase (IC50 = 11.5 × 10−5 M).16 Compounds 13, 14 and 16 were found to be toxic to HeLa cells at concentrations of both 50 μg ml−1 and 5 μg ml−1.17 A later investigation of E. bicolor resulted in the isolation of twelve compounds from the dichloromethane extract and ten from the methanol extract. The dichloromethane extract yielded the seven known compounds (7, 10, 19, 20, 21, 22 and 23) and five novel triterpenoids (24 [the aglycone of 25], 26, 27, 28 and 29). The methanol extract was found to contain the known homoisoflavonoid 30 as well as nine novel lanosterol glycosides (31–39).18
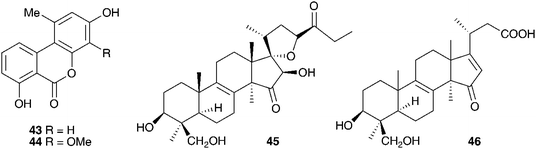
Eucomis autumnalis (Mill.) Chitt. (syn. Eucomis undulata Aiton) bulbs are used in the Eastern Cape of South Africa to treat cancer,19 and are used by Zulu traditional healers as an emetic and enema to treat fevers, as well as for the treatment of skin infections, biliousness, and urinary and respiratory infections. It is also used as a charm against witchcraft.20 Phytochemical investigations of the bulbs of E. autumnalis have resulted in the isolation of three homoisoflavanones (40–42),14,21,22 two dibenzo-α-pyrones (43–44),23 two spirocyclic nortriterpenoids (20 and 45) and an acid, compound 46.24 The structures assigned to compounds 43 and 44 were subsequently revised and reported to be the corresponding xanthones.25 The proposed structure for eucosterol 20 was confirmed by the X-ray structure of the p-bromobenzenesulphonate derivative.26 The extensive use of E. autumnalis by traditional healers for fever and the treatment of skin conditions, in particular wounds, boils and open sores, has led to extensive investigations into the anti-inflammatory and antibacterial effects of extracts of the leaves, bulbs and roots. The inhibition of prostaglandin synthesis was assessed in terms of the inhibition of the cyclooxygenase enzymes COX-1 and COX-2. COX-1 is found in normal cells and serves to produce substances that protect the stomach and kidneys (prostanoids). While both enzymes are involved in the inflammatory process, inhibition of COX-1 is associated with gastro-intestinal and kidney related side effects. As such, a higher selectivity for COX-2 inhibition is preferred.27 Initial testing of the leaves, bulbs and roots of E. autumnalis against COX-1 showed good activity from all extracts (leaf extract IC50 = 15 μg ml−1, root extract 27 μg ml−1 and bulb extract 72 μg ml−1).28,29 A comparison of the COX-1 and COX-2 inhibitory activity of the leaf, root and bulb extracts, showed that the leaf extract inhibited COX-1 to a greater extent while the root and bulb extract showed greater inhibition of COX-2 (COX-2/COX-1 of 0.7 and 0.8 respectively for the root and bulb extracts).27,30 There has been some interest in the biological effects of plant lectins obtained from the bulbs of E. autumnalis. These non-enzymatic proteins can bind, with high specificity and reversibility, mono- and oligosaccharides. Cyclooxygenase is a membrane-bound glycoprotein and as such could be affected by plant lectins. The partially purified lectin-like protein extract of E. autumnalis was found to have very good cyclooxygenase inhibitory activity (COX-1 inhibition of 88%)31 as well as good activity against the Gram-positive bacterium Bacillus subtilis (MIC of 0.2 mg ml−1).32 Due to the widespread use of the bulbs of E. autumnalis by South African traditional healers, extensive work has been carried out on the factors possibly affecting the effectiveness of the bulb extracts. Environmental factors such as sunlight intensity, temperature and watering as well as age of the bulbs and the effect of cold storage have all been examined.33 Bulbs lifted and stored at a low temperature (8–10 °C) showed greater COX-1 inhibitory activity.34 The leaves of young plants showed greater COX-1 inhibitory activity than those of older plants, where the greatest activity was associated with bulbs and roots.35
Six homoisoflavanones (19, 40, 47–50)14,36 and the optically active 2-hydroxy-2-[(4-hydroxyphenyl)methyl]butanedioic acid 51 have been isolated from the bulbs of Eucomis comosa (Houtt.) H. R. Wehrh. (as Eucomis punctata L'Herit).37 The bulb wax of Eucomis comosa yielded the chroman-4-one 5238 while an examination of the methanol extract of the fresh, air-dried bulbs gave five homoisoflavanones (47, 48, 50 and both the E and Z form of 6).39 The same study included Eucomis pallidiflora Baker subsp. pole-evansii (N.E.Br.) Reyneke ex J.C.Manning and Eucomis schijffii Reyneke. The bulbs of Eucomis pallidiflora subsp. pole-evansii contained the homoisoflavanone 53 and the spirocyclic nortriterpenoid 20 while Eucomis schijffii yielded two compounds, the spirocyclic nortriterpenoid 54 and scillascillin 55.39Eucomis montana Compton was investigated and the bulbs were found to contain eleven homoisoflavanones (6, 7, 9, 10, 30, 50, 56, 57, 58, 59 and 60).40 No inhibitory activity against cyclooxygenase enzymes (COX-1 and COX-2) was found for compounds 48, 53 and 55 isolated from E. comosa, E. pallidiflora and E. schijffii respectively.39 Some antibacterial activity has been observed for homoisoflavanones isolated from E. comosa (MIC of 0.52 mM for compound 6 (E isomer) and 0.24 mM for compound 47) and E. schijffii (MIC of 0.50 mM for compound 55).41 The dichloromethane and methanol extracts of the whole plant of Eucomis vandermerwei I.Verd. yielded four known homoisoflavanones (6, 7, 10 and 56).42 Similarly, the whole plant of Eucomis zambesiaca Baker was investigated and this resulted in the isolation and identification of five homoisoflavanones (6, 19, 40, 61 and 62) and three nortriterpenoids (20, 21 and 63).42
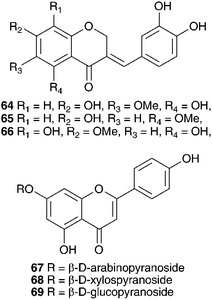
The bulbs of Muscari racemosum Mill. were found to contain homoisoflavanones of the 3-benzylidene-4-chromanone-type (64–66).43 Long-range coupling between C-2 and C-9, was indicative of Z geometry. These homoisoflavanones have been investigated with respect to their effect on lipid peroxidation in vitro.44,45 Strong anti-oxidant activity was noted (IC50 = 0.94–7.98 μM) and this led to an investigation into their antimutagenic/anticarcinogenic activity. It has been suggested that polyphenolics may be cancer preventative due to their antioxidant properties44 and a mixture of these three homoisoflavanones was found to contain significant antimutagenic activity.44 Estrogenic activity was found in an ether extract of the bulbs. The extract induced proliferation of MCF7 cells in a dose dependent manner at concentrations up to 5 μg ml−1. This concentration produced the greatest effect of 181% of the control.46
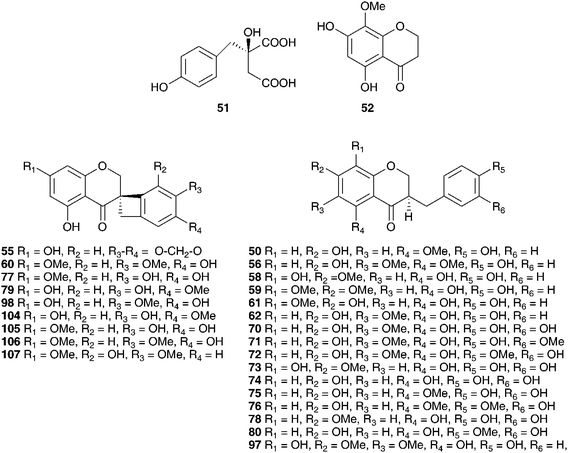
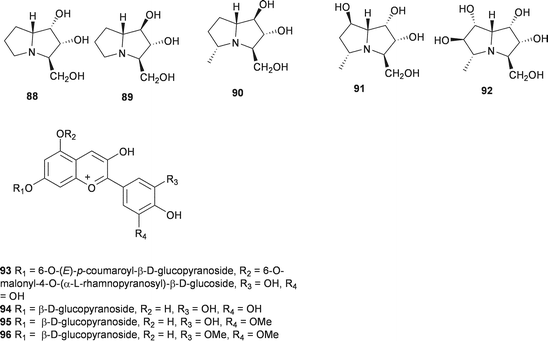
A wide range of compounds has been isolated from the bulbs of Muscari armeniacum Leichtlin ex Baker. These include flavonoids (67–69),47 homoisoflavanones, with an unusual 3′,4′ substitution pattern in the B ring (30, 62, 70–80),48,49 spirocyclic nortriterpenoid glycosides (81–87)50 and polyhydroxylated pyrrolizidine alkaloids (88–92).51 The alkaloids were assessed for their action against glycosidases. Compound 88 was found to be a potent inhibitor of rat intestine lactase (IC50 = 4.4 μM) as well as a moderate inhibitor of α-L-fucosidase (IC50 = 46 μM) and amyloglucosidase (IC50 = 25 μM). Inverting the hydroxyl group at C-1 to give compound 89, enhanced the amyloglucosidase inhibition (IC50 = 8.6 μM) but resulted in the loss of the α-L-fucosidase inhibition. Compounds 90 and 91 were less active.51 Three ribosome-inactivating proteins have been isolated from the bulbs of M. armeniacum. It has been proposed that this type of activity is linked to an anti-viral response.52 The petals of M. armeniacum have yielded four anthocyanins, the new muscarinin A 93 and the known 3-O-β-D-glucopyranoside of delphinidin, petunidin and malvidin (94–96).53–55 The principle water soluble polysaccharide in the bulbs of M. armeniacum (as Muscari szovitsianum Baker) was found to be a neutral glucofructan, with glucose and fructose residues in the ratio 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.56,57
1.56,57
Phytochemical investigations into the bulbs of Muscari botryoides (L.) Mill. confirmed a close taxonomic relationship with Muscari armeniacum. Similar homoisoflavanones (19, 30, 58, 60, 77, 79, 97, 98)48 were isolated as well as similar spirocyclic nortripertenoid glycosides (86, 87, 99, 100, 101, 102, 103).50Muscari neglectum Guss. ex Ten. was found to contain a similar spread of homoisoflavanones in the bulbs (30, 62, 80, 104–107) as well as an interesting scillascillin-type homoisoflavanone with an unusual oxygenation pattern in the B ring (55).58 Ethanol extracts of the bulbs and leaves of Muscari neglectum have been tested for antifungal activity against the wood rots Postia placenta and Trametes versicolor. Significant reduction in wood weight loss was observed for Turkish oriental beech and Scots pine (weight loss dropped from 35–42% in the untreated wood to 5.4–18.3% in the treated wood).59
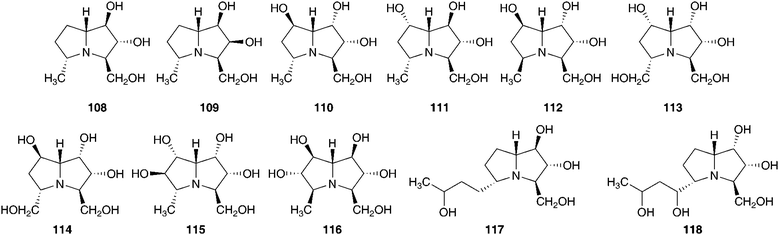
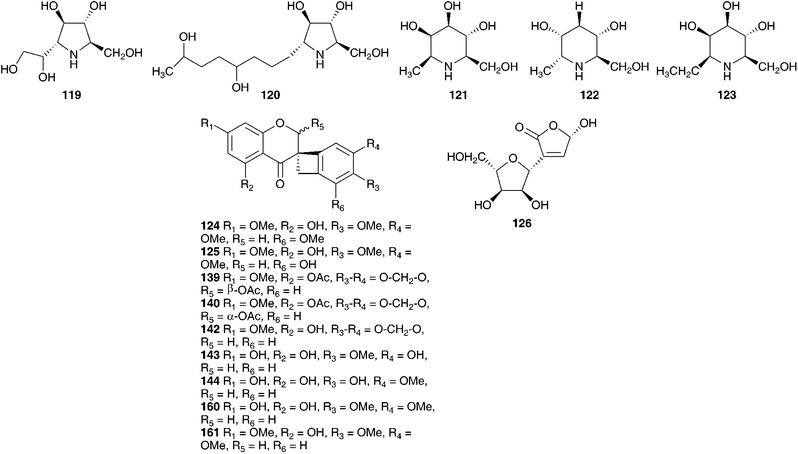
An aqueous ethanol extract of the bulbs of Ledebouria socialis (Baker) Jessop (as Scilla socialis Baker) was found to contain eleven hyacinthacines (108–118) as well as two pyrrolidines (119–120) and three piperidines (121–123).60 Hyacinthacines appear to be restricted to the Hyacinthoideae. These compounds have been found in Othocallis siberica (Haw.) Speta (as Scilla sibirica), Oncostema peruviana (L.) Speta (as Scilla peruviana), Hyacinthoides non-scripta (L.) Chouard ex Rothm., Hyacinthoides hispanica (Mill.) Rothm. (as Scilla campanulata), and Muscari armeniacum. The hyacinthacines are characterised as 7α-R-hydro-1,2-dihydroxy-3-hydroxymethylpyrrolizidines and in general appear to be weak to moderate inhibitors of glycosidases.60 The dichloromethane and methanol extracts of the bulbs of Ledebouria socialis have yielded five compounds, the scillascillin-type homoisoflavanones 124 and 125, phytol, stigmasterol and the polyhydroxylated difuran derivative, polybotrin 126.61 The different types of compounds isolated reflect the different solvents used for extraction, with the more polar extracts yielding the more polar polyhydroxy alkaloids and the less polar extracts the homoisoflavanones and sterols.
Early work by Gunn et al.62 on some southern African Ledebouria species, examined the cardiac effect of Ledebouria cooperi (Hook.f.) Jessop (as Scilla cooperi Hook and as Scilla rogersii Baker) and Ledebouria ovatifolia (Baker) Jessop (as Scilla lanceaefolia Baker). Both species produced a similar action to digitalis when applied to isolated frog and cat hearts as well as in situ frog, cat and dog hearts. The extracts obtained were, however, insufficient in quantity to determine minimum lethal doses and the active components were not identified.
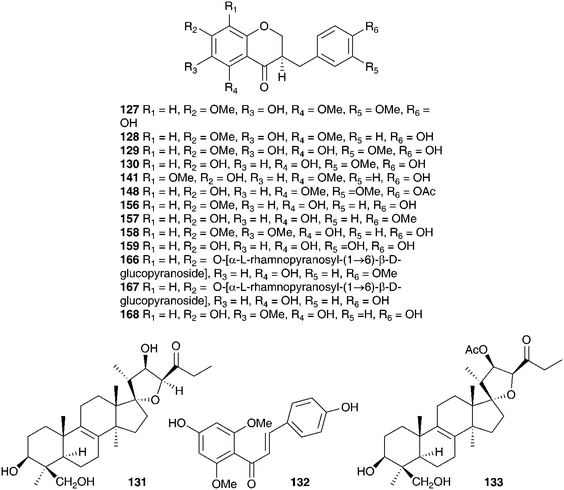
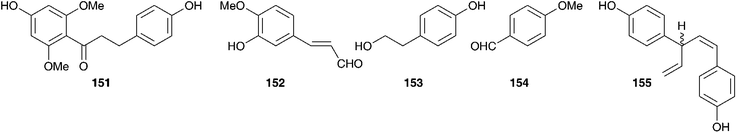
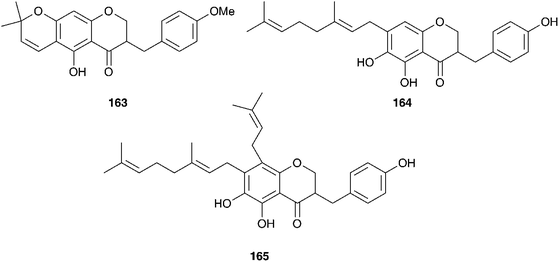
The chemical constituents of a further six southern African Ledebouria species have been investigated. Two samples of the bulbs of Ledebouria zebrina (Baker) S.Venter (as Scilla zebrina Baker), collected from two different regions in South Africa (Durban and Blyde River, Mpumalanga) have been investigated and the chemical differences noted. Both contained homoisoflavanones (Durban bulbs compounds 127 and 128 and the Blyde River bulbs compounds 127, 129, 130). In addition to the homisoflavanones, the Blyde River bulbs also contained spirocyclic nortriterpenoids (131, 21), while the Durban bulbs produced a chalcone 132.63 The bulbs of Ledebouria cooperi yielded the homoisoflavanones 59 and 62 as well as the eucosterol-type spirocyclic nortriterpenoid 133 and malic acid. Ledebouria ovatifolia bulbs were found to contain the chalcones 132 and 134 as well as the homoisoflavanone 30.64,65L. ovatifolia is used by the Zulu to treat gastro-enteritis, influenza and backache. In addition, it has been found to be toxic to sheep.66 Extracts of the bulbs were found to have good antibacterial activity against Gram-positive bacteria (MIC of 0.8–12.5 mg ml−1), but poor anti-inflammatory and poor antihelmintic activity.66,67 A later investigation of the dichloromethane, ethyl acetate and methanol extracts of the bulbs led to the isolation of twenty-nine compounds, including xanthones (135–138), homoisoflavanones (7, 10, 30, 50, 74, 75, 139–148), triterpenoids (133, 149–150), chalcones (132, 151) and simple aromatic compounds (152–154), including polybotrin 126.61,68 Sixteen of the above compounds isolated from L. ovatifolia were assessed for anti-inflammatory activity, in particular the selective inhibition of the COX-2 enzyme. Compounds 75, 137 and 146 were found to significantly inhibit COX-1 and COX-2 (percentage activity relative to the non-inhibitor control of 54–56% for COX-1 and 0% for COX-2), while compound 145 showed good selectivity for the inhibition of COX-2 over COX-1 (IC50 for COX-2 of 2.87 μM, with no statistically significant inhibition for COX-1 up to a concentration of 20 μM).61,68 During the same study, the norlignan hinokiresinol 155, previously isolated from Drimiopsis burkei Baker,69 was synthesised and tested for selective COX-2 inhibitory activity. Significant selective inhibition against COX-2 was observed (percentage activity relative to the non-inhibitor control of 93.8% for COX-1 and 0% for COX-2).61,68Ledebouria leptophylla (Baker) S.Venter (as Ledebouria graminifolia (Baker) Jessop), sourced in Botswana, is used traditionally to treat gastro-enteritis, backache and coughs as well as a treatment for skin irritations and for dressing wounds. A phytochemical investigation of the bulbs found seven 3-benzyl-4-chromanones (30, 62, 128, 156–159), two 3-benzyl-3-hydroxy-4-chromanones (7, 57), two scillascillin-type homisoflavanones (160–161) and two xanthones (136 and 162). Cultivated and wild-sourced bulbs contained the same compounds.70,71 There was initial concern about the presence of the xanthones and speculation as to whether they may have come from a fungal or lichen contamination of the wild bulbs. The fact that the cultured bulbs contained the same xanthones confirmed that they were secondary metabolites of L. leptophylla.72
Within South Africa Ledebouria revoluta (L.f.) Jessop is extremely widespread. It is used by the Sotho to drive away lightening and with the exception of pregnant women, to treat lumbago. The Kwena and Tswana use it to treat wounds and skin conditions. The bulbs were found to contain homoisoflavanones (30, 59, 61 and 157).73Ledebouria floribunda (Baker) Jessop is used by traditional healers in the Eastern Cape of South Africa to treat skin disorders, tuberculosis, urinary tract infections and gastro-enteritis. Three homoisoflavanones, with unusual substituents at C-6, -7 and -8 (163–165) have been isolated from the bulbs as well as two 7-O-diglycosides (166–167) and five additional homoisoflavanones (10, 30, 50, 62, 168). These latter seven homoisoflavanones have shown good antioxidant activity against the DPPH radical (IC50 of 31–273 μg ml−1) and in the β-carotene/linoleic acid system (antioxidant activity of up to 79% after 120 min) as did 164 and 165 against the DPPH radical (IC50 of 23.2 and 28.7 μg ml−1 respectively).74,75
Leopoldia comosa (L.) Parl. is found largely in the Mediterranean region and the bulbs have been used as a food source for generations.76 Evidence for the ancient use of this plant includes the discovery of traces of this species in a Neanderthal grave in Iraq.77 The bulbs (as Muscari comosum Mill.) were examined for antioxidant activity as part of a study on non-cultivated vegetables commonly consumed by Albanians living in southern Italy. Twenty-seven plant species were investigated, with two showing high activity, one of which was L. comosa (inhibitory activity on a DPPH assay of 85%).78 A further study also found good antioxidant activity as well as hypoglycemic activity via the inhibition of carbohydrate digestive enzymes.79 Extracts of L. comosa (as Muscari comosum Mill.) have been found to be effective in combatting the wood rot fungus Postia placenta in samples of Pinus sylvestris L. and Fagus orientalis.80 Phytochemical studies on L. comosa (as Muscari comosum Mill.) have resulted in the isolation of nineteen nortriterpenoids of the lanosterol-type as well as homoisoflavanones.
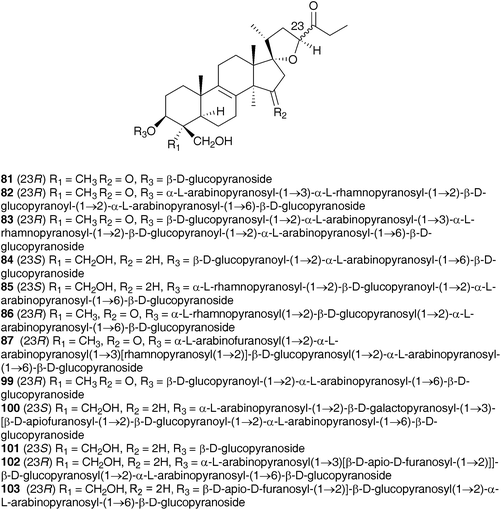
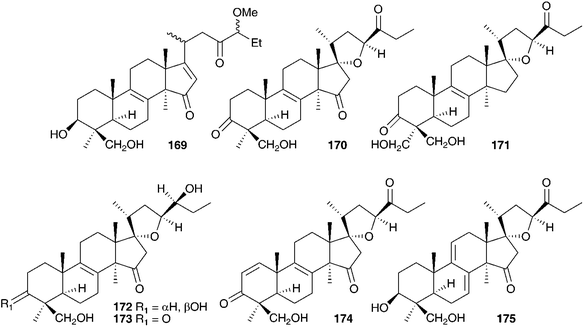
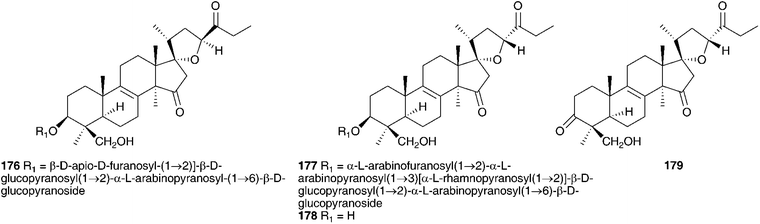
Initial work on the bulbs was complicated by the presence of complex glycosides. Acid catalysed methanolysis of the glycoside mixture resulted in the identification of the aglycones. Eucosterol 20 was found to be the major component, with compounds 21, 54, 169, 170 and 171 present in smaller amounts.76,81–84 Extensive work on the bulbs by Adinolfi et al. resulted in the isolation of the free aglycones present in the bulbs (20, 21, 22, 54, 169–175)85–89 as well as the full structural elucidation of the glycosides (86, 87, 102, 103 and 176).90–94 All the nortriterpenes isolated from Leopoldia comosa mentioned so far have S configuration at C-23, three compounds with R configuration have also been identified, 177,91178 (23R20) and 179 (23R170).89
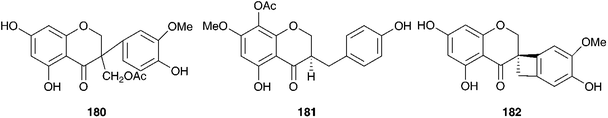
Two classes of homoisoflavanones have been isolated from the bulbs of L. comosa, the 3-benzyl-4-chromones (30, 58, 59, 61, 62, 80, 97, 180–181)95,96 and the scillascillin type (55, 104–107 and 182).97,98 The absolute configuration at C-3 of these homoisoflavanones was determined using circular dichroism and found to be R in all cases.99 For the scillascillin type, this was further confirmed by X-ray analysis.98 The crude extract of the bulbs has been subjected to anti-inflammatory bioassay-directed fractionation using a croton oil-induced mouse ear dermatitis test. The homoisoflavanone rich fraction was found to have high activity and further fractionation resulted in the isolation of 30, 59, 61, 80 and 97, all of which showed activity, with compound 30 being the most active at 41% inhibition (100 μg ear−1).100 Two flavonoids have been isolated from the flowers of L. comosa, 3-glucoarabinosyl- and 3-rhamnoarabinosyl-3,4,5-trihydroxy-7-alkoxyl flavone.101
Leopoldia comosa is used in the Basilicata region of southern Italy to treat toothache and headache.102 One third of the plants used for medicinal purposes in southern Italy are used to treat skin and soft tissue infections. As Staphylococcus aureus is a common cause of such infections, the effect of a number of Italian plants, including the bulbs of Leopoldia comosa, on the inhibition of the S. aureus biofilms has been studied. Significant dose-dependent biofilm inhibition (IC50 = 16 μg ml−1) was noted for L. comosa.103
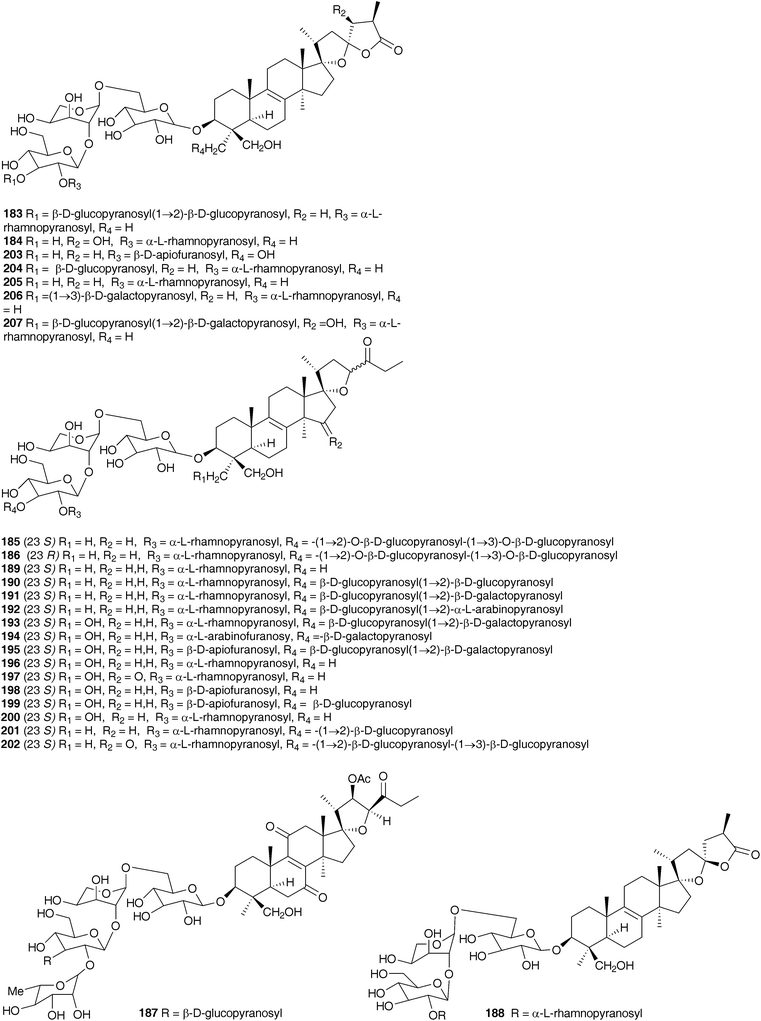
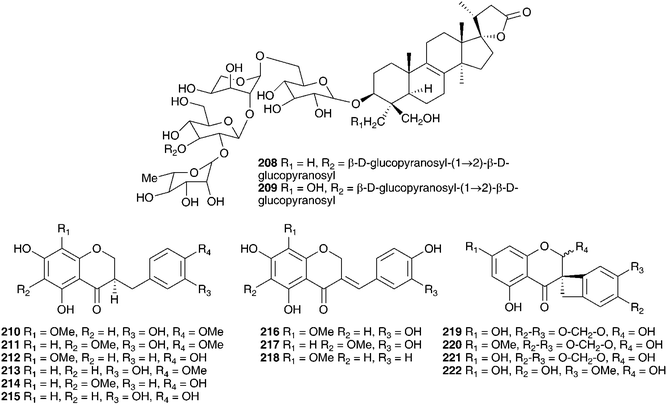
Investigations into the chemistry of the bulbs of Scilla luciliae (Boiss.) Speta (as Chionodoxa gigantea Whittall and as Choinodoxa luciliae Boiss.) has yielded a large number of lanosterol-type spirocyclic nortriterpenoids, many of which have been tested for biological activity. Compounds 183–18616,104 and compounds 13 and 187–18817 have been isolated from the bulbs of Scilla luciliae (as Chionodoxa gigantea). In separate investigations, compounds 13, 184 and 187–19017 as well as compounds 189 and 190–197 and eucosterol 20,105 compounds 198–204,106205–207107 and 208–209108 have been found in the bulbs of this species (as C. luciliae). Compounds 183–186 all showed some inhibitory activity on cyclic AMP phosphodiesterase, with compound 183 giving the best results (IC50 = 11.2 × 10−5 M).104 Cytotoxicity against HeLa cells was assessed for 13, 184, 187, 188, 189, and 190 with the 15-deoxoeucosterol oligosaccharides (13, 187 and 190) showing good activity down to 5 μg ml−1.17 Compounds 198–204 as well as 183 and 185 were tested for activity against HSC-2 human oral squamous carcinoma cells. Compounds 183, 185, 200, 201 and 204 all gave good results with LD50 values in the range 10–23 μg ml−1 (etoposide LD50 = 24 μg ml−1, for etoposide resistant HSC cells). It was noted that the 23R isomer of 185, compound 186 did not show any activity.106 Compounds 208 and 209 showed only weak activity in the same assay (LD50 of 254 and 238 μg ml−1 respectively).108 In addition to the spirocyclic nortriterpenoids, the bulbs of Scilla luciliae (as Chionodoxa luciliae) have been found to contain the homoisoflavanones 210–222.109
The bulbs and aerial parts of Scilla bifolia L. have been studied using HPLC and mass spectrometry. Eighteen polyphenols were identified from extracts of the bulbs and aerial parts (caftaric acid, isoquercitin, routine, myricetol, fistein, quercetol, patuletin, gentisic acid, caffeic acid, chlorogenic acid, p-coumaric acid, ferulic acid, hyperoside, quercitrin, luteolin, kaempferol, apigenin and sinapic acid).110
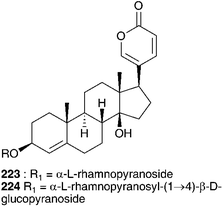
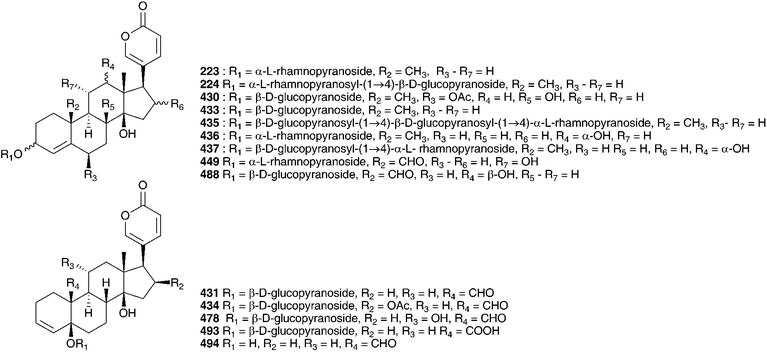
The methanol and aqueous ethanol extracts of the bulbs of Autonoe madeirensis (Menezes) Speta (as Scilla maderensis Menezes), a species endemic to the Portuguese archipelago of Madeira, was found to produce cardiac action on a frog heart and although TLC showed compounds with Rf values and colours very similar to the bufadienolides proscillaridin A 223 and scillaren A 224, typical of the Urgineoideae, the presence of these compounds was not conclusively established.111
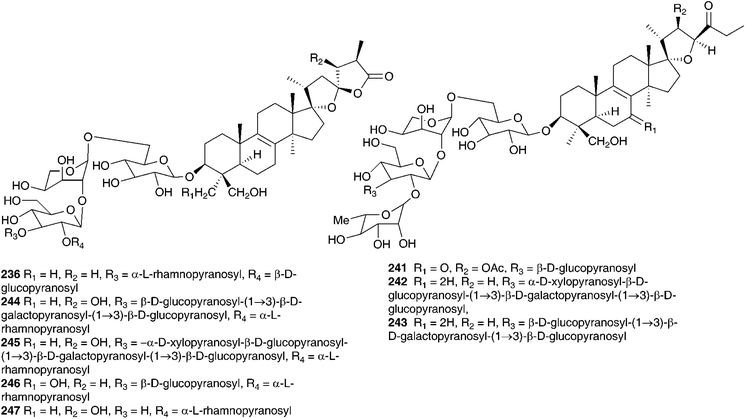
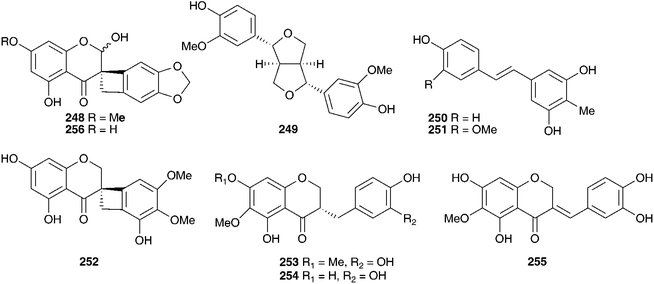
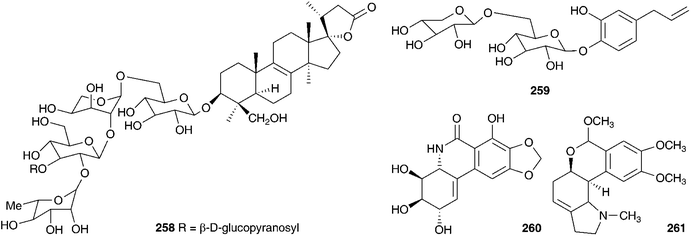
Barnardia japonica (Thunb.) Schult. & Schult.f. (as Scilla scilloides (Lind.) Druce) has been used for a long time by traditional Chinese healers to treat abscesses and to promote circulation.112 This plant also grows extensively in the wild in Japan and Korea and has been used by traditional healers of the latter country.113 The root extract has been evaluated for its potential as an antimicrobial agent, as an anti-inflammatory and as an antioxidant.113 The root extract of B. japonica (as Scilla scilloides) was found to inhibit the growth of Staphylococcus aureus, Salmonella enteritidis, Escherichia coli and Candida parapsilosis down to a concentration of 0.1% of the extract. Antioxidant activity was evaluated by looking at the inhibition of hyaluronidase, an enzyme that initiates the degradation of hyaluronic acid, associated with inflammation. Inhibition was noted at concentrations of root extract of 0.1 (14.8% inhibition) and 1% (48.2% inhibition), but not below. Anti-oxidant activity was assessed by monitoring the oxidation of linoleic acid and good activity was found (anti-oxidative index of 33.2 at a concentration of 1%).113 Extensive phytochemical investigations of the bulbs of B. japonica (as Scilla scilloides) resulted in the isolation of spirocyclic nortriterpenes (21, 22, 131, 225, 226 and 227),114,115 the related oligoglycosides (187, 228–239 and 241–247)112,116–118 and homoisoflavanones (42, 55 and 248).119 Nishida et al.120 reported the isolation of sixteen compounds from the methanol extract of the bulbs, including three nortriterpenes (21, 22, and 225), a lignan 249, a xanthone 137, two homostilbenes (250 and 251) and nine homoisoflavanones (49, 55, C-3 epimer compound of 62, 182, 252, 253,and 254–156). The configuration at C-3 for 253, 254 and the epimer of compound 62 was unusually found to be S by circular dichroism.120 Scillascilloside E-3 239 and scillascilloside E-1 237 have shown potent cytotoxicity against a range of human cancer cells (ED50 1.5–3.0 nM and 1.6–5.9 nM respectively).112,117 Patents have been filed covering the use of B. japonica (as Scilla scilloides) bulb extracts for the treatment of fungal infections121 and as a treatment for cancer.122 A later investigation into the methanol extract of the bulbs resulted in the isolation of two norlanostane-type triterpenoid glycosides (257 and 258), a phenylpropanoid glycoside 259 and two alkaloids (260 and 261).123 The aqueous methanol extract of the bulbs of B. japonica (as Scilla japonica) produced some cardiac action on an isolated toad heart muscle, although the active components responsible were not identified.124
Othocallis siberica (as Scilla sibirica Haw.) is native to southwestern Russia, the Caucasus and Turkey. The bulbs were found to contain 0.04% glycosides and 0.15% alkaloids, with the alkaloid fraction being able to stop the function of an isolated frog heart. The glycoside fraction showed a strong hypertensive action and was a stimulant to respiration.125 The hot water extract of the bulbs produced an acylated glucomannan with D-mannose, D-glucose and an acyl group in a ratio of 7.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
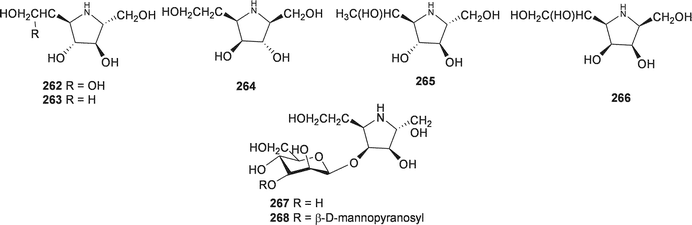
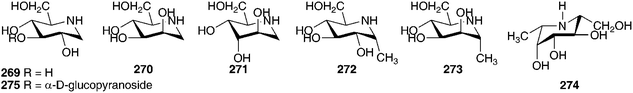
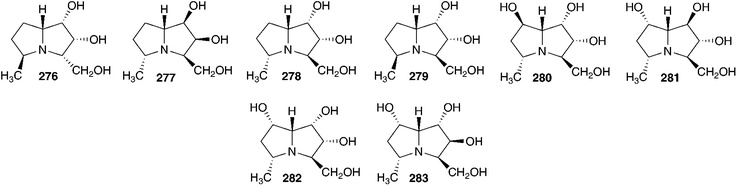
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4.126 As part of their investigations into potential glycosidase inhibitors, Yamashita et al. examined the ethanol extract of the bulbs of O. siberica (as Scilla sibirica). They isolated and identified five pyrrolidines (262–266), two pyrrolidine glycosides (267–268), six piperidines (269–274), one piperidine glycoside (275) and eight pyrrolizidines (276–283).127 These alkaloids were tested for their inhibitory activity against various glycosidases. Compound 262 was found to be a potent inhibitor of bacterial (Caldocellum sacchrolyticum) β-glucosidase and mammalian β-galactosidase (IC50 = 3.2 μg ml−1 and 4.4 μg ml−1 respectively). The loss of the hydroxy group at C-6, to give compound 263, significantly lowered the activity. Moderate or no activity was found for all other compounds isolated.127
2.4.126 As part of their investigations into potential glycosidase inhibitors, Yamashita et al. examined the ethanol extract of the bulbs of O. siberica (as Scilla sibirica). They isolated and identified five pyrrolidines (262–266), two pyrrolidine glycosides (267–268), six piperidines (269–274), one piperidine glycoside (275) and eight pyrrolizidines (276–283).127 These alkaloids were tested for their inhibitory activity against various glycosidases. Compound 262 was found to be a potent inhibitor of bacterial (Caldocellum sacchrolyticum) β-glucosidase and mammalian β-galactosidase (IC50 = 3.2 μg ml−1 and 4.4 μg ml−1 respectively). The loss of the hydroxy group at C-6, to give compound 263, significantly lowered the activity. Moderate or no activity was found for all other compounds isolated.127
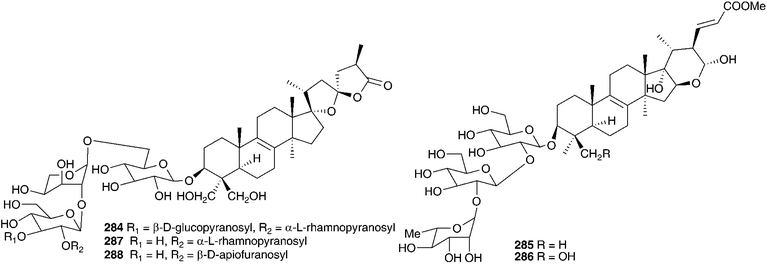
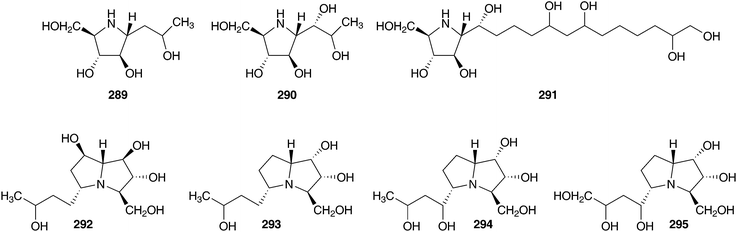
The bulbs of Oncostema peruviana (as Scilla peruviana) were found to contain rearranged lanosterol glycosides (scillasaponin B 284, peruvianoside A 285, peruvianoside B 286, and compounds 187–188 and 287–288)16,17,128,129 as well as a range of polyhydroxylated pyrrolidines (289–291) and pyrrolizidines (292–295).130 Moderate inhibitory activity against cyclic AMP phosphodiesterase was noted for peruvianoside A 285 (IC50 = 23.5 × 10−5 M) and scillasaponin B 286 (IC50 = 14.0 × 10−5 M),16,128 while compound 187, a 15-deoxoeucosterol oligosaccharide, was found to be toxic to HeLa cells at a concentration of 5 μg ml−1.17 The pyrrolidine 291 was found to be a potent inhibitor of bacterial β-glucosidase (IC50 = 80 nM), compounds 292 and 293 showed significant inhibition of yeast α-glucosidase (IC50 = 6.6 and 6.3 μM respectively) with compound 293 also showing good inhibition of bacterial β-glucosidase (IC50 = 5.1 μM).130
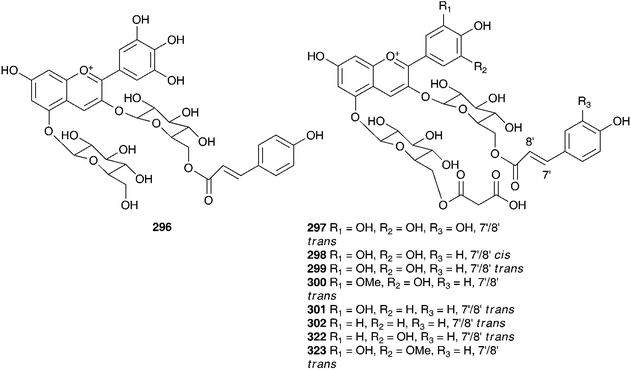
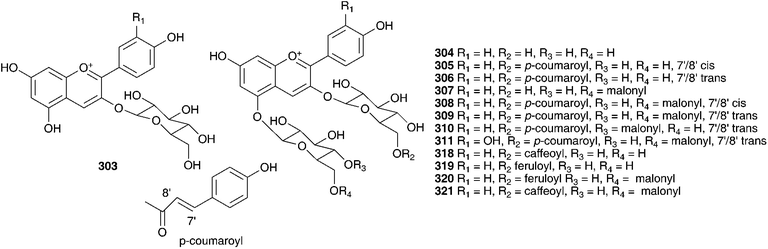
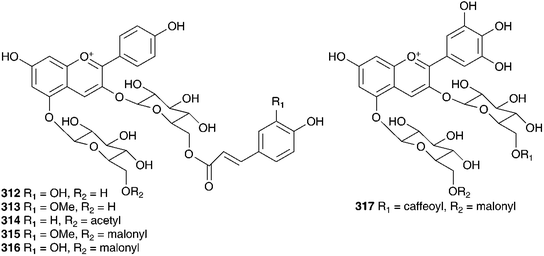
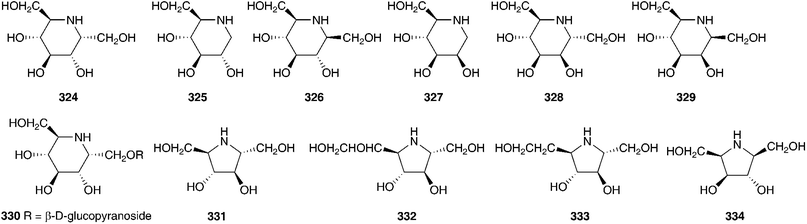
Phytochemical work on the Hyacinthaceae has primarily focused on the bulbs. The exception to this has been the genus Hyacinthus, where most of the work has been done on the flowers. Hyacinthus orientalis is used as an ornamental plant and can have blue, pink, yellow or white flowers. The blue flowers of H. orientalis have been found to contain a range of acylated anthocyanins (296–302).131 The anthocyanin content was also studied in the blue flowers produced in vitro. The same anthocyanins were produced with some small changes in the percentage composition of the different compounds.132 The red flowers of H. orientalis likewise produced a range of acylated anthocyanins (303–316) and, as with the blue flowers, the red flowers produced in vitro produced the same anthocyanins with some differences in composition.133–135 A later investigation of the blue flowers of H. orientalis revealed a further seven anthocyanins (317–323) in addition to those isolated previously.136 Differences in essential oil content between wild H. orientalis flowers and those generated in vitro have been studied. Four common components were found, 1-hepten-3-ol, benzyl alcohol, phenethyl alcohol and cinnamyl alcohol. The main components in the wild flowers were phenethyl alcohol (55% at stage 3 and 48% at stage 4) and cinnamyl alcohol (23% at stage 3 and 29% at stage 4) while the in vitro generated flowers produced 75% phenethyl alcohol as the major component.137 The bulbs of H. orientalis were found to be rich in polyhydroxy alkaloids. α-Homonojirimycin 324 was the major component138 with the compounds 325–334 being found in smaller amounts.138,139 Compound 332 was found to be a good inhibitor of bacterial β-glucosidase (IC50 = 3.8 μM), mammalian β-galactosidases (IC50 = 4.0 μM and 4.4 μM) and mammalian trehalases (5.0 μM and 2.0 μM), while compound 333 inhibited rice α-glucosidase (IC50 = 2.2 μM) and rat intestinal maltase (IC50 = 2.5 μM) and compound 334 inhibited α-L-fucosidase (IC50 = 50 μM).139
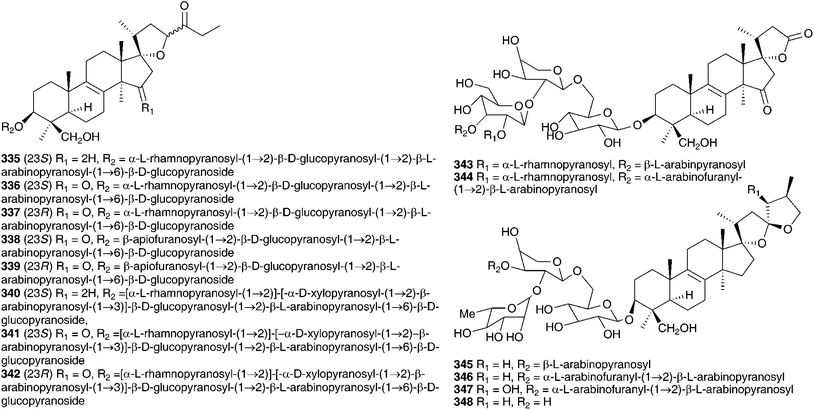
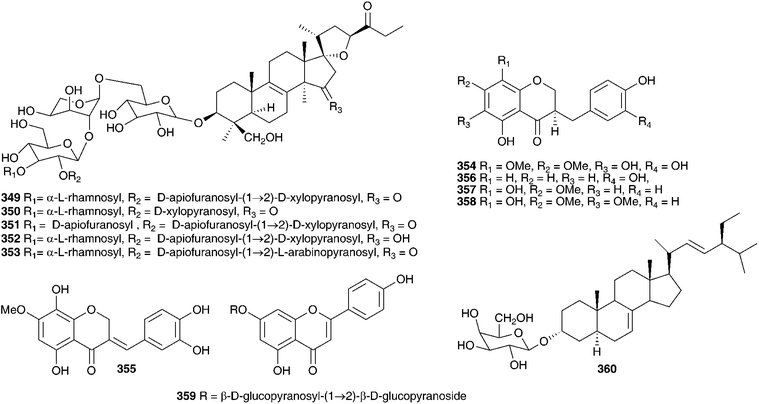
The methanol extract of the bulbs of Bellevalia paradoxa (Fisch. & C.A.Mey.) Boiss. (as Muscari paradoxum (Fisch. et C.A. Mey.) K.Koch) yielded eight 27-norlanostane glycosides (335–342)55 and seven tetranorlanosterol glycosides (25, 343–348).54 All were tested for cytotoxicity against HSC-2 human oral squamous cell carcinoma. Compounds 25 and 343–344 showed no activity while compounds 345–348 gave IC50 values of 6.3–59 μg ml−1, with the standard etoposide giving an IC50 of 24 μg ml−1. Compounds 335, 336, 338, 340 and 341, all 23S isomers, showed good activity relative to etoposide, but the corresponding 23R isomers showed no activity up to a dose of 100 μg ml−1. The methanol extract of the bulbs of Bellevalia romana (L.) Sweet produced oligoglycosides of the eucosterol-type, four new compounds bellevaliosides A 349, B 350, C 351 and D 352 as well as compounds 86, 87 and 353, previously isolated from Muscari species.140 In addition to the spirocyclic nortriterpenoids, five homoisoflavanones (354–358) were isolated.141 The bulbs of Autonoe madeirensis (as Scilla maderensis Menezes), have produced a slightly unusual phytochemical profile. An investigation of the ethanol extract of the bulbs resulted in the isolation of a range of 2-hydroxy di- and tricarboxylic acids and esters, cis- and trans-hydroxycinnamate esters and a flavone diglucoside 359.142 A later study of the bulbs found 24S-ethyl-5α-cholesta-7,22E-dien-3-ol-β-galactopyranoside 360 to be present.143 Dias et al. isolated the pyrimidine derivative 2-(4′-aminobenzenamine)-pyrimidine and found it to be an α-adrenoreceptor antagonist.144
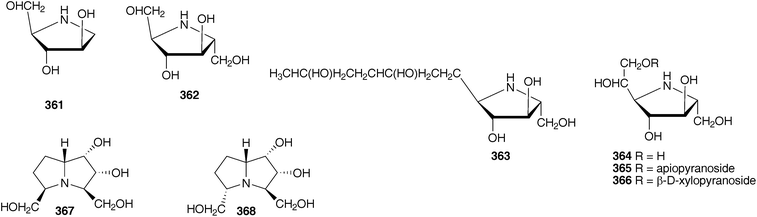
Work on the bulbs of Hyacinthoides hispanica (as Scilla campanulata) has focused on the isolation and structure determination of a mannose-specific lectin. Crystals have been grown and the structure determined by X-ray diffraction studies.145–149 In addition to the mannose-binding lectin, a fetuin-binding lectin has been isolated and identified.150 Polyhydroxylated alkaloids have been isolated from the ethanol extract of the bulbs of H. hispanica (as Scilla campanulata) and tested for glycosidase inhibitory activity. Compounds 361–368 were identified with compound 364 showing good inhibition of Caldocellum saccharolyticum β-glucosidase (IC50 = 3.8 μM) and bovine liver β-galactosidase (IC50 = 4.4 μM). Compound 366 was more active than compound 364 against Caldocellum saccharolyticum β-glucosidase (IC50 = 0.34 μM), but less so of bovine liver β-galactosidase (IC50 = 24 μM).151
Hyacinthoides non-scripta (bluebells) has long been known to be toxic to livestock. Horses suffer from abdominal pain and dysentery while cattle suffer from lethargy and dullness after consuming the plants. The toxic principles are the polyhydroxy pyrrolidine and pyrrolizidine alkaloids, common to many Hyacinthoideae.151,152 Alkaloids 361–367, also found in Hyacinthoides hispanica (as Scilla campanulata), have been isolated from the leaves, stalks and immature fruits of the bluebells.151,152 A glucomannan has been isolated from the seeds of H. non-scripta. It is a linear polymer with D-glucopyranose and D-mannopyranose residues occurring in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3.153
1.3.153
Southern African genera that have received the attention of phytochemists are Drimiopsis, Resnova, Veltheimia, Merwilla, Lachenalia, Pseudoprospero, Schizocarphus and Spetaea. The range of compounds isolated is typical of the subfamily Hyacinthoideae, including homoisoflavanones, spirocyclic nortriterpenoids and xanthones, as well as some more unusual alkaloids. Three species of Drimiopsis have been studied, Drimiopsis maculata Lindl. & Paxton, Drimiopsis burkei and Drimiopsis barteri Baker. Drimiopsis maculata is a southern African species used by traditional healers for treating a variety of complaints, including stomach complaints in children and the relief of constipation. It has not been found to be toxic to livestock.25 The bulbs of this plant have yielded scillascillin-type homoisoflavanones (compound 106, previously isolated from Muscari botryoides and compound 182, previously isolated from M. botryoides and M. armeniacum),154 xanthones (drimiopsins A–E, 163, 369, 138 and 370–372),25 three homoisoflavanones (30, 59, 373) and a norlignan 374.69Drimiopsis burkei is widespread throughout Botswana and Zimbabwe, occurring southward towards the Eastern Cape Province of South Africa.69 In Botswana it is known as thejane and is used to treat stomach complaints.155 A sample of this plant collected in KwaZulu-Natal, South Africa was found to contain the norlignan hinokiresinol 155 and a homoisoflavanone 375,69 while a sample collected in Mochudi, Botswana was found to contain the scillascillin-type homoisoflavanones (248 and 376), xanthones (137, 138, 369, 377–378), the norlignan hinokiresinol 155 and the homoisoflavanones (E- eucomin 6, 104, 128 and 379–382).155Drimiopsis barteri is the only genus member to occur in Cameroon, where it is used to treat fever.156 The bulbs have been found to contain the unusual isoquinoline alkaloids (383 and 384) as well as the more expected scillascillin-type homoisoflavanones (161, 376, 385–387), 3-benzyl-4-chromanones (61, 214 and 388) and Z-eucomin 6. While the configuration at C-3 was not specified, the data compared favourably to known compounds of this type and would appear to be R.155,156 The homoisoflavanones 144, 373, 375, 387 and 389–391, were tested against the Gram-positive, methicillin-resistant Staphylococcus aureus. Compound 391 was found to have significant inhibitory activity with an MIC value of 0.47 mM. Compounds 389 and 375 showed some activity, but the quantities available were insufficient for conclusive testing.41 The homoisoflavanone 389, showed significant vasodilating effects when tested on rat aortic rings (inhibition of the contraction elicited in aorta rings by high K+ gave pIC50 of 5.31 M). This result would serve to validate the use of these bulbs for the treatment of conditions involving vasoconstriction.157Resnova humifusa (Baker) U.Müll.-Doblies & D.Müll.-Doblies, a South African species, earlier placed in Drimiopsis by Jessop40 was found to contain nine homoisoflavanones (7, 10, 30, 56–60, 392). The same study reported seven to be common with those found in the South African species Eucomis montana (7, 10, 30, 56, 58, 59, 60), which in addition contained compound 8.40 A later study on the methanol extract of the bulbs of this plant resulted in the isolation of four homoisoflavanones (50, 58, 59, 61), a chalcone 132 and a tetrahydropyran derivative 393.42
Veltheimia bracteata Harv. ex Baker is indigenous to South Africa. The petroleum ether and diethyl ether extracts of bulbs (as Veltheimia viridifolia) grown from seeds in Berlin have yielded two homoisoflavanones (394 and muscomin, 97)158 as well as a spirocyclic nortriterpenoid 133 and its pentaglycoside 395 from the petrol and chloroform extracts respectively.159 The homoisoflavanone 394, was found to exhibit good inhibitory activity against phosphodiesterase (PDE).158 Compound 394 was found to inhibit the two PDE-isoenzymes by 70% and 75% at a concentration of 100 μmol l−1.158Veltheimia capensis (L.) DC., native to the Western Cape, South Africa, has been found to contain an indolizidine alkaloid, steviamine 396. This alkaloid is also present in Stevia rebaudiana Bertoni (Asteraceae), but such alkaloids have not yet been isolated from any other Hyacinthaceae taxa.160
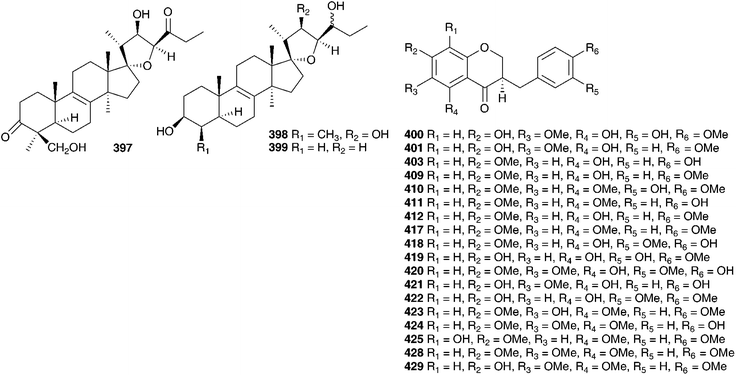
Merwilla plumbea (Lindl.) Speta is widely used by southern African traditional healers. The Zulu use it as a purgative and to facilitate full term labour. The Sotho eat the cooked bulbs as an aperient, use bulb decoctions in enemas to treat internal tumours and for the treatment of lung disease in cattle. Powdered bulbs are rubbed on sprains and fractures by the Southern Sotho, while the Tswana believe that bulbs rubbed on the body will make them resistant to witchcraft. The Swati use a lotion prepared from the bulbs to treat veldsores and boils.161,162 Concern for the sustainability of Merwilla plumbea has led Ncube et al. to include the bulbs of this plant in a study to compare the antibacterial, anticandidal and cyclooxygenase inhibitory activity of the leaves and the, more commonly harvested, bulbs of some popular medicinal plants, harvested during different seasons in the year. Antimicrobial activities between the leaves and bulbs of Merwilla plumbea were found to be comparable over all four seasons and as such they concluded that the leaves could be used in many cases as a substitute for the bulbs. Likewise both the leaves and bulbs produced good cyclooxygenase inhibitory activity throughout the year.163,164 A further study found that in vitro propagation of these plants could, under the right conditions, result in an increase in the flavonoid and gallotannin levels found in the shoots, which would allow for the successful mass propagation of these plants containing elevated levels of the potentially medicinally active components.110,161 A phytochemical investigation of the bulbs (as Scilla natalensis Planch.) found two homoisoflavanones 62 and 72, seven known eucosterol-type spirocyclic nortriterpenoids and the new spirocyclic nortriterpenoids 397, 398 and 399.161 An extract of the lectin-like proteins from the bulbs of M. plumbea (as Merwilla natalensis Planch.) have been found to exhibit moderate inhibitory activity (47%) against cyclooxygenase (COX-1).31 In a separate study, the homoisoflavanones 62 and 400 have been isolated from M. plumbea (as Scilla natalensis Planch.).162 In addition M. plumbea (as S. natalensis) has been shown to have good in vitro activity against the parasite Schistosoma haematobium (LC = 0.4 mg ml−1).66 Three homoisoflavanones (7, 62, 401) have been isolated from Merwilla dracomontana (Hilliard & B.L. Burtt) Speta (as Scilla dracomontana Hillard and Burtt).162 A southern African member of the genus Merwilla, under its basionym Scilla krausii Baker, was found to contain the homoisoflavanone, 80.162
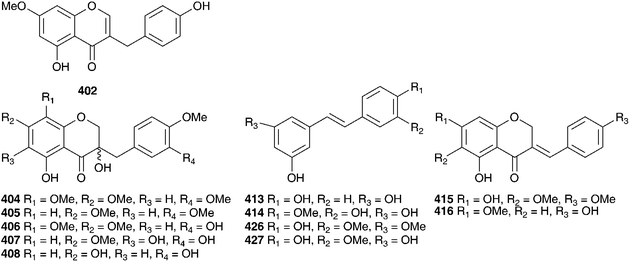
Lachenalia is the largest endemic genus of the Hyacinthaceae in southern Africa. Surprisingly, very little phytochemical work has been done on the genus.165 A study of the leaves of Lachenalia unifolia Jacq. has yielded flavone sulphates (luteolin-3′-sulphate and -7,3′-disulphate, tricetin-3′-sulphate and -7,3′disulphate, diosmetin-3′-sulphate and -7,3′-disulphate)166 while the bulbs of L. rubida Jacq., collected from the Western Cape, South Africa, were found to contain the homoisoflavanones 402 and 403.165 The bulbs of Pseudoprospero firmifolium (Baker) Speta have been found to contain five 3-hydroxy-3-benzyl-4-chromanone type homoisoflavanones, all as racemic mixtures (404–408) as well as the spirocyclic nortriterpenoid, 15-deoxoeucosterol.167 A subsequent study on the bulbs of Pseudoprospero firmifolium subsp. natalensis J.C. Manning confirmed the previous findings for the typical subspecies but, in addition, found the homoisoflavanone 59 in the dichloromethane extract.42
Schizocarphus nervosus (Burch.) van der Merwe is widespread in southern Africa. Although toxic to livestock,1 it plays an important role in traditional medicine within the region. The bulbs are used by the Zulu to treat dysentery and nervous conditions in children as well as treat rheumatic fever.168 In Botswana the bulbs are used to enhance fertility and to treat infections.169 The methanol extract of the bulbs of S. nervosus (as Scilla nervosa (Burch.) Jessop) has been tested for anti-inflammatory and anti-microbial activity. The crude methanol extract displayed a strong, but short-acting effect on a croton-oil induced contact dermatitis in a mouse ear (66% reduction of oedema after 3 h) and the nonpolar components were active against Staphylococcus aureus (IC50 = 1.8 μg ml−1), Klebsiellia pneumonia (IC50 = 2.0 μg ml−1) and Candida albicans (IC50 = 1.0 μg ml−1). Little discrimination was noted between the Gram-positive and Gram-negative bacteria, but some specificity for the yeast was observed.169 Five homoisoflavanones (49 and 409–412) and two stilbenoids (413, 414) have been isolated from the bulbs.168 A further thirteen homoisoflavanones (41, 49 and 415–425) and three stilbenes (414 and 426–427) have been isolated from the bulbs of S. nervosus (as Scilla nervosa (Burch.) Jessop subsp. rigidifolia).170,171 Compound 419 was found to be highly active against colon cancer (HT-29 cell line ED50 = 0.88 μg ml−1) and breast cancer (MDA-MB-435 cell line ED50 = 0.42 μg ml−1).170 The yellow inter-bulbscale deposits of this subspecies were likewise investigated and eight homoisoflavanones were isolated, two of which were novel (428–429).172 There has been some evidence presented to indicate that the bulbs may cause damage to liver cells.173 A homoisoflavanone 400 has been isolated from Spetaea lachenaliiflora Wetschnig & Pfosser (as Scilla plumbea Lindl.). This is a little known species, neither toxic nor known to be used by traditional healers.65
2 The Urgineoideae
The subfamily Urgineoideae, with more than 100 species,7 has received considerable taxonomic attention in recent years, with widely differing opinions subsequently offered on generic circumscriptions. Speta recognised eleven genera,174 several of which have been studied from both a phytochemical and a pharmacological perspective. More than any other compound type, the bufadienolides characterise the Urgineoideae. Bufadienolides are C-24 steroids possessing an α-pyrone group (doubly unsaturated six-membered lactone ring) at position 17β. The presence of these highly toxic cardioactive steroids has led to these plants being used by man in antiquity, with the ancient Egyptians reportedly using preparations to treat heart disease.175 Research into this subfamily was further stimulated by the sometimes fatal toxicity associated with various species, both to stock176–178 and to humans.179,180 The systematic position of the sea onion, Charydbis maritima (L.) Speta, has long been debated.181 The ancient Greeks referred to the plant as skilla, the Romans as scilla and for much of the early research on this plant, the names Urginea maritima or Scilla maritima were applied. Subsequent work by Pfosser and Speta181 provided evidence for two genera in the subfamily Urgineoideae in the Mediterranean region, Urginea Steinh. and Charydbis Speta. Phytochemical investigations to date support this generic distinction.With much of the early research on Charybdis maritima focused on the plant then known as Scilla maritima, the trivial names of isolates of this urgineoid accordingly reflect this nomenclatural association. Notably, Scilla is a distinct genus currently assigned to the subfamily Hyacinthoideae, and has a very different chemical profile. Kopaczewski isolated a toxic component from the bulbs of C. maritima (as Scilla maritima) which he termed scillitin.182 It was found to be a diuretic but the whole extract from the bulbs was found to be more poisonous to guinea pigs and rabbits than scillitin itself.183 In contrast, Smith found scillitin to be more toxic to rats than the crude extract.184 The bufadienolides scillaren A 224, scilliroside 430, proscillaridin A 223 and scillaren F 431 have also been isolated from C. maritima (reported as Scilla maritima and/or Urginia maritima).185–190 Using HPLC, with proscillaridin A 223 as a reference, Tittel and Wagner showed the possible presence of bufadienolides in a plant identified as Scilla rubra L. (here considered synonymous with C. maritima).191 Petrol extracts of bulbs of this species (as Scilla maritima and Urginia maritima) were found to contain fatty acids (lauric acid, myristic acid, palmitic acid, palmitoleic acid, stearic acid, oleic acid, linoleic acid and linolenic acid) and sterols (sitosterol, stigmasterol, campesterol and cholesterol).192–194
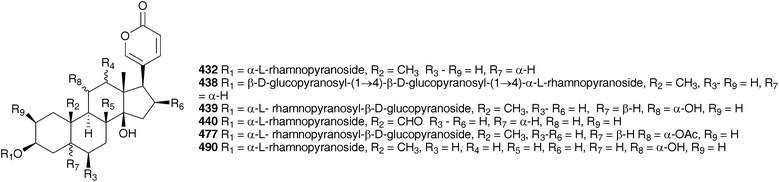
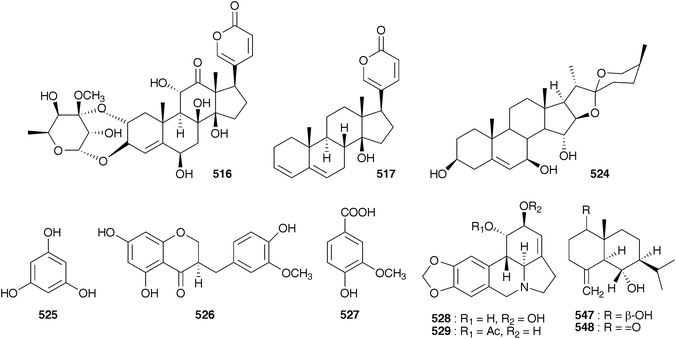
Investigations carried out in the early 1920s focused primarily on Charydbis maritima (squill). Both the extracts of fresh bulbs and dried powdered bulbs showed digitalis-like activity on the heart when tested on frogs and cats.195,196 Markwalder196 found no difference in activity between the fresh and dried bulbs and noted that neither the dried outer leaves nor the heart of the bulb exhibited much activity, while the mature middle layers of the bulb were rich in active components. The structure of scillaren A 224, the principle active component of C. maritima (reported as Urginia maritima) was elucidated by Stoll et al. in 1933.197 The interesting activity shown by the squill bufadienolides resulted in some comparative studies with digitoxigenin and strophanthidin as well as the effects of combination therapy.198,199 Extensive work on the bulbs of C. maritima (as Urginia maritima) over the following years resulted in the isolation and identification of a large number and variety of bufadienolides. They can be divided into those having a double bond at C-4 (e.g., 224), those having a double bond at C-3 (e.g., 431) and those without either (dihydro C-4) (e.g., 432). Without exception, within C. maritima they contain a β-hydroxy group at C-14, a trans junction of rings B and C and a cis junction of rings C and D. They have been isolated as the aglycones as well as a variety of glycosides, with scillaren A 224 and proscillaridin A 223 being present in all specimens of C. maritima investigated. Samples of the plant from across its Mediterranean, European and Middle Eastern range have been investigated and this would account for the great diversity of bufadienolides isolated.
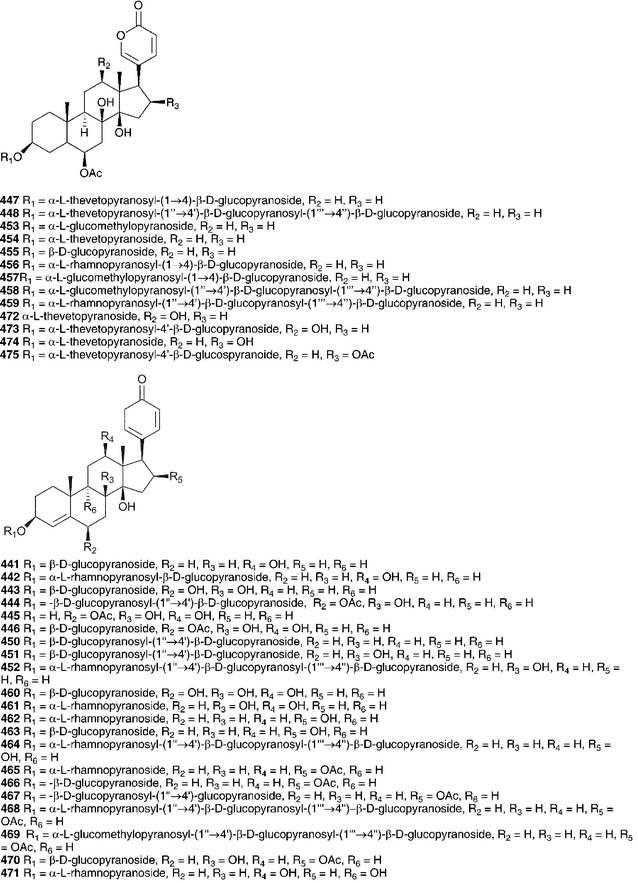
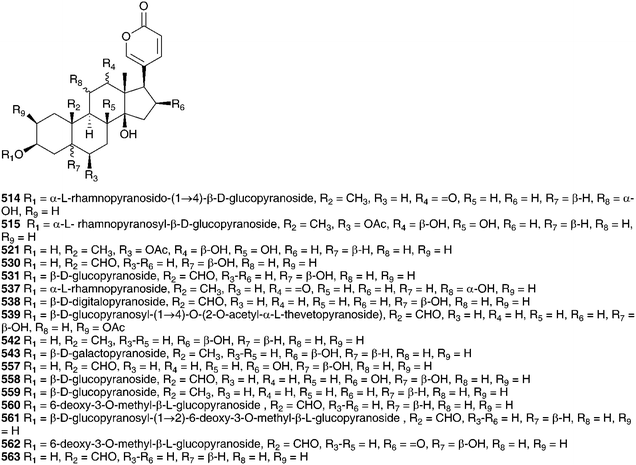
Von Wartburg and Renz200,201 isolated five bufadienolides from C. maritima (as Urginia maritima), scilliroside 430, scillaren A 224, scillaren F 431, proscillaridin A 223 and scillarenin-β-D-glucopyranoside 433. The structures reported at that time were subsequently revised and the structures shown here reflect that revision.202,203 Gorlich204 found proscillaridin A 223, scillaren A 224, scilliglaucoside (scillaren F) 431 and scillicyanoside 434 in crystalline form as well as minor amounts of scillicoeloside (no structure given) and glucoscillaren A 435. Later work on this plant resulted in the isolation of further minor components, scilliphaeoside 436 and glucoscilliphaeoside 437.205 A reinvestigation of the bulbs of C. maritima (as Urginia maritima) in 1991 by Krenn et al.206 resulted in an additional four new bufadienolides, identified as 5α-4,5-dihydroproscillaridin A 432, 5α-4,5-dihydroglucoscillaren A 438, gamabufotalin-3-O-α-L-rhamnopyranosyl-β-D-glucopyranoside 439 and 19-oxo-5α-4,5-dihydroproscillaridin A 440. Forty-one bufadienolides were isolated from an Egyptian sample of C. maritima (as Urginia maritima).207 Proscillaridin A 223, scillaren A 224 and scilliroside 430 were found as expected. In addition to 15 known bufadienolides found (223, 224, 430, 434, 435, 436, 441–449), 10 new glucosides of known aglycones (450–459) and sixteen new compounds were isolated (460–475). An unusual 9-hydroxy bufadienolide 476 was later isolated, also from Egyptian material.208 From 2000 until 2012 a total of eleven additional new bufadienolides (477–487) have been isolated from C. maritima (as Urginia maritima). Two of the compounds 477 and 478 were isolated from Spanish material209 and nine (479–487) from a plant cultivated in Japan (provenance undisclosed).210 A comparative study of the bulbs of C. maritima collected in the northern and southern regions of the Mediterranean area showed some differences in both the quantity and type of bufadienolides isolated.211 Both samples contained scilliroside 430, scillarenin 3-O-β-D-glucopyranoside 433, proscillaren A 223, scilliphaesidin 3-β-D-glucopyranoside 441, scilliglaucoside 431, scilliphaoside 436 and 12-epi-scilliphaeoside. In addition, the bulbs from Tunisia contained glucoscilliphaeoside 437, 12-epi-glucoscilliphaeoside, 12β-hydroxyscilliglaucosidin 3-O-β-D-glucopyranoside 488, 12-epi-scilliphaeosidin 3-O-β-D-glucopyranoside 443 and 12-epi-scilliphaeosidin 3-O-α-L-rhamnopyranosyl-α-L-rhamnopyrano- side 489. Bulbs sourced from Sardegna also contained gamabufotalin 3-O-α-L-rhamnopyranoside 490, scillirubrosidin 3-O-α-L-rhamnopyranoside 491, scillirubroside 492, 12β-hydroxyscilliroside 446, 5α-4,5-dihydroscillirosidin 3-O-α-L-thevetopyranosyl-β-D-glucopyranoside 475, deacetylscilliroside 443, 10-carboxy-5β-14β-hydroxybufa-3,20,22-trienolide 5-O-β-D-glucopyranoside 493 and scilliglaucogenin 494.
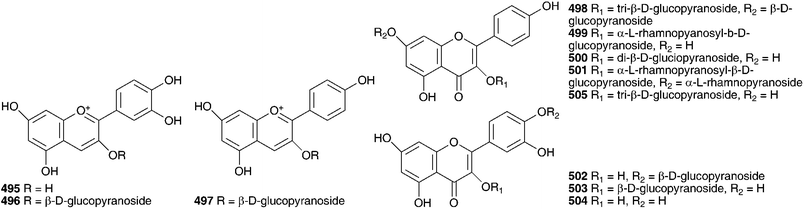
The flavonoid, anthocyanin and sterol content of the bulbs of C. maritima (as Urginia maritima) have been investigated. The ethanol extract of the bulbs was studied by gas-liquid chromatography and the sterols identified by comparison to known reference compounds. Sitosterol, stigmasterol, campesterol, cholesterol, Δ5-avenasterol and Δ7-avenasterol were found to be present.193,194 Red bulbs of C. maritima (as Urginia maritima), collected in the Balearic Islands contained cyanidin 495, cyanidin-3-monoglucoside 496 and pelargonidin-3-monoglucoside 497, both in the free form and acylated with p-coumaric acid.212–216 The proportion of anthocyanin was found to be higher in tetraploids than in triploids or hexaploids.217
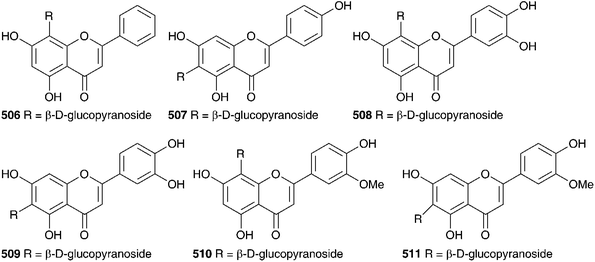
The flavonoids isolated included kaempferol-7-O-β-D-glucopyranoside-3-O-β-D-triglucopyranoside 498, kaempferol-7-O-β-D-glucopyranoside-3-O-α-L-rhamnopyranosyl-β-D-glucopyranoside 499, kaempferol-7-O-β-D-glucopyranoside-3-O-β-D-diglucopyranoside 500 and kaempferol-7-O-α-L-rhamnopyranoside-3-O-α-L-rhamnosyl-β-D-glucopyranoside 501.218 In addition the flavonoid O-glycosides 502–505, have been isolated.219 Five C-glycosylflavones have been isolated from the ethyl acetate extract of the bulbs. They have been identified as vitexin 506, isovitexin 507, orientin 508, isoorientin 509, scoparin 510 and isoscoparin 511.220–223 Fernandez et al. identified twenty-five flavonoids in a Spanish sample of C. maritima bulbs (as Urginia maritima). They followed a parallel series: pelargonidin, kaempferol, cyaniding, quercetin and taxifolin. A complex mixture of both C- and O-glycosides was obtained.224 A fructan, sinistrin, has been isolated from C. maritima (as Urginia maritima) and found to be a mixed type β-D-fructan, with (2–1) linked and (2–6) linked unbranched β-D-fructofuranosyl and α-D-glucopyranoside residues.225,226
C. maritima (reported as Urginia maritima) has been used as a form of rodenticide since the 13th century.202 The toxic component has been found to be scilliroside 430, which affects the cardiovascular and central nervous systems, resulting in death.202 Scilliroside has an emetic effect on humans, cats, dogs and pigeons, but rats and mice are unable to vomit and as such they die within a few hours of ingesting the bulbs. The flavonoid fraction has been found to have a diuretic effect227 and the ethanolic extract of Spanish squill has shown good insecticidal activity against the stored grain pest Tribolium castaneum (red flour beetle).228 Twelve wild populations of the bulbs were studied with the red bulbs giving 60–90% mortality if applied at 20–30 μg insect−1.228 An extract of the bulbs of C. maritima (as Urginia maritima) growing in Turkey has been found to exhibit greater toxicity than cisplatin against the A549 non-small cell lung cancer (NSCLC) cell line.229 Tissue cultures of C. maritima (as Urginia maritima) grown both in the dark and under light showed no sign of the bufadienolides commonly associated with this species. The cultures grown in the light did though produce anthocyanins.230
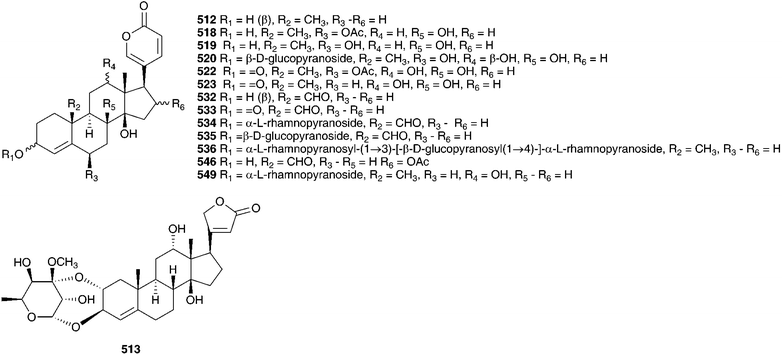
The range of bufadienolides found in the bulbs of Charybdis hesperia (Webb & Berthel.) Speta (as Urginea hesperia Webb & Berthel.) indicates a close relationship to C. maritima. Thirteen bufadienolides have been isolated including scillarenin (512) and a variety of associated glycosides.231Urginea fugax (Moris) Steinh. is a species which is widespread in the Mediterranean area. In contrast to C. maritima and C. hesperia, cardenolides have been isolated from the bulbs.232 The new cardenolide fugaxin 513 has a rubellin-like carbohydrate linkage between C-3 and C-2, unlike any seen in C. maritima and C. hesperia, which lends chemotaxonomic support to recognition of both Urginea and Charybdis in the Mediterranean region.181 Six bufadienoides have been isolated from the bulbs of Charybdis pancration (Steinh.) Speta (as Urginea pancration), scilliglaucoside 431, scillirubroside 492, scilliroside 430, 5α-4,5-dihydroscillirosidin 3-O-α-L-thevetopyranosyl-β-D-glucopyranoside 475, arenobufagin-3β-O-L-rhamnopyranosyl-4′-β-D-glucopyranoside 514 and the glycoside 515.233,234Charybdis aphylla (Forssk.) Speta (as Urginea aphylla (Forssk.) Speta), growing in the northeastern Mediterranean region was found to contain eleven bufadienolides, scillicyanoside 434, gamabufotalin-3-O-α-L-rhamnopyranoside 490, scilliglaucoside 431, gamabufotalin-3-O-α-L-rhamnosyl-β-D-glucopyranoside 439, proscillaridin A 223, scilliphaeosidin-3-O-β-D-glucopyranoside 437, scillarenin-3-O-β-D-glucopyranoside 433, 16β-O-acetylgamabufotalin-3-O-α-L-rhamnopyranoside (16β-O-acetyl 436), 12β-hydroxyscilliroside 446 and 5α-4,5-dihydroxyscillirosidin-3-O-α-L-thevetopyranosyl-β-D-glucopyranosyl-β-D-glucopyranoside (475 glucoside).235 The similarity in chemical composition of the bulbs of Charybdis from across the European region highlights their close relationship.
As early as 1923, Juritz reported the presence of a glucoside in the toxic South Africa plant Boosia macrocentra (Baker) Speta (as Urginea macrocentra Baker) with digitalis-like activity and he noted that it could be used as a substitute for squill.236 This plant has also been implicated in stock mortality and it is used by the Zulu as a vermifuge (as Drimia macrocentra).237 The major component was identified as rubellin 516 by NMR spectroscopy.237 In 1927, work by Watt238 on South African Sekanama burkei (Baker) Speta (as Urginea burkei), yielded two glucosides, a red compound found to be toxic to animals and having a digitalis-like effect on the heart as well as a diuretic effect, and a white compound which resulted in paralysis of the central nervous system and respiration. Additional work on S. burkei (as Urginea burkei) and Urgineopsis modesta (Baker) Speta (as Urginea rubella Baker) in the 1940s and 1950s resulted in the isolation and identification of two cardiac glycosides, transvaalin and rubellin 516 respectively.239–243 Rubellin 516 was found to behave as a cardiac poison towards rats while transvaalin was found to kill rats primarily by its effect on the central nervous system.241,243,244 Rubellin was found to have a digitalis-like action on cats.245 Further work by Louw et al.246 on transvaalin led to the conclusion that it was either isomeric with scillaren A 224 or a stable complex of scillaren A 224 and scilliroside 430. This was based on melting point analysis, toxicity tests and the fact that hydrolysis of transvaalin led to the same products as the hydrolysis of scillaren A, namely scillaridin A 517 and scillabiose (disaccharide). Zoller and Tamm247 and Tschesehe and Hottemann240 all concluded that transvaalin and scillaren A were identical compounds. The structure of rubellin 516 was fully elucidated by Steyn et al. in 1986248 using high-field NMR spectroscopy. Later work by Krenn et al.249 on Sekanama sanguinea (Schinz) Speta (as Urginea sanguinea Schinz) yielded eight bufadienolides as well as a steroidal sapogenin and stigmasterol. The first six bufadienolides, namely scillirosidin 518, deacetylscillirosiden 519, 12β-hydroxyscillirosidin 445, 12β-hydroxydeacetylscillirosidin 520, 12β-hydroxyscilliroside 446 and 5α-4,5-dihydro-12β-hydroxyscillirosidin 521 had been isolated previously from other members of the Urgineoideae. Two new compounds, 12β-hydroxyscillirosidin-3-one 522 and 12β-hydroxyscillirubrosidin-3-one 523 were identified using NMR spectroscopy. The steroidal saponin, 7β,15α-dihydroxyyamogenin 524 was the first such compound to be isolated from a member of the Urgineoideae. Majinda et al.250 investigated another specimen of the same plant, and found stigmasterol, phloroglucinol 525, phloroglucinol 1-β-O-glucopyranoside, scillaren A 224 and the novel 5α-4,5-dihydroscillaren A. In addition, salicylic acid and 3-hydroxy-4-methylbenzoic acid were isolated. An ethnopharmacological study of S. sanguinea, using patient records from Ga-Rankuwa Hospital in South Africa revealed a number of cases of poisoning as a result of ingestion. The amount usually prescribed by traditional healers does not result in adverse side effects, but the patients admitted to the hospital had taken more than the prescribed dose. A brine shrimp assay showed a seasonal variation in toxicity, with the plant being more toxic in February (late Summer) than in September (Spring).251Sekanama sanguinea has traditionally been used for many different conditions, including asthma and it is often taken by pregnant women. A study by Marx et al.252 (as Urginea sanguinea) showed significant damage to chick embryos after exposure to this plant. They concluded that it should be used with extreme care by traditional healers.253 Undoubtedly the bufadienolides dominate the chemistry of the Urgineoideae, including the Sekanama species. However, the isolation of a novel homoisoflavanoid from the bulbs of Sekanama delagoensis (Baker) Speta (as Drimia delagoensis (Baker) Jessop) indicates that a broader range of secondary metabolites can be expected, if not commonly found.254 The homoisoflavonoid 526 and 3-methoxy-4-hydroxybenzoic acid 527, were isolated from the dichloromethane and methanol extracts of the bulbs respectively.254
Other sub-Saharan members of the Urgineoideae to receive attention include Urginavia altissima (L.f.) Speta (as Urginea altissima (L.f.) Baker and Drimia altissima (L.f.) Baker), Fusifilum physodes (Jacq.) Raf. ex Speta (as Urginea physodes (Jacq.) Baker), Urginavia epigea (R.A.Dyer) Speta (as Urginea epigea R.A.Dyer), Urginea lydenburgensis R.A.Dyer and Urginea riparia Baker. Characteristic bufadienolides have been isolated and identified, many of which include either an aldehyde group at C-10 or a rubellin type carbohydrate group doubly linked to the aglycone at positions 2α and 3β. The best known of this group is the widespread Urginavia altissima (often referred to as Drimia altissima or Urginea altissima). Rat populations need to be controlled to limit threats to public health and minimise crop damage. The toxicity of U. altissima has been investigated with respect to the field rat, Arvicanthis abyssinicus.255 Synthetic rodenticides affect non-target species and can be expensive. The powdered bulb was tested and found to be toxic at levels of 5% of the daily food intake. If the percentage was lower, no mortality was noted. Urginavia brachystachys (Baker) Speta has (as Drimia brachystachys (Baker) Stedje) reportedly been used as an arrow poison.256
Both bufadienolides and alkaloids have been isolated from U. altissima. The alkaloids, lycorine 528 and acetylcaranine 529 have not been found by any subsequent investigators and the identity of the plant investigated by Miyakado and co-workers257 has been called into question as bulbs of U. altissima may be easily confused with those of some Amaryllidaceae, especially the genus Crinum L.258,259 Six bufadienolides have been isolated from U. altissima collected in Kenya, all of which have an aldehyde at C-10 (530–535).260,261 A South African sample of this plant was found to contain 12β-hydroxyscillirosidin 445,258 which has been isolated from S. sanguinea previously and urginin 536 with an interesting trisaccharide substituent at C-3.258 A specimen from Ethiopia yielded different bufadienolides again, gamabufotalin-3-O-α-L-rhamnopyranoside 490 and arenobufagin-3-O-α-L-rhamnopyranoside 537.259 It would appear that significant regional differences exist in the type of bufadienolide found in U. altissima. U. altissima is widely used for the treatment of gout, rheumatism and a number of respiratory conditions, including asthma.258 This plant is also used by Zimbabwean traditional healers to treat skin conditions. It has been found to produce a mild inflammation and itching if rubbed on the skin. This effect has been found to be due to the presence of calcium oxalate raphides in the bulbs and as such the appropriateness of its use as a skin treatment has been questioned.262
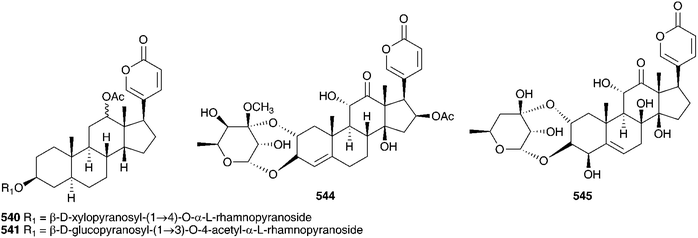
Fusifilum physodes (as Urginea physodes (Jacq.) Baker) was found to contain four bufadienolides, physodines A 538, B 539, C 540 and D 541. Two of which, physodines A and B, both having a C-10 aldehyde group, were found to be toxic to weaned guinea pigs (fractions not causing death in 48 h at 200 mg kg−1 were considered non toxic). Physodines C and D were of interest in that they were the first naturally occurring 14-deoxybufadienolides to be isolated.263 An ethanolic extract of the bulbs has been shown to have antifungal activity.264 The methanol extract of the bulbs of Fusifilum depressum (Baker) U.Müll.-Doblies J.S.Tang & D.Müll.-Doblies (as Drimia depressa (Baker) Jessop) was found to contain two bufadienolides, 542 and 543.265 The leaves of this plant are toxic to cattle, sheep and rabbits and the plants are used by the Sotho as a good luck charm or to cause harm to one's enemy.265 Novel rubellin type bufadienolides, riparianin 544 and lydenburgenin 545, have been isolated from Urginea riparia and Urginea lydenburgensis R.A.Dyer respectively.237,266 In addition, Urginea lydenburgensis was found to contain the novel bufadienolide, scillicyanosidin 546, with an aldehyde group at C-10. Riparianin 544 showed moderate activity against MCF7 (breast), TK10 (renal) and UACC62 (melanoma) cell lines (total growth inhibition of <3.5, <7 and <10.5 ppm respectively). Urginavia epigea is used locally to treat backaches or as a soap. The smoke from smouldering bulbs is also used as a treatment for headaches and colds.267 In addition to the buadienolide 517,268 two eudesmane sesquiterpenoids were isolated, 547 and 548. These were the first to be reported from the Hyacinthaceae and due to their occurrence in U. epigea but not the oft-confused U. altissima, provide species level taxonomic markers.267 The bufadienolide 517, has an unusual 3,5-diene system, readily obtained synthetically, but not commonly isolated directly from plant sources.268 The butanol extract of the bulbs of U. epigea was found to have significant molluscicidal activity against the bilharzia-carrying snail, Bulinus africanus. This result has raised some concern with regards its use as soap and the potential toxic affect it may have on other aquatic biota.269
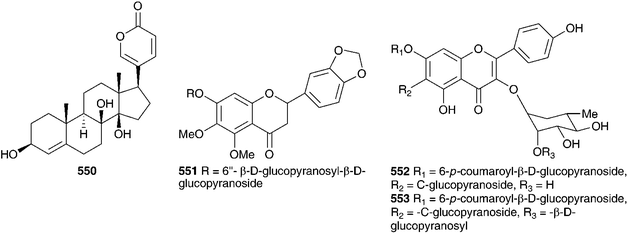
An Indian member of the Urgineoideae, known locally as ‘jungli-piyaz’ or ‘bhuikanda’ and Indian squill has been used traditionally for stomach complaints, as a cardiac stimulant, a diuretic, as well as to treat asthma, cough or bronchitis and various infections.270 Commercially available samples vary with some plants having a truncated bulb and others a scaly bulb.271 For much of the work reported, the plant has been identified as Urginea indica (Roxb.) Kunth or Scilla indica Baker. The name Indurgia indica (Roxb.) Speta is currently accepted. Extracts of the bulbs have shown some activity against Neisseria gonorrhoeae infections272 and have had outstanding antimicrobial activity attributed to them in a survey of medicinally used plants in India.273 The ethanolic extract of the bulbs of I. indica (as Scilla indica Baker) has been found to have good antioxidant activity (protection against lipid peroxidation induced by ferrous sulphate in a dose-dependant manner, with 49% protection for hydroxyl radical-mediated hydroxylation at a concentration of 250 μg ml−1) as well as to possess cardiac activity on a par with ouabain.271,274 Rao and Rangaswami have detected bufadienolides in extracts of the bulbs of I. indica (as Scilla indica Roxb.) and have isolated scillaren A 224 as the principle active component.275,276 Jha and Sen, however, found no trace of scillaren A 224 in a plant identified as Scilla indica at the time.277 The principle bufadienolides isolated from this plant (as Urginia indica Kunth) by various investigators are scillaren A 224 and proscillaridin A 223,275,278–281 with scillaren A being reported as early as 1956. These are also the principle bufadienolides of the European squill C. maritima. In small doses, the action of I. indica is the same as C. maritima, in that it slows the heart beat and increases urine flow and it can successfully be used as a substitute for C. maritima.282 Jha and Sen278,280 have studied the twelve different cytotypes of I. indica (as Urginea indica), belonging to four ploidy levels. Scillaren A 224 and proscillaridin A 223 were present in all twelve. Bulbs were investigated through three successive years. The tetraploids showed the highest concentration of these bufadienolides, followed by diploids and then triploids. Significant variation in the bufadienolide concentration was also noted between samples collected in northern and southern India, with the northern samples showing much lower levels of proscillaridin A 223 and scillaren A 224.283 In addition, two minor components, scilliphaeoside 436 and anhydroscilliphaeosidin, were found to be present in the tetraploid cytotypes and scilliphaeoside 436 in the triploids. Likewise the sterol content was investigated with only minor differences noted between the ploidy levels. Stigmasterol predominated, with sitosterol and campesterol being found in the tetraploid cytotypes and some of the triploids respectively.284,285 Kopp and Danner281 found the bulbs of I. indica (as Urginia indica) to contain six bufadienolides, scillarenin 512, proscillaren A 223, scillarenin A 224, scilliphaeosidin 3-O-α-L-rhamnopyranoside 549, scilliglaucosidin 3-O-α-L-rhamnopyranoside 534 and scilliglaucosidin 3-O-β-D-glucopyranoside 535, while Saxena and Chaturvedi isolated 6-desacetoxy scillirosidin 550.286 In addition to the bufadienolides and sterols, Sultana et al. have isolated three novel flavonoid glycosides (551–553) from bulbs collected in Bangladesh.287
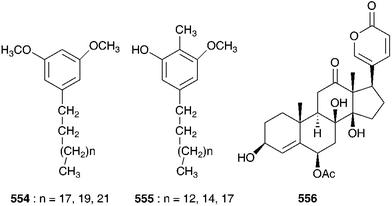
Seasonal variation in the glycosidal content of the bulbs has been observed with the highest such levels found during October at the onset of dormancy. The levels steadily decrease to reach a minimum in June.288 Sterol content has been found to peak during the pre-flowering and pre-vegetative growth stages and it has been suggested that the sterols could be a trigger for the onset of reproductive and vegetative growth in the species.289 Significant antifungal activity has been noted and attributed to the presence of a 29-kDa glycoprotein which inhibited growth of Fusarium oxysporum at a concentration of 10 μg protein well−1.290,291 This protein has in addition shown good antitumour activity against the growth of an ascites tumour and mouse mammary carcinoma (tested in vivo using 6–8 week old female mice).292 The bulbs of I. indica (as Urginia indica L.) collected in Nigeria were found to contain six alkylresorcinols of the structure types shown in 554 and 555.293 An ethanolic extract of I. indica (as Scilla indica), has reportedly shown good inhibition of the Semliki Forest Virus and the active principal found to be a diosgenin saponin.294
The genus Drimia Jacq. is closely related to Urginea. Drimia robusta Baker is widely used in southern Africa by traditional healers. It has been used as a diuretic, as a treatment for feverish colds, to promote the healing of broken bones, to treat diseases of the uterus and to relieve stabbing pains in the chest.258,295 Luyt et al.295 investigated the biological activity of this plant in order to substantiate its use in traditional medicine. Anti-bacterial activity was found to be present in the ethyl acetate extract of the bulbs. An ethanolic extract of the bulbs showed higher inhibitory activity than indomethacin in the screening for inhibition of cyclooxygenase. This inhibition of prostaglandin synthesis (tested for with the cyclooxygenase assay) could explain the plant's use as a treatment for feverish colds. Further work on the COX-1 inhibition of a water extract of the bulbs showed activity above 70%.264 Evidence for the presence of proscillaridin A 223 was presented: TLC and NMR data were compared with a standard sample. Luyt et al.296 then compared bulbs derived from tissue culture with those grown naturally. A standard proscillaridin A 223 was used for comparison and the UV spectra and qualitative TLC results were compared. The results indicated that the bufadienolide content was the same in the in vitro-derived plants as in those grown naturally. This is in contrast to the work done on C. maritima, where no bufadienolides were found in the tissue cultured bulbs.230 Phytochemical studies of the bulbs of D. robusta yielded eight bufadienolides, 12β-hydroxyscillirosidin 445, 6β-acetoxy-3β,8β,14β-trihydroxy-12-oxobufa-4,20,22-trienolide 556, scilliroside 430, 12β-hydroxyscilliroside 446, hellebrigenin-3-O-β-glucopyranoside 531, 16β-hydroxyhellebrigenin 557, 16β-hydroxyhellebrigenin-3-O-β-glucopyranoside 558 and 5β,16β-dihydroxybufalin-3-O-β-glucopyranoside 559 as well as three common aromatic acids, 4-hydroxy-3-methoxybenzoic acid, 3,4-dihydroxybenzoic acid and trans-3-(4′-hydroxyphenyl)-2-propenoic acid.258,268,297 The toxicity of Rhodocodon madagascariensis Baker, a plant endemic to Madagascar, has been tested against the embryo-larvae of medaka fish (Oryzias latipes) and an LD50 of 1 mg ml−1 was established.298
Work on the genus Bowiea Harv. ex Hook.f. has centred on Bowiea volubilis Harv. ex Hook.f. Most of the investigations were carried out in the 1950s and early 1960s. This African species is toxic and as early as 1938, the presence of a cardioactive glucoside was reported.299 Katz and Tschesche et al. have between them reported the presence of several bufadienolides (bovisides A 560, B, C, D, bovogenin E, bowieasubstanz G, glucobovoside A 561, nabogenin (scilliglaucosidin) 532, bovoruboside 562 and bovogenin A 563) between the years of 1950 and 1959.300–314 Structures have been reported for nabogenin 532, bovoruboside 562, bovoside A 560, glucobovoside A 561 and bovogenin A 563, although the structure reported for bovoruboside is not certain. The cardioactive glycosides from B. volubilis attracted much attention in the early years of their discovery. The activity was initially found to be in the region of strophanthin,315 the action was as rapid and lasting with a cumulative effect, though not as much as digitoxin.316–318 Solutions of bovoside A were found to be unstable, due to the aldehyde group at C-19 being vulnerable to oxidation. Reduction of this group to the alcohol (bovosidol A) gave a stable solution which could then be assessed for activity.319,320 Material from East Africa (as Bowiea kilimandscharica Mildbr.) was initially reported to contain two bufadienolides, kilimanscharogenins A and B. Katz concluded that kilimanscharogenin A was the same compound as bovogenin A 563.321
Chen and Henderson322,323 carried out a comparative survey of the cardioactivity of twenty-nine bufadienolides and cardenolides from various sources. Included in the survey were bufadienolides from B. volubilis. The tests were carried out on etherised cats by slow continuous intravenous injection and the electrocardiograms were recorded to confirm the digitalis-like action of these compounds. The potency of each compound was determined in terms of lethal dose (LD), the amount (mg kg−1) needed to kill a cat of approximately 2 kg in 30–60 min. The bufadienolides were found to be more potent than the cardenolides, with the glycosides seldom showing more activity than the aglycones. The methyl C-10 analogues were less active than the compounds with an aldehyde group in the C-10 position and 3-epimerisation and 5α-configuration reduced the activity. A later study by Chen increased the number of cardenolides and bufadienolides to fifty and similar profiles were obtained using the same assay system.324
3 The Ornithogaloideae
The Ornithogaloideae are widespread throughout Africa, Europe and southwest Asia. This group consists of approximately 280 species,5 with a recent phylogenetic analysis by Martínez-Azorín et al.6 recognising nineteen monophyletic genera. Phytochemical studies on the Ornithogaloideae have centred on Ornithogalum L. and Galtonia Decne., primarily driven by the significant biological activity attributed to the compounds isolated. In addition, a small amount of work has been done on Albuca L., Eliokarmos Raf., Stellarioides Medik., Loncomelos Raf. and Honorius Gray. The types of compounds isolated are strongly linked to the geographic region the plant species derive from, with the African taxa containing primarily cholestane glycosides and spirosterols, and the European taxa cardenolides. Some flavonoids and sterols have been isolated from plants sourced from all regions studied. Additionally, a limited number of homoisoflavanones have been identified from the bulbs.The genus Albuca L. is widespread in southern Africa, with some species occurring in the Arabian Peninsula. The bulbs of Albuca fastigiata Dryand. have been investigated and only one compound was isolated, a homoisoflavonoid, 564.325Albuca fastigiata is found on the East coast of South Africa and is used by the Zulu as a protective charm and to treat malicious food poisoning.325 Another southern African species, Albuca setosa Jacq., has been tested for anti-inflammatory activity in a carrageenan-induced rat paw edema assay.326 This plant is used by traditional healers to treat wounds, among other conditions, and was found to significantly inhibit carrageenan-induced paw edema for a 3-hour challenge (43–55% over 3 h at 150 mg kg−1 and 68–85% for 300 mg kg−1). Albuca setosa has also been used by traditional healers in the Nkonkobe municipality in South Africa for the treatment of diabetes.327 As part of a study on the steroidal saponins of various monocot families, Okanishi et al.328 found Albuca nelsonii N.E.Br. to contain ruscogenin 565. This was identified by comparison of melting points, mixed melting points, optical rotations, infrared and mass spectra with an authentic sample.
Galtonia candicans (Baker) Decne. is native to South Africa, and was investigated by the laboratories of Kuroda and Mimaki329–333 who used bioassay-directed fractionation to isolate the active components of the bulbs. The methanol extract was found to be active against leukemia HL-60 cells (IC50 = 0.017 μM). This was further partitioned between n-butanol and water, with the activity being retained in the n-butanol fraction (IC50 = 0.0056 μM). This was found to contain the hexacyclic rearranged cholestane diglycoside, candicanoside A 566, the polyoxygenated 5β-cholestane diglycoside galtonioside A 567, six cholestane bisdesmosides 568–573 and six cholestane glycosides 574–579.329–333 Candicanoside A 566 and galtonioside A 567 were found to have potent activity against leukemia HL-60 cells (IC50 = 0.032 μM and 0.057 μM respectively) and further testing on a 38 cell line assay showed differential cytotoxicities against breast cancer, CNS cancer and lung cancer lines, while colon cancer, ovarian cancer and stomach cancer cell lines were resistant to them.330,331 Compound 569 showed moderate activity against leukemia HL-60 cell lines ((IC50 = 6.8 μM)332 and compounds 575–578 showed good activity using the same assay (IC50 = 0.00012 μM, 0.00048 μM, 0.0024 μM and 0.053 μM respectively).333 Tang and Yu have achieved the total synthesis of candicanoside A 566, using a 27-step synthesis.334,335 Another southern African species of Galtonia that has received some attention is Galtonia princeps (Baker) Decne.336 A methanol extract of the bulbs yielded the cholestane derivative 580. Both the bulbs and aerial leaves of Galtonia viridiflora I.Verd. were found to contain monoglycosides of caffeic acid, principally rhamnose and glucose.337
Work on the bulbs of Galtonia saundersiae (Baker) Mart.-Azorín, M.B.Crespo & Juan (as Ornithogalum saundersiae), a native of the East coast of South Africa, began in the early 1990s with the isolation of three acylated cholestane glycosides 581–583. All three were examined for their inhibitory effect on cyclic AMP phosphodiesterase. Compounds 582 and 583 showed good activity (IC50 = 0.00025 μM and 0.00020 μM respectively), suggesting that the presence of a benzoyl group attached to the sugar moiety is essential for activity.338 Six additional cholestane glycosides 584–589 were isolated and tested for their inhibitory activity on cyclic AMP phosphodiesterase. Compounds 585 and 588, with the acyl group at the C-3 hydroxyl position of the rhamnose group, showed good activity (IC50 = 9.9 × 10−5 μM and 10.9 × 10−5 μM respectively), as did compound 589 (40% inhibition at 0.08 mg ml−1), while the other compounds were inactive.339,340 Compound 590, isolated from the bulbs of G. saundersiae (as Ornithogalum saundersiae), showed significant inhibition (IC50 = 3.1 μM) of the proliferation of peripheral blood lymphocytes produced by a patient with chronic renal failure, suggesting potential as an immunosuppressive agent. Rearranged cholestane glycosides (591–599) with a six-membered hemiacetal ring E, between C-16 and C-23, have been isolated. The conformation of ring E was determined through molecular mechanics and molecular dynamics calculations.341 These compounds were tested for anti-tumour activity, primarily against leukaemia HL-60 cells and MOLT-4 cells. Compounds 591 (IC50 = 21.0 nM and 18.0 nM), 592 (IC50 = 9.2 nM and 3.2 nM) and 597–599 (IC50 = 0.019, 0.063 and 0.052 μM for HL-60 cells) showed significant cytotoxic activity, primarily through the induction of apoptosis. These results would indicate that the presence of an aromatic ester group at the glycoside moiety is essential for good activity.342–348 Further investigation of the methanol extract of the bulbs resulted in the isolated of the related cholestane glycoside 600.349 In addition to the rearranged cholestane glycosides mentioned above, ten new (575, 601–609) and three previously isolated (567–569) cholestane glycosides, with a glycosidic moiety at C-16 have been tested for their inhibitory activity against leukemia HL-60 cells and MOLT-4 cells. All these compounds showed significant activity, with a loss of activity noted each time the ester group on the sugar moiety was removed.350–353 Due to the significant anti-tumour activity shown by these plant extracts, a number of patents have been filed in Japan and China covering the extract methods used, the use of the saponins and, in some cases, the synthesis of the active components.354–358 In particular, 567 (called OSW1) has been the subject of much synthetic work. The full synthesis of the compound itself has been accomplished, and a large number of analogues have been produced for structure–activity relationship studies. Studies indicate that OSW1 acts by damaging the mitochondria and inducing apoptosis through an increase of cytosolic calcium and thus the activation of calcium dependent apoptosis. It has been demonstrated that OSW1 is active against some cells that are resistant to conventional anti-cancer agents, in particular fludarabine-resistant chronic lymphocytic leukemia cells.359 In addition to the work on the anti-tumour activity of G. saundersiae, the ethanol extract of the whole dried plant has been tested for its protective effects against acute hepatic failure. The authors concluded that it did provide some benefit, primarily through suppressing oxidative stress, lipid peroxidation and apoptosis of hepatocytes, as well as reducing inflammation.360,361
Species from the genus Ornithogalum have received substantial attention. While early work on the genus began in the 1950s on the European members of the genus, later work has included species from Turkey and Iran.
Work on the European species of Ornithogalum was initially centred on Ornithogalum umbellatum L. (common Star of Bethlehem). European species of Ornithogalum are characterised by the presence of cardenolides, with some flavonoids also identified. Extracts of the bulbs of O. umbellatum were found to have a digitalis-like effect on frog, rabbit and cat hearts. In all cases, a positive inotropic action was noted, decreased conduction and finally systolic standstill.362–364 A clinical trial found that when tablets of O. umbellatum were substituted for digitoxin there was a loss of activity and fluid retention increased. When the tablets were coated to protect them from stomach acid, the digitalis-like action was re-established but at only half the expected strength. It was concluded that only half the active components were being absorbed. When compared to digitoxin, the drug showed less slowing of the heart, a greater diuretic effect and less gastrointestinal nausea.365 The principal active component of O. umbellatum was found to be the cardenolide convallatoxin 610.366 The cytotoxicity of convallatoxin 610 was studied. It was found to have an IC50 of 002 μg ml−1 when assayed against Eagle's KB strain of human carcinoma and structure–activity studies showed that the glycosidic portion was essential for activity and that the β-hydroxy groups at C-5 and C-14 and the aldehyde at C-10 significantly enhanced the activity.367 The convallatoxin 610 content in the bulbs can be increased by defloration of the plant.368 In total, sixteen cardenolides (610–626) have been isolated from the bulbs of O. umbellatum L.366,369–371 Later phytochemical investigations of the bulbs resulted in the isolation and identification of two flavonoids (627 and 628)372 while the four flavonoids (627–630) were isolated from the leaves.372,373 Compounds 629 and 630 were also isolated from the leaves of Ornithogalum algeriense Boiss. and Ornithogalum kochii Parl.373 Sabudak and Oyman reported the presence of 3-O-(2′-methoxy-4′-(2-pentenal))-phenylsitosterol in the bulbs of O. umbellatum374 and a study of the roots colonized by the fungus Glomus intraradices found a complex range of apocarotenoids.375 A range of cardenolides have been isolated from the species Loncomelos magnum (Krasch. & Schischk.) Speta (as Ornithogalum magnum) (610, 614, 631–635),376–382Honorius nutans (L.) Gray (as Ornithogalum nutans) (610, 631, 632, 636–664),383,384Ornithogalum gussonei Ten. (631, 635, 665)385 and Honorius boucheanum (Kunth) Holub (as Ornithogalum boucheanum) (666–673).386 The aerial parts of O. gussonei yielded two flavonoids saponaretin 674 and saponarin 675.387 A terpene glucoside (676) has been isolated from Ornithogalum montanum Cyr.388 and the main water soluble polysaccharide of the bulbs of Ornithogalum ponticum (Zahar.) Speta was found to be a neutral glucofructan with a glucose![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fructose ratio of 30
fructose ratio of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.57,389
1.57,389
A comparative study on the Bulgarian species Ornithogalum nanum Sibth. & Sm., O. gussonei, Loncomelos narbonense (L.) Raf. (as O. narbonense), and O. montanum Cyr. resulted in cardenolides (677–681) being found in the bulbs of O. nanum and O. gussonei but not in the bulbs of the other two species.390
The leaves and bulbs of the Turkish species Ornithogalum alpigenum Stapf. have been tested for antioxidant activity, antimicrobial activity and as free radical scavengers. The leaf extracts were found to be good free radical scavengers (90.9%) and the bulb extracts (methanol, ethanol, acetone and benzene) showed variable activity against Candida albicans, Bacillus subtilis and Bacillus cereus. The highest antioxidant activity was found in the methanol extract of the bulbs (88.1% on a β-carotene-linoleic acid system) and the lowest in the benzene extract of the bulbs.391 The Iranian species Ornithogalum procerum Stapf. and Ornithogalum cuspidatum Bertol. have received some attention. The aerial parts of O. cuspidatum are used in Iran as food additives as well as an anti-irritant and relaxant to soothe the throat when suffering from a dry cough. The essential oils in the leaves, flowers and bulbs have been determined by GC-MS. The flowers and bulbs contained primarily saturated hydrocarbons, while the leaves contained oxygenated hydrocarbons and terpenoid compounds.392 In addition, a GS-MS analysis of the methanol extract of the bulbs has allowed for the identification of thirteen phytosterols (cholesterol, 3,5-didehydrostigmastan-6,22-diene, 4,4-dimethyl-5α-cholest-7-en-3-one, 4β-methylcholesterol, 5-cholestene-3β,7β-diol, campesterol, cholest-4-ene-3,6-dione, stigmast-4-en-3-one, stigmasta-3,5-dien-7-one, stigmasterol, Δ5-ergostenol, β-sitosterol and γ-sitosterol).393 A GC-MS analysis of the aerial parts and bulbs of O. procerum resulted in the identification of twenty-three essential oils in the aerial parts and four polysterol-type compounds in the bulbs.394Ornithogalum sintensii Freyn, another Iranian species, has been tested for antioxidant activity. A higher phenolic and flavonoid content in the aerial parts of the plant relative to the bulbs, accounted for the greater antioxidant activity in those extracts (IC50 for DPPH radical scavenging activity of 368 and 669 μg ml−1 for leaves and bulbs respectively).395
Eliokarmos thyrsoides (Jacq.) Raf. is found in southwestern South Africa. Although not reportedly used by traditional healers it has been strongly linked to stock poisoning.1 Accordingly, the bulbs have been the subject of many phytochemical investigations. An initial investigation of the methanol extract of the bulbs in 1992 (as Ornithogalum thyrsoides) resulted in the isolation of four cholestane bisdesmosides (682–685), with 685 showing some inhibitory activity on cyclic AMP phosphodiesterase (IC50 = 15.3 × 10−5 M).396 Following the discovery that the methanol extract of the bulbs showed potent cytotoxicity against leukemia HL-60 cells (IC50 = 0.79 μg ml−1), fractionation of this extract resulted in the isolation of twelve bisdesmosidic cholestane glycosides (577–578, 686–694),397 seven cholestane glycosides (695–701) and nine spirocyclic glycosides (702–710).398,399 The bisdesmosidic cholestane glycosides with an aromatic acyl group at the C-16 diglycoside were found to be the most cytotoxic (686 and 576) with IC50 values of 0.00016 and 0.00013 μg ml−1 respectively. Compounds 687 and 696, the deacyl derivatives of compounds 688 and 689 and compounds 691 and 692 respectively did not show any activity at all.400 Only moderate activity against leukemia HL-60 cells was found for the spirocyclic glycosides (IC50 of 1.6–5.3 μg ml−1).401 An arabinoglucuronomannoglycan has been isolated from the leaves of E. thyrsoides (as O. thyrsoides).402 South African bulbs of Stellarioides longibracteata (Jacq.) Speta are used extensively by the Zulu as an anti-inflammatory, and regionally to treat diabetes. A phytochemical investigation of the bulbs (as Ornithogalum longibracteatum Jacq.) revealed one major compound, the homoisoflavanone 711.403 The water extract of the bulbs produced a significant increase in glucose utilisation in Chang liver cells.404Stellarioides longibracteata was introduced to China many years ago and it is used in Chinese folk medicine to treat cancer, as an antimicrobial, anti-inflammatory and to treat hepatitis and parotitis.405 This species was known earlier as Ornithogalum caudatum Ait. and as such many papers and in particular patents, have been filed under that name. Spirosterols, caudaside A 712 and hecogenin 713 have been isolated as well as β-sitosterol406 and three homoisoflavanones (714–716).407 Compounds 714–716 were tested for anti-tumour activity against P388 (mouse leukemia) and A-549 (human pulmonary adenocarcinoma) but no activities were noted. Later investigations of the bulbs of S. longibracteata (as O. caudatum) resulted in the isolation of n-butyl pyroglumate 717, 26 additional known flavonoids, sterols and acids408 and a monoterpene lactone (6-hydroxy-4,4,7α-trimethyl-5,6,7,7α-tetrahydro-4H-benzofuran-2-one).409 A number of patents have been filed in China and Russia outlining methods for the extraction of S. longibracteata (as O. caudatum) and use as a treatment for cancer,410,411 cholecystitis and cholecystitis-related diseases412 and for the isolation of the flavonoid components for use as an anti-inflammatory and analgesic.413,414 Extracts from S. longibracteata (as O. caudatum) have shown some benefit in the treatment of liver conditions.415,416 Four water soluble polysaccharide fractions have been extracted from S. longibracteata (as O. caudatum) and have been found to have potential as antitumour agents.417–419 Further work on the anti-cancer effects of this species has shown inhibition of MDA-MB-231 breast cancer cells in a dose and time dependant manner.420 The bulbs of Stellarioides tenuifolia (Redoute) Speta (as Ornithogalum tenuifolium Delaroche) yielded a crystalline steroidal saponin, 718, with an unusual 5β configuration.421 This unusual configuration at C-5, together with the 1β,3α hydroxyl group configuration, results in a molecule with a shape that is able to undergo self-recognition, similar to single-strand DNA, and thus form a supramolecular zipper.
A review of steroidal glycosides isolated from various monocot families (including Galtonia saundersiae, as Ornithogalum saundersiae) and their biological activities has been published as well as a review of cholestane glycosides from G. saundersiae, G. candicans and Eliokarmos thyrsoides (as Ornithogalum thyrsoides).422,423
4 The Oziroëoideae
The genus Oziroë Raf. (syn. Fortunatia Macbr.), is widespread in South America and can be found in Peru, Chile, Bolivia, Paraguay and north to central Argentina.424 No phytochemical investigations have been recorded on these plant species.5 Conclusions
Research into the chemistry of the Hyacinthaceae has spanned over 90 years and resulted in the isolation of over 700 compounds. The need to understand the chemistry was largely fuelled by both the toxicity of the plants to stock and the significant ethnomedicinal applications associated with representative species. In addition to the isolation of many compounds of medicinal significance, the phytochemical studies have shed light on the classification of many of the species. A thorough understanding of the chemistry and pharmacology of these important plant species allows for the potential discovery of new medical applications and further aids the conservation of those taxa threatened with unsustainable utilisation by indicating alternatives with similarly active constituents.6 Acknowledgements
This review was initiated during a Rockefeller Foundation Bellagio Academic Writing Residency, which Dulcie Mulholland gratefully acknowledges. Dr Mario Martínez-Azorín of CIBIO at the Universidad de Alicante, Spain, kindly commented on taxonomic queries, particularly with respect to the Ornithogaloideae; the third author (Neil Crouch), however, takes responsibility for final decisions on current names, and their relation to synonyms.7 References
- T. S. Pohl, N. R. Crouch and D. A. Mulholland, Curr. Org. Chem., 2000, 4, 1287–1324 CrossRef CAS.
- Angiosperm Phylogeny Group, Bot. J. Linn. Soc., 2003, 141, 399–436 CrossRef.
- Angiosperm Phylogeny Group, Bot. J. Linn. Soc., 2009, 161, 105–121 CrossRef.
- M. Pfosser and F. Speta, Ann. Mo. Bot. Gard., 1999, 86, 852–875 CrossRef.
- F. Speta, The Families and Genera of Vascular Plants, Springer, Berlin, 1998, vol. 3, pp. 261–285 Search PubMed.
- M. Martínez-Azorín, B. Crespo Manuel, A. Juan and M. F. Fay, Ann. Bot., 2011, 107, 1–37 CrossRef.
- S. Ali Syed, Y. Yu, M. Pfosser and W. Wetschnig, Ann. Bot., 2012, 109, 95–107 CrossRef.
- M. W. Chase, J. L. Reveal and M. F. Fay, Bot. J. Linn. Soc., 2009, 161, 132–136 CrossRef.
- B. M. Abegaz, J. Mutanyatta and M. Nindi, Nat. Prod. Commun., 2007, 2, 475–498 CAS.
- K. du Toit, S. E. Drewes and J. Bodenstein, Nat. Prod. Res., 2010, 24, 457–490 CrossRef CAS.
- W. Heller and C. H. Tamm, Fortschr. Chem. Org. Naturst., 1979, 40, 106–136 Search PubMed.
- P. Boehler and C. H. Tamm, Tetrahedron Lett., 1967, 8, 3479–3483 CrossRef.
- H. P. Weber, Helv. Chim. Acta, 1977, 60, 1288–1392 Search PubMed.
- C. H. Tamm, Arzneim. Forsch., 1972, 22, 1776–1784 CAS.
- W. Heller, P. Andermatt, W. A. Schaad and C. H. Tamm, Helv. Chim. Acta, 1976, 59, 2048–2058 CrossRef CAS.
- Y. Mimaki, K. Ori, S. Kubo, Y. Sashida, T. Nikaido, L. G. Song and T. Ohmoto, Chem. Lett., 1992, 1863–1866 CrossRef CAS.
- Y. Mimaki, H. Nishino, K. Ori, M. Kuroda, T. Matsui and Y. Sashida, Chem. Pharm. Bull., 1994, 42, 327–332 CrossRef CAS.
- J. K. Sihra, PhD Thesis, University of Surrey, 2012.
- S. Koduru, D. S. Grierson and A. J. Afolayan, Curr. Sci., 2007, 92, 906–908 Search PubMed.
- R. Williams, N. R. Crouch and G. Pettit, ISBA Bulletin, 1996, 44, 26–31 Search PubMed.
- W. T. L. Sidwell and C. H. Tamm, Tetrahedron Lett., 1970, 11, 475–478 CrossRef.
- L. Farkas, A. Gottsegen, M. Nogradi and J. Strelisky, Tetrahedron, 1971, 27, 5049–5056 CrossRef CAS.
- W. T. L. Sidwell, H. Fritz and C. H. Tamm, Helv. Chim. Acta, 1971, 54, 207–215 CrossRef CAS.
- R. Ziegler and C. H. Tamm, Helv. Chim. Acta, 1976, 59, 1997–2011 CrossRef CAS.
- D. A. Mulholland, C. Koorbanally, N. R. Crouch and P. Sandor, J. Nat. Prod., 2004, 67, 1726–1728 CrossRef CAS.
- W. T. L. Sidwell, C. H. Tamm, R. Ziegler, J. Finer and J. Clardy, J. Am. Chem. Soc., 1975, 97, 3518–3519 CrossRef CAS.
- J. L. S. Taylor and J. van Staden, S. Afr. J. Bot., 2002, 68, 80–85 CAS.
- A. K. Jager, A. Hutchings and J. van Staden, J. Ethnopharmacol., 1996, 52, 95–100 CrossRef CAS.
- J. L. S. Taylor and J. van Staden, J. Ethnopharmacol., 2001, 75, 257–265 CrossRef CAS.
- A. K. Jager and J. van Staden, Phytochem. Rev., 2005, 4, 39–46 CrossRef CAS.
- M. Gaidamashvili and J. van Staden, S. Afr. J. Bot., 2006, 72, 661–663 CrossRef CAS.
- M. Gaidamashvili and J. van Staden, J. Ethnopharmacol., 2002, 80, 131–135 CrossRef CAS.
- A. R. Ndhlala, R. B. Mulaudzi, M. G. Kulkarni and J. Van Staden, S. Afr. J. Bot., 2012, 79, 1–8 CrossRef.
- J. L. S. Taylor and J. van Staden, S. Afr. J. Bot., 2002, 68, 157–162 CAS.
- J. L. S. Taylor and J. Van Staden, Plant Growth Regul., 2001, 34, 39–47 CrossRef CAS.
- R. E. Finckh and C. H. Tamm, Experientia, 1970, 26, 472–473 CrossRef CAS.
- W. Heller and C. H. Tamm, Helv. Chim. Acta, 1974, 57, 1766–1784 CrossRef CAS.
- W. Heller and C. H. Tamm, Helv. Chim. Acta, 1978, 61, 1257–1261 CrossRef CAS.
- N. A. Koorbanally, N. R. Crouch, A. Langois, K. Du Toit, D. A. Mulholland and S. E. Drewes, S. Afr. J. Bot., 2006, 72, 428–433 CrossRef.
- N. A. Koorbanally, N. R. Crouch, A. Harilal, B. Pillay and D. A. Mulholland, Biochem. Syst. Ecol., 2006, 34, 114–118 CrossRef CAS.
- K. Du Toit, E. E. Elgorashi, S. F. Malan, D. A. Mulholland, S. E. Drewes and J. Van Staden, S. Afr. J. Bot., 2007, 73, 236–241 CrossRef CAS.
- J. K. Sihra, PhD Thesis, University of Surrey, Guildford, UK, 2012.
- I. Masterova, V. Suchy, D. Uhrin, K. Ubik, Z. Grancaiova and B. Bobovnicky, Phytochemistry, 1991, 30, 713–714 CrossRef CAS.
- E. Miadokova, I. Masterova, V. Vlckova, V. Duhova and J. Toth, J. Ethnopharmacol., 2002, 81, 381–386 CrossRef CAS.
- I. Juranek, V. Suchy, D. Stara, I. Masterova and Z. Grancaiova, Die Pharmazie, 1993, 48, 310–311 CAS.
- M. Urbancikova, I. Masterova and J. Toth, Fitoterapia, 2002, 73, 724–726 CrossRef CAS.
- M. Ellnain-Wojtaszek, Z. Kowalewski and L. Skrzypczakowa, Ann. Pharm. (Poznan), 1978, 13, 171–175 CAS.
- M. Adinolfi, M. M. Corsaro, R. Lanzetta, G. Laonigro, L. Mangoni and M. Parrilli, Phytochemistry, 1986, 26, 285–290 CrossRef CAS.
- Z. Grancaiova, I. Materova and V. Suchy, Fitoterapia, 1992, 63, 380 CAS.
- M. Adinolfi, G. Barone, M. M. Corsaro, L. Mangoni, R. Lanzetta and M. Parrilli, Can. J. Chem., 1988, 66, 2787–2793 CrossRef CAS.
- N. Asano, H. Kuroi, K. Ikeda, H. Kizu, Y. Kameda, A. Kato, I. Adachi, A. A. Watson, R. J. Nash and G. W. J. Fleet, Tetrahedron: Asymmetry, 2000, 11, 1–8 CrossRef CAS.
- F. J. Arias, P. Antolin, C. de Torre, B. Barriuso, R. Iglesias, M. A. Rojo, J. Miguel Ferreras, E. Benvenuto, E. Mendez and T. Girbes, Int. J. Biochem. Cell Biol., 2003, 35, 61–78 CrossRef CAS.
- K. Yoshida, H. Aoki, K. Kameda and T. Kondo, ITE Lett. Batteries, New Technol. Med., 2002, 3, 35–38 CAS.
- K. Ori, M. Koroda, Y. Mimaki, H. Sakagami and Y. Sashida, Phytochemistry, 2003, 64, 1351–1359 CrossRef CAS.
- M. Kuroda, Y. Mimaki, K. Ori, H. Sakagami and Y. Sashida, J. Nat. Prod., 2004, 67, 2099–2103 CrossRef CAS.
- V. V. Barbakadze, I. L. Targamadze and A. I. Usov, Bioorg. Khim., 1996, 22, 441–445 CAS.
- V. Barbakadze and A. Usov, Bull. Georgian Natl. Acad. Sci., 2001, 163, 484–487 CAS.
- G. Barone, M. M. Corsaro, R. Lanzetta and M. Parrilli, Phytochemistry, 1988, 27, 921–923 CrossRef CAS.
- O. Goktas, E. Ozen, E. Baysal, R. Mammadov and M. H. Alma, Wood Res. (Bratislava, Slovakia), 2010, 55, 53–62 CAS.
- A. Kato, N. Kato, I. Adachi, J. Hollinshead, G. W. J. Fleet, C. Kuriyama, K. Ikeda, N. Asano and R. J. Nash, J. Nat. Prod., 2007, 70, 993–997 CrossRef CAS.
- C. P. Waller, PhD Thesis, University of Surrey, Guildford, UK, 2012.
- J. W. C. Gunn, M. Goldberg and J. H. Ferguson, Trans. R. Soc. S. Afr., 1924, 12, 1–3 CrossRef CAS.
- D. A. Mulholland, N. R. Crouch, C. Koorbanally, N. Moodley and T. Pohl, Biochem. Syst. Ecol., 2006, 34, 251–255 CrossRef CAS.
- T. Pohl, PhD Thesis, University of Natal, South Africa, 2002.
- T. Pohl, C. Koorbanally, N. R. Crouch and D. A. Mulholland, Biochem. Syst. Ecol., 2001, 29, 857–860 CrossRef CAS.
- S. G. Sparg, J. van Staden and A. K. Jager, J. Ethnopharmacol., 2002, 80, 95–101 CrossRef CAS.
- L. V. Buwa and J. van Staden, J. Ethnopharmacol., 2006, 103, 139–142 CrossRef CAS.
- C. P. Waller, A. E. Thumser, M. K. Langat, N. R. Crouch and D. A. Mulholland, Phytochemistry , 2013, in press Search PubMed.
- C. Koorbanally, D. A. Mulholland and N. R. Crouch, Biochem. Syst. Ecol., 2006, 34, 588–592 CrossRef CAS.
- J. Mutanyatta, G. Matapa Bhagali, D. D. Shushu and M. B. Abegaz, Phytochemistry, 2003, 62, 797–804 CrossRef CAS.
- D. D. Shushu, J. Mutanyatta and B. M. Abegaz, J. Herbs, Spices Med. Plants, 2005, 11, 97–107 CrossRef CAS.
- B. M. Abegaz, Phytochem. Rev., 2002, 1, 299–310 CrossRef CAS.
- N. Moodley, N. R. Crouch, D. A. Mulholland, D. Slade and D. Ferreira, S. Afr. J. Bot., 2006, 72, 517–520 CrossRef CAS.
- I. Calvo Maria, Fitoterapia, 2009, 80, 394–398 CrossRef.
- I. Calvo Maria, Fitoterapia, 2009, 80, 96–101 CrossRef.
- M. Parrilli, R. Lanzetta, V. Dovinola, M. Adinolfi and L. Mangoni, Can. J. Chem., 1981, 59, 2261–2265 CrossRef CAS.
- J. Lietava, J. Ethnopharmacol., 1992, 35, 263–266 CrossRef CAS.
- A. Pieroni, V. Janiak, C. M. Durr, S. Ludeke, E. Trachsel and M. Heinrich, Phytother. Res., 2002, 16, 467–473 CrossRef CAS.
- M. R. Loizzo, R. Tundis, F. Menichini, A. Pugliese, M. Bonesi, U. Solimene and F. Menichini, Int. J. Food Sci. Nutr., 2010, 61, 780–791 CrossRef CAS.
- R. M. Mamedov and O. M. Gektash, Khim. Rastit. Syr'ya, 2011, 141–144 CAS.
- M. Parrilli, M. Adinolfi, V. Dovinola and L. Mangoni, Gazz. Chim. Ital., 1979, 109, 391–393 CAS.
- M. Parrilli, M. Adinolfi, V. Dovinola and L. Mangoni, Rend. Accad. Sci. Fis. Mat., Naples, 1979, 45, 527–532 Search PubMed.
- M. Parrilli, M. Adinolfi and L. Mangoni, Gazz. Chim. Ital., 1979, 109, 611–613 CAS.
- M. Parrilli, R. Lanzetta, M. Adinolfi and L. Mangoni, Tetrahedron, 1980, 36, 3591–3596 CrossRef CAS.
- M. Adinolfi, G. Barone, R. Lanzetta, G. Laonigro, M. Parilli and L. Mangoni, Rend. Accad. Sci. Fis. Mat., Naples, 1983, 50, 295–296 CAS.
- M. Adinolfi, G. Barone, R. Lanzetta, G. Laonigro, L. Mangoni and M. Parrilli, J. Nat. Prod., 1983, 46, 559–562 CrossRef CAS.
- M. Adinolfi, G. Barone, R. Lanzetta, G. Laonigro, L. Mangoni and M. Parrilli, J. Nat. Prod., 1984, 47, 721–723 CrossRef CAS.
- M. Adinolfi, G. Barone, R. Lanzetta, G. Laonigro, L. Mangoni and M. Parrilli, J. Nat. Prod., 1984, 47, 544–546 CrossRef CAS.
- M. Adinolfi, G. Barone, R. Lanzetta, G. Laonigro, L. Mangoni and M. Parrilli, J. Nat. Prod., 1984, 47, 100–105 CrossRef CAS.
- M. Adinolfi, G. Barone, R. Lanzetta, G. Laonigro, M. Parrilli and L. Mangoni, Rend. Accad. Sci. Fis. Mat., Naples, 1983, 50, 293–294 CAS.
- M. Adinolfi, G. Barone, M. M. Corsaro, R. Lanzetta, L. Mangoni and M. Parrilli, Can. J. Chem., 1987, 65, 2317–2326 CrossRef CAS.
- M. Adinolfi, G. Barone, R. Lanzetta, G. Laonigro, L. Mangoni and M. Parrilli, Can. J. Chem., 1983, 61, 2633–2637 CrossRef CAS.
- M. Adinolfi, G. Barone, R. Lanzetta, G. Laonigro, L. Mangoni and M. Parrilli, Can. J. Chem., 1984, 62, 1223–1226 CrossRef CAS.
- R. Lanzetta, G. Laonigro, M. Parrilli and E. Breitmaier, Can. J. Chem., 1984, 62, 2874–2878 CrossRef CAS.
- G. Barone, M. Belardini, R. Lanzetta, G. Laonigro and M. Parrilli, Rend. Accad. Sci. Fis. Mat., Naples, 1983, 50, 297–298 CAS.
- M. Adinolfi, G. Barone, M. Belardini, R. Lanzetta, G. Laonigro and M. Parrilli, Phytochemistry, 1984, 23, 2091–2093 CrossRef CAS.
- M. Adinolfi, G. Barone, M. Belardini, R. Lanzetta, G. Laonigro and M. Parrilli, Phytochemistry, 1985, 24, 2423–2426 CrossRef CAS.
- M. Adinolfi, G. Barone, F. Giordano, R. Lanzetta and M. Parrilli, Tetrahedron, 1990, 46, 6565–6574 CrossRef CAS.
- M. Adinolfi, G. Barone, M. M. Corsaro, L. Mangoni, R. Lanzetta and M. Parrilli, Tetrahedron, 1988, 44, 4981–4988 CrossRef CAS.
- R. Della Loggia, P. Del Negro, A. Tubaro, G. Barone and M. Parrilli, Planta Med., 1989, 55, 587–588 CrossRef.
- S. H. Hilal, M. M. Shabana and A. S. Ibrahim, Egypt. J. Pharm. Sci., 1985, 24, 67–74 Search PubMed.
- M. Guarrera Paolo, G. Salerno and G. Caneva, J. Ethnopharmacol., 2005, 99, 367–378 CrossRef.
- C. L. Quave, L. R. W. Plano, T. Pantuso and B. C. Bennett, J. Ethnopharmacol., 2008, 118, 418–428 CrossRef CAS.
- Y. Mimaki, S. Kubo, Y. Kinoshita, Y. Sashida, L. G. Song, T. Nikaido and T. Ohmoto, Phytochemistry, 1993, 34, 791–797 CrossRef CAS.
- G. Barone, M. M. Corsaro, R. Lanzetta, L. Mangoni and M. Parrilli, Phytochemistry, 1993, 33, 431–436 CrossRef CAS.
- K. Ori, M. Kuroda, Y. Mimaki, H. Sakagami and Y. Sashida, Chem. Pharm. Bull., 2003, 51, 92–95 CrossRef CAS.
- M. Adinolfi, M. M. Corsaro, R. Lanzetta, A. Mancino, L. Mangoni and M. Parrilli, Phytochemistry, 1993, 34, 773–778 CrossRef CAS.
- M. Kuroda, Y. Mimaki, K. Ori, H. Koshino, T. Nukada, H. Sakagami and Y. Sashida, Tetrahedron, 2002, 58, 6735–6740 CrossRef CAS.
- M. M. Corsaro, R. Lanzetta, A. Mancino and M. Parrilli, Phytochemistry, 1992, 31, 1395–1397 CrossRef CAS.
- P. Baskaran, B. Ncube and J. van Staden, Plant Growth Regul., 2012, 67, 235–245 CrossRef CAS.
- C. Dias, J. A. Borralho Graca and M. Lurdes Goncalves, J. Ethnopharmacol., 2000, 71, 487–492 CrossRef CAS.
- S.-M. Lee, H.-K. Chun, C.-H. Lee, B.-S. Min, E.-S. Lee and Y.-H. Kho, Chem. Pharm. Bull., 2002, 50, 1245–1249 CrossRef CAS.
- E.-J. Yeo, K.-T. Kim, Y. S. Han, S.-Y. Nah and H.-D. Paik, Food Sci. Biotechnol., 2006, 15, 639–642 CAS.
- I. Kouno, N. Noda, Y. Ida, M. Sholichin, K. Miyahara, T. Komori and T. Kawasaki, Liebigs Ann. Chem., 1982, 306–314 CrossRef CAS.
- M. Sholichin, K. Miyahara and T. Kawasaki, Heterocycles, 1982, 17, 251–257 CrossRef CAS.
- M. Sholichin, K. Miyahara and T. Kawasaki, Chem. Pharm. Bull., 1985, 33, 1756–1759 CrossRef CAS.
- S.-M. Lee, H.-J. Lee, H.-K. Chun, C.-H. Lee, S.-J. Kang, H.-Y. Maeng and Y.-H. Kho, Saengyak Hakhoechi, 2001, 32, 189–192 CAS.
- M. Ono, D. Toyohisa, T. Morishita, H. Horita, S. Yasuda, Y. Nishida, T. Tanaka, M. Okawa, J. Kinjo, H. Yoshimitsu and T. Nohara, Chem. Pharm. Bull., 2011, 59, 1348–1354 CrossRef CAS.
- I. Kouno, T. Komori and T. Kawasaki, Tetrahedron Lett., 1973, 14, 4569–4572 CrossRef.
- Y. Nishida, M. Eto, H. Miyashita, T. Ikeda, K. Yamaguchi, H. Yoshimitsu, T. Nohara and M. Ono, Chem. Pharm. Bull., 2008, 56, 1022–1025 CrossRef CAS.
- H. B. Lee and S. M. Lee,KR Pat., 2010-35527, 2010.
- H. G. Jun, Y. H. Ko, C. H. Lee, H. J. Lee and S. M. Lee, KR Pat., 2001-1197, 2001.
- M. T. Ono, Y. Takatsu, T. Ochiai, S. Yasuda, Y. Nishida, T. Tanaka, M. Okawa, J. Kinjo, H. Yoshimitsu and T. Nohara, Chem. Pharm. Bull., 2012, 60, 1314–1319 CrossRef CAS.
- T. Kurihara and H. Ito, Annu. Rep. Tohoku Coll. Pharm., 1968, 39–47 CAS.
- A. I. Ismailov, Azerb. Med. Zh., 1961, 1, 57–60 Search PubMed.
- V. V. Barbakadze, I. L. Targamadze and A. I. Usov, Bioorg. Khim., 1996, 22, 58–61 CAS.
- T. Yamashita, K. Yasuda, H. Kizu, Y. Kameda, A. Watson, R. J. Nash, G. W. J. Fleet and N. Asano, J. Nat. Prod., 2002, 65, 1875–1881 CrossRef CAS.
- Y. Mimaki, K. Ori, Y. Sashida, T. Nikaido, L. G. Song and T. Ohmoto, Bull. Chem. Soc. Jpn., 1993, 66, 1182–1186 CrossRef CAS.
- Y. Mimaki, K. Ori, Y. Sashida, T. Nikaido, L. G. Song and T. Ohmoto, Chem. Lett., 1992, 1999–2000 CrossRef CAS.
- N. Asano, K. Ikeda, M. Kasahara, Y. Arai and H. Kizu, J. Nat. Prod., 2004, 67, 846–850 CrossRef CAS.
- K. Hosokawa, Y. Fukunaga, E. Fukushi and J. Kawabata, Phytochemistry, 1995, 38, 1293–1298 CrossRef CAS.
- K. Hosokawa, Y. Fukunaga, E. Fukushi and J. Kawabata, Phytochemistry, 1996, 41, 1531–1533 CrossRef CAS.
- K. Hosokawa, Y. Fukunaga, E. Fukushi and J. Kawabata, Phytochemistry, 1995, 40, 567–571 CrossRef CAS.
- K. Hosokawa, Y. Fukunaga, E. Fukushi and J. Kawabata, Phytochemistry, 1995, 39, 1437–1441 CrossRef CAS.
- K. Hosokawa, Y. Fukunaga, E. Fukushi and J. Kawabata, Phytochemistry, 1996, 42, 671–672 CrossRef CAS.
- K. Hosokawa, Recent Res. Dev. Phytochem., 1998, 2, 135–141 CAS.
- K. Hosokawa and Y. Fukunaga, Plant Cell Rep., 1995, 14, 575–579 CrossRef CAS.
- G. C. Kite, C. Sellwood, P. Wilkin and M. S. J. Simmonds, Biochem. Syst. Ecol., 1998, 26, 357–359 CrossRef CAS.
- N. Asano, A. Kato, M. Miyauchi, H. Kizu, Y. Kameda, A. A. Watson, R. J. Nash and G. W. J. Fleet, J. Nat. Prod., 1998, 61, 625–628 CrossRef CAS.
- M. Adinolfi, G. Barone, L. Dellagreca, L. Mangoni, R. Lanzetta and M. Parrilli, Gazz. Chim. Ital., 1990, 120, 427–433 CAS.
- M. Adinolfi, T. Aquila, G. Barone, R. Lanzetta and M. Parrilli, Phytochemistry, 1989, 28, 3244–3246 CrossRef CAS.
- C. Dias, M. Dias, C. Borges, M. A. Almoster Ferreira, A. Paulo and J. Nascimento, J. Mass Spectrom., 2003, 38, 1240–1244 CrossRef CAS.
- A. Paulo, C. Dias, M. L. Jimeno, C. Borges and J. Nascimento, Fitoterapia, 2005, 76, 765–767 CrossRef CAS.
- C. Dias, A. Paulo, J. Nascimento, P. Houghton, J. E. Hawkes and M. L. Goncalves, Planta Med., 2003, 69, 1060–1062 CrossRef CAS.
- S. D. Wood, A. K. Allen, L. M. Wright and C. D. Reynolds, Lectins: Biol., Biochem., Clin. Biochem., 1997, 11, 86–90 CAS.
- S. D. Wood, L. M. Wright, C. D. Reynolds, P. J. Rizkallah, A. K. Allen, W. J. Peumans and E. J. M. Van Damme, Acta Crystallogr., Sect. D: Biol. Crystallogr., 1999, 55, 1264–1272 CrossRef CAS.
- L. M. Wright, P. J. Rizkallah, S. D. Wood and C. D. Reynolds, Acta Crystallogr., Sect. D: Biol. Crystallogr., 1998, 54, 665–667 CrossRef CAS.
- L. M. Wright, S. D. Wood, C. D. Reynolds, P. J. Rizkallah and A. K. Allen, Acta Crystallogr., Sect. D: Biol. Crystallogr., 1998, 54, 90–92 CrossRef CAS.
- L. M. Wright, S. D. Wood, C. D. Reynolds, P. J. Rizkallah, W. J. Peumans, E. J. M. Van Damme and A. K. Allen, Acta Crystallogr., Sect. D: Biol. Crystallogr., 1996, 52, 1021–1023 CrossRef CAS.
- L. M. Wright, C. D. Reynolds, P. J. Rizkallah, A. K. Allen, E. J. Van Damme, M. J. Donovan and W. J. Peumans, FEBS Lett., 2000, 468, 19–22 CrossRef CAS.
- A. Kato, I. Adachi, M. Miyauchi, K. Ikeda, T. Komae, H. Kizu, Y. Kameda, A. A. Watson, R. J. Nash, M. R. Wormald, G. W. J. Fleet and N. Asano, Carbohydr. Res., 1999, 316, 95–103 CrossRef CAS.
- A. A. Watson, R. J. Nash, M. R. Wormald, D. J. Harvey, S. Dealler, E. Lees, N. Asano, H. Kizu, A. Kato, R. C. Griffiths, A. J. Cairns and G. W. J. Fleet, Phytochemistry, 1997, 46, 255–259 CrossRef CAS.
- J. L. Thompson and J. K. N. Jones, Can. J. Chem., 1964, 42, 1088–1091 CrossRef CAS.
- C. Koorbanally, N. R. Crouch and D. A. Mulholland, Biochem. Syst. Ecol., 2001, 29, 539–541 CrossRef CAS.
- D. Ngamga, J. Bipa, P. Lebatha, C. Hiza, J. Mutanyatta, M. Bezabih, P. Tane and B. M. Abegaz, Nat. Prod. Commun., 2008, 3, 769–777 CAS.
- D. Ngamga, P. Tane, M. Bezabih and B. M. Abegaz, Planta Med., 2006, 72, 1009–1009 Search PubMed.
- F. Fusi, A. Ferrara, C. Koorbanally, N. R. Crouch, D. A. Mulholland and G. Sgaragli, J. Pharm. Pharmacol., 2008, 60, 489–497 CrossRef CAS.
- G. Amschler, A. W. Frahm, A. Hatzelmann, U. Kilian, D. Mueller-Doblies and U. Mueller-Doblies, Planta Med., 1996, 62, 534–539 CrossRef CAS.
- G. Amschler, A. W. Frahm, D. Muller-Doblies and U. Muller-Doblies, Phytochemistry, 1998, 47, 429–436 CrossRef CAS.
- A. Michalik, J. Hollinshead, L. Jones, G. W. J. Fleet, C.-Y. Yu, X.-G. Hu, R. van Well, G. Horne, F. X. Wilson, A. Kato, S. F. Jenkinson and R. J. Nash, Phytochem. Lett., 2010, 3, 136–138 CrossRef CAS.
- N. Moodley, D. A. Mulholland and N. R. Crouch, J. Nat. Prod., 2004, 67, 918–920 CrossRef CAS.
- N. R. Crouch, V. Bangani and D. A. Mulholland, Phytochemistry, 1999, 51, 943–946 CrossRef CAS.
- B. Ncube, J. F. Finnie and J. van Staden, S. Afr. J. Bot., 2011, 77, 387–396 CrossRef CAS.
- B. Ncube, J. F. Finnie and J. van Staden, S. Afr. J. Bot., 2012, 78, 246–251 CrossRef CAS.
- A. Langlois, D. A. Mulholland, G. D. Duncan, N. R. Crouch and T. J. Edwards, Biochem. Syst. Ecol., 2005, 33, 961–966 CrossRef CAS.
- C. A. Williams, J. B. Harborne and T. S. Crosby, Phytochemistry, 1976, 15, 349–350 CrossRef CAS.
- C. Koorbanally, S. Sewjee, D. A. Mulholland, N. R. Crouch and A. Dold, Phytochemistry, 2007, 68, 2753–2756 CrossRef CAS.
- V. Bangani, N. R. Crouch and D. A. Mulholland, Phytochemistry, 1999, 51, 947–951 CrossRef CAS.
- K. du Toit, A. Kweyama and J. Bodenstein, S. Afr. J. Sci., 2011, 107, 96–100 CrossRef CAS.
- B. M. Abegaz, Phytochem. Rev., 2002, 1, 299–310 CrossRef CAS.
- A. Silayo, B. T. Ngadjui and B. M. Abegaz, Phytochemistry, 1999, 52, 947–955 CrossRef CAS.
- S. O. Famuyiwa, K. F. Sichilongo, S. O. Yeboah and B. M. Abegaz, Phytochem. Lett., 2012, 5, 591–595 CrossRef CAS.
- P. Pillay, A. Phulukdaree, A. A. Chuturgoon, K. du Toit and J. Bodenstein, J. Ethnopharmacol., 2012 Search PubMed.
- F. Speta, Phyton (Horn, Austria), 1998, 38, 1–141 Search PubMed.
- L. Krenn and B. Kopp, Phytochemistry, 1998, 48, 1–29 CrossRef CAS.
- S. J. van der Walt, Onderstepoort J. Vet. Res., 1944, 20, 75–83 CAS.
- N. Sapeika, S. Afr. Pharm. J., 1951, 1, 28–29 Search PubMed.
- D. G. Steyn, 15th Annual Report of the Director of Veterinary Services, 1929, pp. 777–803 Search PubMed.
- C. F. Juritz, 12th Annual Meeting of the South African Association for the Advancement of Science, 1914, 109–150 Search PubMed.
- A. Hutchings and S. E. Terblanche, S. Afr. Med. J., 1989, 75, 41–96 Search PubMed.
- M. F. Pfosser and F. Speta, Plant Syst. Evol., 2004, 246, 245–263 Search PubMed.
- W. Kopaczewski, C. R. Hebd. Seances Acad. Sci., 1914, 158, 1520–1522 CAS.
- W. Kopaczewski, Biochem. Z., 1914, 66, 501–508 CAS.
- F. W. Smith, Analyst, 1921, 46, 178–179 RSC.
- O. Krestynova-Telupilova and F. Santavy, Ceska Slov. Farm., 1957, 6, 207–209 CAS.
- K. J. Kraus, E. Mutschler and H. Rochelmeyer, Arzneim. Forsch., 1969, 19, 322–328 CAS.
- K. J. Kraus, E. Mutschler and H. Rochelmeyer, Pharm. Ztg., 1969, 114, 417–419 CAS.
- F. Kaczmarek and I. Zygmunt, Herba Pol., 1974, 20, 330–338 CAS.
- E. Gliozheni and T. Bisha, Bul. Shkencave Nat., 1972, 26, 43–50 CAS.
- C. H. Boehringer, FR Pat. 1967-123308, 1967.
- G. Tittel and H. Wagner, Planta Med., 1980, 39, 125–134 CrossRef CAS.
- M. Couladi and A. Loukis, Fitoterapia, 1987, 58, 57–58 CAS.
- A. Aliaga, M. Fernandez, F. A. Vega and C. Dios, Acta Pol. Pharm., 1987, 44, 560–562 CAS.
- M. Fernandez, M. Oses and C. Dios, An. R. Acad. Farm., 1987, 53, 292–295 CAS.
- K. Okushima, Arch. Exp. Pathol. Pharmakol., 1922, 95, 258–266 CAS.
- J. Markwalder, Klin. Wochenschr., 1922, 1, 212–215 CrossRef CAS.
- A. Stoll, E. Suter, W. Kreis, B. B. Bussemaker and A. Hofmann, Helv. Chim. Acta, 1933, 16, 703–733 CrossRef CAS.
- W. Anders and G. Zeise, Med. Klin., 1952, 47, 1061–1063 CAS.
- H. Haas, Med. Klin., 1967, 62, 121–126 CAS.
- A. von Wartburg, Helv. Chim. Acta, 1966, 49, 30–42 CrossRef CAS.
- A. von Wartburg and J. Renz, Helv. Chim. Acta, 1959 Search PubMed.
- A. J. Verbiscar, J. Patel, T. F. Banigan and R. A. Schatz, J. Agric. Food Chem., 1986, 34, 973–979 CrossRef CAS.
- H. Lichti, P. Niklaus and A. von Wartburg, Helv. Chim. Acta, 1973, 56, 2083–2087 CrossRef CAS.
- B. Gorlich, Arzneim. Forsch., 1960, 10, 770–774 CAS.
- A. von Wartburg, M. Kuhn and K. Huber, Helv. Chim. Acta, 1968, 51, 1317–1328 CrossRef CAS.
- L. Krenn, R. Ferth, W. Robien and B. Kopp, Planta Med., 1991, 57, 560–565 CrossRef CAS.
- B. Kopp, L. Krenn, M. Draxler, A. Hoyer, R. Terkola, P. Vallaster and W. Robien, Phytochemistry, 1996, 42, 513–522 CrossRef CAS.
- L. Krenn, B. Kopp, S. Steurer and M. Schubert-Zsilavecz, J. Nat. Prod., 1996, 59, 612–613 CrossRef CAS.
- L. Krenn, M. Jelovina and B. Kopp, Fitoterapia, 2000, 71, 126–129 CrossRef CAS.
- M. Iizuka, T. Warashina and T. Noro, Chem. Pharm. Bull., 2001, 49, 282–286 CrossRef CAS.
- L. Krenn, B. Kopp, A. Deim, W. Robien and W. Kubelka, Planta Med., 1994, 60, 63–69 CrossRef CAS.
- I. Garcia-Jalon, F. A. Vega, M. Fernandez and T. Arrupe, Cienc. Ind. Farm., 1974, 6, 43–52 CAS.
- I. Garcia-Jalon, F. A. Vega, M. Fernandez and L. Martinez Valls, Cienc. Ind. Farm., 1973, 5, 260–269 CAS.
- F. A. Vega, C. Martin and I. Garcia-Jalon, An. R. Acad. Farm., 1969, 35, 291–296 CAS.
- F. A. Vega, I. Garcia-Jalon, M. Fernandez and J. Renedo, Phytochemistry, 1972, 11, 2896 CrossRef CAS.
- F. A. Vega, C. Martin, M. Fernandez and I. Garcia-Jalon, Galenica Acta, 1970, 23, 3–48 CAS ; (43-44-45), 79–96.
- F. A. Vega and M. Fernandez, Naturwissenschaften, 1964, 51, 483–484 CrossRef CAS.
- M. Fernandez, F. A. Vega, T. Arrupe and J. Renedo, Sci. Pharm., 1976, 44, 307–314 CAS.
- M. Fernandez, F. A. Vega, T. Arrupe and J. Renedo, Phytochemistry, 1972, 11, 1534 CrossRef CAS.
- M. Couladis, E. Verykokidou-Vitsaropoulou and S. Philianos, Fitoterapia, 1993, 64, 92 CAS.
- F. A. Vega, An. R. Acad. Farm., 1976, 42, 81–94 CAS.
- M. Fernandez, J. Renedo, T. Arrupe and F. A. Vega, Phytochemistry, 1975, 14, 586–586 CrossRef CAS.
- M. Fernandez, J. Renedo, T. Arrupe and F. A. Vega, Ciencia & Industria Farmaceutica, 1974, 6, 386–390 CAS.
- M. Fernandez, F. A. Vega, T. Arrupe and J. Renedo, Galenica Acta, 1971, 24, 45–57 CAS.
- T. Spies, W. Praznik, A. Hofinger, F. Altmann, E. Nitsch and R. Wutka, Carbohydr. Res., 1992, 235, 221–230 CrossRef CAS.
- W. Praznik and T. Spies, Carbohydr. Res., 1993, 243, 91–97 CrossRef CAS.
- D. Fos, J. Giraldez, M. J. Renedo, M. Fernandez and F. A. Vega, Cienc. Ind. Farm., 1979, 11, 141–146 CAS.
- M. J. Pascual-Villalobos and M. Fernandez, Ind. Crops Prod., 1999, 10, 115–120 CrossRef.
- H. Bozcuk, M. Ozdogan, O. Aykurt, F. Topcuoglu, H. Ozturk, D. Ekinci, A. Karadeniz, A. Mutlu and D. Burgucu, Turk. J. Med. Sci., 2011, 41, 101–108 Search PubMed.
- S. E. Shyr and E. J. Staba, Planta Med., 1976, 29, 86–90 CrossRef CAS.
- L. Krenn, M. Jambrits and B. Kopp, Planta Med., 1988, 54, 227–232 CrossRef CAS.
- L. Krenn, A. Hufner, A. Kastenhuber and F. Speta, Phytochemistry, 2004, 65, 2881–2884 CrossRef CAS.
- B. Kopp, M. Unterluggauer, W. Robien and W. Kubelka, Planta Med., 1990, 56, 193–197 CrossRef CAS.
- L. Krenn, M. Bamberger and B. Kopp, Planta Med., 1992, 58, 284–285 CrossRef CAS.
- L. Krenn, B. Kopp, E. Griesmayer-Camus and W. Kubelka, Sci. Pharm., 1992, 60, 65–72 CAS.
- C. F. Juritz, Chem. News J. Ind. Sci., 1923, 126, 69–70 CAS.
- N. Moodley, N. R. Crouch and D. A. Mulholland, Phytochemistry, 2007, 68, 2415–2419 CrossRef CAS.
- J. M. Watt, Arch. Exp. Pathol. Pharmakol., 1927, 120, 65–76 CrossRef CAS.
- P. G. J. Louw, Onderstepoort J. Vet. Res., 1953, 26, 141–148 CAS.
- R. Tschesche and K. H. Hottemann, Chem. Ber., 1953, 86, 392–395 CrossRef CAS.
- N. Sapeika, S. Afr. J. Med. Sci., 1944, 9, 31–32 CAS.
- P. G. J. Louw, Onderstepoort J. Vet. Res., 1949, 22, 313–319 CAS.
- P. G. J. Louw, Nature, 1949, 163, 30–31 CrossRef CAS.
- N. Sapeika, S. Afr. Med. J., 1950, 24, 912–913 CAS.
- B. Isaacson, S. Afr. Med. J., 1950, 24, 901–912 CAS.
- P. G. J. Louw, Onderstepoort J. Vet. Res., 1952, 25, 123–133 CAS.
- P. Zoller and C. H. Tamm, Helv. Chim. Acta, 1953, 36, 1744–1756 CrossRef CAS.
- P. S. Steyn, F. R. van Heerden and R. Vleggaar, S. Afr. J. Chem., 1986, 39, 143–146 CAS.
- L. Krenn, B. Kopp, M. Bamberger, E. Brustmann and W. Kubelka, Nat. Prod. Lett., 1993, 3, 139–143 CrossRef CAS.
- R. R. T. Majinda, R. D. Waigh and P. G. Waterman, Planta Med., 1997, 63, 188–190 CrossRef CAS.
- G. N. Foukaridis, E. Osuch, L. Mathibe and P. Tsipa, J. Ethnopharmacol., 1995, 49, 77–79 CrossRef CAS.
- J. Marx, E. Pretorius and M. J. Bester, J. Ethnopharmacol., 2006, 104, 315–321 CrossRef CAS.
- J. Marx, E. Pretorius, W. J. Espag and M. J. Bester, Environ. Toxicol. Pharmacol., 2005, 20, 26–34 CrossRef CAS.
- C. Koorbanally, D. A. Mulholland and N. R. Crouch, Biochem. Syst. Ecol., 2005, 33, 743–748 CrossRef CAS.
- M. Teshome, H. Kassa and K. Charles, Ethiop. J. Health Dev., 2010, 24, 175–179 Search PubMed.
- H. D. Neuwinger, African Ethnobotany: Poisons and Drugs, Chapman and Hall, London, 1994 Search PubMed.
- M. Miyakado, T. Kato, N. Ohno and K. Koshimizu, Phytochemistry, 1975, 14, 2717 CrossRef CAS.
- T. Pohl, C. Koorbanally, N. R. Crouch and D. A. Mulholland, Phytochemistry, 2001, 58, 557–561 CrossRef CAS.
- E. Dagne, W. Mammo, M. Alemu and I. Casser, Bull. Chem. Soc. Ethiop., 1994, 8, 85–89 CAS.
- K. Shimada, E. Umezawa, T. Nambara and S. M. Kupchan, Chem. Pharm. Bull., 1979, 27, 3111–3114 CrossRef CAS.
- H. Lichti and A. von Wartburg, Helv. Chim. Acta, 1960, 43, 1666–1681 CrossRef CAS.
- A. L. Cogne, A. Marston, S. Mavi and K. Hostettmann, J. Ethnopharmacol., 2001, 75, 51–53 CrossRef CAS.
- F. R. Van Heerden, R. Vleggaar and L. A. P. Anderson, S. Afr. J. Chem., 1988, 41, 145–151 CAS.
- A. R. Ndhlala, J. F. Finnie and J. van Staden, J. Ethnopharmacol., 2011, 133, 663–674 CrossRef CAS.
- N. R. Crouch, A. Langlois and D. A. Mulholland, Phytochemistry, 2007, 68, 1731–1734 CrossRef CAS.
- N. R. Crouch, K. du Toit, D. A. Mulholland and S. E. Drewes, Phytochemistry, 2006, 67, 2140–2145 CrossRef CAS.
- C. Koorbanally, D. A. Mulholland and N. R. Crouch, Biochem. Syst. Ecol., 2005, 33, 295–299 CrossRef CAS.
- N. A. Koorbanally, C. Koorbanally, A. Harilal, D. A. Mulholland and N. R. Crouch, Phytochemistry, 2004, 65, 3069–3073 CrossRef CAS.
- O. O. G. Amusan, J. D. Msonthi and L. P. Makhubu, Fitoterapia, 1997, 68, 185–186 Search PubMed.
- S. Abbas, S. Bashir, A. Khan, H. Mehmood Malik and H. Gilani Anwarul, Phytother. Res., 2012, 26, 704–708 CrossRef CAS.
- Y. B. Tripathi, A. V. Singh and G. P. Dubey, Current Science, 2001, 80, 1267–1269 CAS.
- P. Shokeen, M. Bala and V. Tandon, Int. J. Antimicrob. Agents, 2009, 33, 86–91 CrossRef CAS.
- H. N. Thatoi, S. K. Panda, S. K. Rath and S. K. Dutta, Asian J. Plant Sci., 2008, 7, 260–267 CrossRef.
- S. Rangaswami and S. S. Subramanian, J. Sci. Ind. Res., 1955, 14B, 131 CAS.
- S. Rangaswami and S. S. Subramanian, J. Sci. Ind. Res., 1956, 15C, 80–81 CAS.
- R. V. Rao and S. Rangaswami, Tetrahedron Lett., 1967, 8, 4563–4565 CrossRef.
- S. Jha and S. Sen, Curr. Sci., 1980, 49, 273–274 Search PubMed.
- S. Jha and S. Sen, Planta Med., 1983, 47, 43–45 CrossRef CAS.
- S. Rangaswami and S. S. Subramanian, J. Sci. Ind. Res., 1954, 13B, 150 CAS.
- S. Jha and S. Sen, Phytochemistry, 1981, 20, 524–526 CrossRef CAS.
- B. Kopp and M. Danner, Sci. Pharm., 1983, 51, 227–237 CAS.
- D. B. Deb and S. Dasgupta, Q. J. Crude Drug Res., 1976, 14, 49–60 CAS.
- S. Jha and S. Sen, Nucleus (Calcutta), 1982, 25, 190–192 CAS.
- S. Jha and S. Sen, Indian J. Exp. Biol., 1980, 18, 291–292 CAS.
- S. Jha and S. Sen, Phytochemistry, 1981, 20, 1442–1443 CrossRef CAS.
- V. K. Saxena and P. K. Chaturvedi, J. Inst. Chem. (India), 1992, 64, 35–37 CAS.
- N. Sultana, K. Akter, N. Nahar, M. S. H. Khan, M. Mosihuzzaman, H. Sohrab Md and K. Krohn, Nat. Prod. Res., 2010, 24, 1018–1026 CrossRef CAS.
- M. M. Patil and S. G. Torne, Curr. Sci., 1980, 49, 276–277 CAS.
- S. Jha and S. Sen, Acta Bot. Indica, 1981, 9, 258–264 Search PubMed.
- S. R. Shenoy, M. N. S. Kameshwari, S. Swaminathan and M. N. Gupta, Biotechnol. Prog., 2006, 22, 631–637 CrossRef CAS.
- A. V. Deepak, G. Thippeswamy, M. N. Shivakameshwari and P. Salimath Bharathi, Biochem. Biophys. Res. Commun., 2003, 311, 735–742 CrossRef CAS.
- A. V. Deepak and P. Salimath Bharathi, Biochimie, 2006, 88, 297–307 CrossRef CAS.
- H. Mikail, P. Magiatis and A. Skaltsounis, Planta Med., 2010, 76, 1242–1242 Search PubMed.
- M. M. Abid Ali Khan, S. N. H. Zaidi, S. A. Musanna and N. Singh, Asian J. Exp. Biol. Sci., 2012, 3, 476–480 CAS.
- R. P. Luyt, A. K. Jager and J. van Staden, S. Afr. J. Bot., 1999, 65, 443–445 CAS.
- R. P. Luyt, A. K. Jager and J. van Staden, S. Afr. J. Bot., 1999, 65, 443–445 CAS.
- L. Krenn, V. Stapf and B. Kopp, Sci. Pharm., 2000, 68, 421–427 CAS.
- L. Rakotobe, M. Berkal, H. Huet, C. Djediat, V. Jeannoda, L. Mambu, F. Crespeau and M. Edery, Food Chem. Toxicol., 2009, 47, 2289–2293 CrossRef CAS.
- R. Jaretzky and F. Scheermesser, DE Patent 1935-J52167, 1935.
- A. Katz, Pharm. Acta Helv., 1954, 29, 77–80 CAS.
- A. Katz, Experientia, 1956, 12, 285–286 CrossRef CAS.
- A. Katz, Helv. Chim. Acta, 1950, 33, 1420–1428 CrossRef CAS.
- A. Katz, Helv. Chim. Acta, 1953, 36, 1417–1423 CrossRef CAS.
- A. Katz, Helv. Chim. Acta, 1953, 36, 1344–1352 CrossRef CAS.
- A. Katz, Helv. Chim. Acta, 1954, 37, 833–836 CrossRef CAS.
- A. Katz, Helv. Chim. Acta, 1954, 37, 451–454 CrossRef CAS.
- A. Katz, Helv. Chim. Acta, 1955, 38, 1565–1572 CrossRef CAS.
- A. Katz, Helv. Chim. Acta, 1957, 40, 831–846 CrossRef CAS.
- A. Katz, Helv. Chim. Acta, 1958, 41, 1399–1404 CrossRef CAS.
- R. Tschesche and U. Dolberg, Chem. Ber., 1957, 90, 2378–2382 CrossRef CAS.
- R. Tschesche and U. Dolberg, Chem. Ber., 1958, 91, 2512–2523 CrossRef CAS.
- R. Tschesche, H. W. Sarau and K. Sellhorn, Chem. Ber., 1955, 88, 1612–1619 CrossRef CAS.
- R. Tschesche and K. Sellhorn, Chem. Ber., 1953, 86, 54–62 CrossRef CAS.
- R. Tschesche, S. Goenechea and G. Snatzke, Justus Liebigs Ann. Chem., 1964, 674, 176–184 CrossRef CAS.
- G. Schenck and F. Rattinger, US Patent 1951-21868, 1951.
- M. A. Angarskaya, P. I. Bezruk and D. A. Tkachenko, Farmakol. i Toksikol., Sb., (Kiev Zdorov'c), 1964, 1, 20–25 Search PubMed.
- F. Hildebrandt and E. Paas, Naunyn-Schmiedebergs Arch. Pharmakol. Exp. Pathol., 1953, 220, 482–489 CAS.
- F. Hildebrandt and W. Reinartz, Naunyn-Schmiedebergs Arch. Pharmakol. Exp. Pathol., 1953, 220, 290–294 CAS.
- A. Katz, CH Patent 324078, 1957.
- A. Katz, CH Patent 330660, 1958.
- A. Katz, Pharm. Acta Helv., 1954, 29, 369–378 CAS.
- K. K. Chen and F. G. Henderson, J. Pharmacol., 1951, 103, 420 CAS.
- K. K. Chen and F. G. Henderson, J. Med. Chem., 1961, 3, 111–127 CrossRef CAS.
- K. K. Chen, J. Med. Chem., 1970, 13, 1029–1034 CrossRef CAS.
- C. Koorbanally, D. A. Mulholland and N. R. Crouch, Biochem. Syst. Ecol., 2005, 33, 545–549 CrossRef CAS.
- E. J. Ndebia, E. Umapathy, B. N. Nkeh-Chungag and J. E. Iputo, J. Med. Plants Res., 2011, 5, 4658–4664 Search PubMed.
- S. O. Oyedemi, G. Bradley and A. J. Afolayan, J. Med. Plants Res., 2009, 3, 1040–1044 Search PubMed.
- T. Okanishi, A. Akahori, F. Yasuda, Y. Takeuchi and T. Iwao, Chem. Pharm. Bull., 1975, 23, 575–579 CrossRef CAS.
- M. Kuroda, Y. Mimaki, Y. Sashida, T. Yamori and T. Tsuruo, Tennen Yuki Kagobutsu Toronkai Koen Yoshishu, 1999, 41, 529–534 Search PubMed.
- Y. Mimaki, M. Kuroda, Y. Sashida, T. Yamori and T. Tsuruo, Helv. Chim. Acta, 2000, 83, 2698–2704 CrossRef CAS.
- M. Kuroda, Y. Mimaki, Y. Sashida, T. Yamori and T. Tsuruo, Tetrahedron Lett., 2000, 41, 251–255 CrossRef CAS.
- Y. Mimaki, M. Kuroda, A. Yokosuka and Y. Sashida, J. Nat. Prod., 2001, 64, 1069–1072 CrossRef CAS.
- M. Kuroda, Y. Mimaki, A. Yokosuka and Y. Sashida, Chem. Pharm. Bull., 2001, 49, 1042–1046 CrossRef CAS.
- P. Tang and B. Yu, Angew. Chem., Int. Ed., 2007, 46, 2527–2530 CrossRef CAS.
- P. Tang and B. Yu, Eur. J. Org. Chem., 2009, 259–269 CrossRef CAS.
- K. du Toit, PhD Thesis, University of Natal, South Africa, 2004.
- F. Klar, Planta Med., 2011, 77, 1274–1274 Search PubMed.
- S. Kubo, Y. Mimaki, M. Terao, Y. Sashida, T. Nikaido and T. Ohmoto, Phytochemistry, 1992, 31, 3969–3973 CrossRef CAS.
- S. Kubo, Y. Mimaki, Y. Sashida, T. Nikaido and T. Ohmoto, Chem. Pharm. Bull., 1992, 40, 2469–2472 CrossRef CAS.
- M. Kuroda, Y. Mimaki, Y. Sashida, T. Nikaido and T. Ohmoto, Tetrahedron Lett., 1993, 34, 6073–6076 CrossRef CAS.
- M. Kuroda, Y. Mimaki, Y. Sashida, T. Hirano, K. Oka and A. Dobashi, Chem. Pharm. Bull., 1995, 43, 1257–1259 CrossRef CAS.
- M. Kuroda, Y. Mimaki and Y. Sashida, Phytochemistry, 1999, 52, 435–443 CrossRef CAS.
- T. Hirano, K. Oka, Y. Mimaki, M. Kuroda and Y. Sashida, Life Sci., 1996, 58, 789–798 CrossRef CAS.
- M. Kuroda, Y. Mimaki, Y. Sashida, T. Hirano, K. Oka, A. Dobashi, H.-Y. Li and N. Harada, Tetrahedron, 1997, 53, 11549–11562 CrossRef CAS.
- Y. Mimaki and Y. Sashida, Adv. Exp. Med. Biol., 1996, 404, 101–110 CrossRef CAS.
- Y. Mimaki, M. Kuroda, A. Kameyama, Y. Sashida, T. Hirano, K. Oka and A. Dobashi, Bioorg. Med. Chem. Lett., 1996, 6, 2635–2638 CrossRef CAS.
- Y. Mimaki, M. Kuroda, Y. Sashida, T. Hirano, K. Oka, A. Dobashi, H. Koshino and J. Uzawa, Tetrahedron Lett., 1996, 37, 1245–1248 CrossRef CAS.
- Y. Sashida, Stud. Plant Sci., 1999, 6, 201–211 CAS.
- M. Kuroda, Y. Mimaki, H. Koshino and Y. Sashida, Heterocycles, 2002, 56, 531–536 CrossRef CAS.
- Y. Mimaki, M. Kuroda, A. Kameyama, Y. Sashida, T. Hirano, K. Oka, K. Koike and T. Nikaido, Biosci., Biotechnol., Biochem., 1996, 60, 1049–1050 CrossRef CAS.
- Y. Mimaki, M. Kuroda, A. Kameyama, Y. Sashida, T. Hirano, K. Oka, R. Maekawa, T. Wada, K. Sugita and J. A. Beutler, Bioorg. Med. Chem. Lett., 1997, 7, 633–636 CrossRef CAS.
- M. Kuroda, Y. Mimaki and Y. Sashida, Phytochemistry, 1999, 52, 445–452 CrossRef CAS.
- M. Kuroda, Y. Mimaki, A. Yokosuka, Y. Sashida and J. A. Beutler, J. Nat. Prod., 2001, 64, 88–91 CrossRef CAS.
- Y. Sashita, K. Oka, T. Hirano, Y. Mimaki, A. Kuroda, A. Fujii and Y. Myata, JP Patent 1996-109329, 1996.
- Y. Sashita, K. Oka, T. Hirano, Y. Mimaki, A. Kuroda, A. Fujii and Y. Myata, JP Patent 1996-107254, 1996.
- Y. Sashita, K. Oka, T. Hirano, Y. Mimaki, A. Kuroda, A. Fujii and Y. Myata, JP Patent 1996-107255, 1996.
- Y. Hui, Z. Yang and Q. Ge, CN Patent 2006-10147244, 2006.
- Y. Hui, Z. Yang, C. Yu, Q. Ge and L. Bao, CN Patent 2006-10116980, 2006.
- Y. Zhou, C. Garcia-Prieto, D. A. Carney, R.-H. Xu, H. Pelicano, Y. Kang, W. Yu, C. Lou, S. Kondo, J. Liu, D. M. Harris, Z. Estrov, M. J. Keating, Z. Jin and P. Huang, J. Natl. Cancer Inst., 2005, 97, 1781–1785 CrossRef CAS.
- W. Ying, Y.-L. Wu, X.-C. Feng, L.-H. Lian, Y.-Z. Jiang and J.-X. Nan, J. Ethnopharmacol., 2010, 132, 450–455 CrossRef CAS.
- Y. Wan, Y.-L. Wu, L.-H. Lian and J.-X. Nan, Chin. J. Nat. Med., 2012, 10, 0177–0184 CrossRef.
- R. A. Waud, J. Pharmacol. Exp. Ther., 1951, 101, 36–36 Search PubMed.
- R. A. Waud and J. Boyd, J. Pharmacol. Exp. Ther., 1954, 111, 147–151 CAS.
- R. A. Waud and J. Boyd, Rev. Can. Biol., 1952, 11, 88 CAS.
- A. Vogelsang, Can. Med. Assoc. J., 1955, 73, 295–296 CAS.
- H. Mrozik, R. A. Waud, O. Schindler and T. Reichstein, Helv. Chim. Acta, 1959, 42, 683–696 CrossRef CAS.
- R. B. Kelly, E. G. Daniels and L. B. Spaulding, J. Med. Chem., 1965, 8, 547–548 CrossRef CAS.
- F. E. Hart and G. R. Paterson, Can. J. Pharm. Sci., 1968, 3, 15–18 CAS.
- J. A. Smith and G. R. Paterson, J. Pharm. Pharmacol., 1967, 19, 221–225 CrossRef CAS.
- R. Ferth and B. Kopp, Die Pharmazie, 1992, 47, 626–629 CAS.
- Y. Buchvarov, M. Pargov and K. Akhtardzhiev, Farmatsiya (Sofia, Bulg.), 1976, 26, 31–34 CAS.
- O. Gasic, V. Simanek, V. Lukic, D. Walterova, S. Kevresan, V. Hanus and B. Pal, Zb. Matice Srp. Prir. Nauke, 1989, 76, 21–26 CAS.
- O. Azzioui, R. L. Braemer and M. Paris, Biochem. Syst. Ecol., 1989, 17, 449–450 CrossRef CAS.
- T. Sabudak and U. Oyman, Turk. J. Chem., 2002, 26, 453–455 CAS.
- W. Schliemann, J. Schmidt, M. Nimtz, V. Wray, T. Fester and D. Strack, Phytochemistry, 2006, 67, 1196–1205 CrossRef CAS.
- N. F. Komissarenko, Khim. Prir. Soedin., 1971, 7, 38–40 CAS.
- N. F. Komissarenko, Postep Dziedzinie Leku Rosl., Pr. Ref. Dosw. Wygloszone Symp., 1972, 67–71 CAS.
- I. G. Zoz and N. A. Chernykh, Farm. Zh. (Kiev), 1969, 24, 77–78 CAS.
- N. F. Komissarenko, Khim. Prir. Soedin., 1969, 5, 46–48 CAS.
- N. F. Komissarenko, Khim. Prir. Soedin., 1965, 156–160 CAS.
- N. F. Komissarenko, Khim. Prir. Soedin., 1969, 5, 46–48 CAS.
- N. F. Komissarenko, Khim. Prir. Soedin., 1972, 397–398 CAS.
- R. Ferth, A. Baumann, W. Robien and B. Kopp, Z. Naturforsch., B: J. Chem. Sci., 1992, 47, 1459–1467 CAS.
- R. Ferth, A. Baumann, W. Robien and B. Kopp, Z. Naturforsch., B: J. Chem. Sci., 1992, 47, 1444–1458 CAS.
- N. F. Komissarenko and P. E. Krivenchuk, Khim. Prir. Soedin., 1974, 10, 257 Search PubMed.
- U. Ghannamy, B. Kopp, W. Robien and W. Kubelka, Planta Med., 1987, 53, 172–178 CrossRef CAS.
- V. A. Bandyukova, Chem. Nat. Compd., 1979, 15, 639 CrossRef.
- M. Nicoletti, L. Tomassini and S. Foddai, Planta Med., 1992, 58, 472 CrossRef CAS.
- V. V. Barbakadze, E. P. Kemertelidze, H. E. Dekanosidze and A. I. Usov, Bioorg. Khim., 1993, 19, 912–916 CAS.
- Y. Buchvarov, E. Balabanova-Radonova, I. Radenkova and S. Vankov, Farmatsiya (Sofia, Bulg.), 1984, 34, 33–37 CAS.
- A. A. Makasci, R. Mammadov, O. Dusen and H. I. Isik, J. Med. Plants Res., 2010, 4, 1637–1642 Search PubMed.
- E. Nazifi, A. Movafeghi, H. Nazemiyeh, S. Asnaashari, S. Bamdad Moghadam and A. Delazar, Ulum-i Daroei, 2010, 16, 37–44 CAS.
- A. Delazar, E. Nazifi, A. Movafeghi, H. Nazemiyeh, S. Hemmati, L. Nahar and S. D. Sarker, Bol. Latinoam. Caribe Plant. Med. Aromat., 2010, 9, 87–92 CAS.
- A. Delazar, E. Nazifi, A. Movafeghi, L. Nahar, H. Nazemiyeh, S. B. Moghadam, S. Asnaashari and S. D. Sarker, Daru, J. Fac. Pharm., Tehran Univ. Med. Sci., 2009, 17, 33–36 CAS.
- M. A. Ebrahimzadeh, S. M. Nabavi, S. F. Nabavi and B. Eslami, Trop. J. Pharm. Res., 2010, 9, 141–148 Search PubMed.
- S. Kubo, Y. Mimaki, Y. Sashida, T. Nikaido and T. Ohmoto, Bull. Chem. Soc. Jpn., 1992, 65, 1120–1124 CrossRef CAS.
- M. Kuroda, Y. Mimaki, A. Yokosuka, F. Hasegawa and Y. Sashida, J. Nat. Prod., 2002, 65, 1417–1423 CrossRef CAS.
- M. Kuroda, Y. Mimaki, K. Ori, H. Sakagami and Y. Sashida, J. Nat. Prod., 2004, 67, 1690–1696 CrossRef CAS.
- M. Kuroda, K. Ori and Y. Mimaki, Steroids, 2006, 71, 199–205 CrossRef CAS.
- M. Kuroda, Y. Mimaki, A. Yokosuka, F. Hasegawa and Y. Sashida, J. Nat. Prod., 2002, 65, 1417–1423 CrossRef CAS.
- M. Kuroda, Y. Mimaki, K. Ori, H. Sakagami and Y. Sashida, J. Nat. Prod., 2004, 67, 1690–1696 CrossRef CAS.
- W. T. Mabusela and A. M. Stephen, Carbohydr. Res., 1990, 207, 332–335 CrossRef CAS.
- D. A. Mulholland, N. R. Crouch, T. Pohl and E. Ndlovu, Biochem. Syst. Ecol., 2004, 32, 499–502 CrossRef CAS.
- M. van Huyssteen, P. J Milner, E. E. Campbell and M. van de Venter, Afr. Networks Ethnomed., 2011, 8, 150–158 Search PubMed.
- Y. Tang, B. Yu, J. Hu, T. Wu and H. Hui, J. Nat. Prod., 2002, 65, 218–220 CrossRef CAS.
- T. Xu, Y. Xu, D. Liu and D. Xu, Yaoxue Xuebao, 2000, 35, 32–36 CAS.
- Y. P. Tang, B. Yu, J. Hu, T. Wu and H. Z. Hui, J. Nat. Prod., 2002, 65, 218–220 CrossRef CAS.
- Y. Tang, B. Yu, J. Hu, T. Wu and Y. Hui, J. Chin. Pharm. Sci., 2001, 10, 169–171 CAS.
- J. F. Bai, Z. Q. Liu, S. M. Wang, F. R. Song and S. Y. Liu, Chem. J. Chin. Univ., 2005, 26, 1817–1819 CAS.
- Z. Han, CN Patent 2001-18727, 2001.
- Z. Han, CN Patent 2006-10115717, 2006.
- X. Lu and Z. Han, CN Patent 2004-10074137, 2004.
- G. I. Mynkina, RU Patent 1999-108770, 1999.
- J. Sun, G. Lu and L. Xiu, CN Patent 2010-10177347, 2010.
- X. Jin, G. Cui, C. Qian, X. Du and Y. Wu, Shizhen Guoyi Guoyao, 2010, 21, 124–126 CAS.
- C. Qian, X. Jin, Y. Wu and X. Li, Shizhen Guoyi Guoyao, 2010, 21, 636–638 CAS.
- L. Shi, J. Li, W.-X. Liu, Y. Wang, Z.-Q. Liu and S.-Y. Liu, Chem. Res. Chin. Univ., 2003, 19, 286–289 CAS.
- R. Chen, S. Li, Z. Liu, H. Dong, Y. Li and S. Yang, Zhongguo Yaoxue Zazhi (Beijing, China), 2011, 46, 1630–1634 CAS.
- R. Chen, F. Meng, Z. Liu, R. Chen and M. Zhang, Carbohydr. Polym., 2010, 80, 845–851 CrossRef CAS.
- X. Liu and Y.-H. Zhang, Shizhen Guoyi Guoyao, 2012, 23, 1388–1390 CAS.
- O. Q. Munro, K. du Toit, S. E. Drewes, N. R. Crouch and D. A. Mulholland, New J. Chem., 2006, 30, 197–207 RSC.
- Y. Sashida, Stud. Plant Sci., 1999, 6, 201–211 CrossRef CAS.
- Y. Mimaki, Nat. Prod. Commun., 2006, 1, 247–253 CAS.
- E. R. Guaglianone and S. Arroyo-Leuenberger, Darwiniana, 2002, 40, 61–76 Search PubMed.
Footnote |
| † 55 families under APGII came to be known as ‘bracketed families’. These were optional segregates of families that could be circumscribed in a larger sense. |
| This journal is © The Royal Society of Chemistry 2013 |