Bisphenylsulfone-based molecular assemblies: polar columnar liquid crystals aligned in electric fields and fibrous aggregates in organic solvents†‡
Masafumi
Yoshio
*,
Reiku
Konishi
,
Takeshi
Sakamoto
and
Takashi
Kato
*
Department of Chemistry and Biotechnology, School of Engineering, The University of Tokyo, Hongo, Bunkyo-ku Tokyo 113-8656, Japan. E-mail: yoshio@chembio.t.u-tokyo.ac.jp; kato@chiral.t.u-tokyo.ac.jp
First published on 17th October 2012
Abstract
Bisphenylsulfone-based hexacatenal molecules self-assemble to form thermotropic columnar liquid-crystalline phases through the dipole–dipole interactions of sulfonyl groups and nanosegregation of aliphatic and aromatic moieties. The vertical alignment of the columns has been achieved by applying electric fields to the samples sandwiched between ITO substrates. In addition, organogels are obtained by the formation of the self-assembled fibrous aggregates of a sulfone derivative in organic solvents such as dodecylbenzene. The dipole–dipole interactions of the sulfonyl moieties play a key role in the formation of these one-dimensional molecular assemblies.
Introduction
Liquid crystals that form ordered and fluid states have been widely used for information display devices.1 Recently, new directions of liquid crystals have been explored in a variety of fields.2 In these applications, nanostructured liquid crystals such as columnar, smectic, and cubic phases have attracted attention. For example, functionalization of columnar and cubic liquid-crystalline nanostructures3 is emerging as a new route to produce advanced materials applicable for ion4 and electron5 transportation, chemical separation,6 and water purification.7 For such functionalization, it is important to design and control intermolecular noncovalent interactions,2c,8 nanosegregation behavior,2c,9 and macroscopic molecular orientation.2c,3c Moreover in the material design, it is of interest to use a variety of shapes of molecules.2c,10Physical gels have also attracted a great deal of attention.11 Fibrous aggregates formed by one-dimensional (1D) self-assembly of molecules in solvents lead to the formation of physical gels, which are functional soft materials.12
Moreover, self-assembled molecules that exhibit both liquid crystallinity and gelation properties of solvents have recently been developed.13 Our intention here is to employ a bisphenylsulfone moiety as a building block for the construction of self-assembled soft materials such as liquid crystals and gelators. The bisphenylsulfone moiety, that is thermally stable, has been used as an aromatic monomeric unit for engineering plastics such as liquid-crystalline polyesters14 and poly(ether sulfone)s.15 But in the case of liquid crystal polymers, the moiety is categorized as a nonmesogenic unit because of the lack of linearity of the unit as it has a bent structure with two aromatic rings. We considered that the nonlinearity was not a problem for liquid crystallinity if the moiety is used for the formation of 1D molecular assemblies.
In particular, we have expected that the dipolar interactions between the polar sulfone moieties can stabilize their 1D assemblies significantly. Sulfone derivatives have large dipole moments (1.6–6.2 D).16 They have versatile applications including self-assembled monolayers,16 electrolytes for lithium-ion batteries,17 electron acceptors for organic semiconductors,18 and water purification membranes19 due to their polarity.
Polar 1D molecular assemblies such as columnar liquid crystals20 and fibrous molecular solid aggregates21 have gained particular interest, because their functionalities may be tuned by electric fields. The polar moieties such as amide, urea, carbonate, cyano, and vanadyl groups have been introduced into discotic, wedge-shaped, and polycatenal molecules to produce electric-field responsive columnar liquid crystals.20 In our previous work, carbonate-containing liquid crystals were developed as ion transporting materials that align in electric fields.20b
Herein, we present the first example of bisphenylsulfone-based molecular assemblies. Thermotropic columnar liquid crystals and fibrous aggregates are formed by 1D self-assembly of sulfonyl moieties through the dipole–dipole interactions. The electric field alignment of the sulfone-based columnar liquid crystals has been successfully achieved.
Results and discussions
The thermal properties of 1a,b and 2a,b (Fig. 1) were examined by DSC (see ESI‡) and polarizing optical microscopy. Table 1 summarizes their thermal properties. Ether compound 1a exhibits an enantiotropic columnar liquid-crystalline phase at room temperature both on heating and cooling processes. No crystallization occurs when compound 1a is cooled to −50 °C. A fan-shaped texture characteristic of the hexagonal columnar phase is observed under crossed polarizers as shown in Fig. 2, although some part shows a dark image because of the vertical alignment of the columns. The spontaneous vertical orientation of the columns on the glass substrate may be induced by the hydrogen bonding interaction between the sulfonyl group of 1a and the silanol group of the glass surface. Compounds 1b and 2b show monotropic columnar liquid-crystalline phases only on cooling, whereas no mesomorphic properties are observed for ester compound 2a. | ||
| Fig. 1 Molecular structures of bisphenylsulfone ethers 1a,b and esters 2a,b. | ||
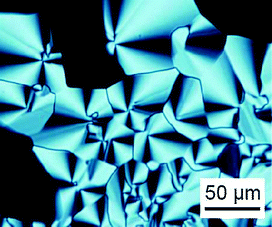 | ||
| Fig. 2 Polarized optical microscopic image of 1a in the columnar phase at 25 °C. | ||
Powder X-ray diffraction (XRD) measurements were performed for the mesomorphic compounds 1a and 1b (see ESI‡). These compounds in the liquid-crystalline states show two peaks with a reciprocal d-spacing ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in the small-angle region, which corresponds to the (10) and (20) reflections, respectively. No peak assigned to the (11) reflection of the columns is observed. In the wide-angle region around 20°, a halo due to the scattering from short correlations between the molten aromatic cores and aliphatic side chains is observed. For example, compound 1a shows two peaks at 32 and 16 Å in the small-angle region.
2 in the small-angle region, which corresponds to the (10) and (20) reflections, respectively. No peak assigned to the (11) reflection of the columns is observed. In the wide-angle region around 20°, a halo due to the scattering from short correlations between the molten aromatic cores and aliphatic side chains is observed. For example, compound 1a shows two peaks at 32 and 16 Å in the small-angle region.
2D transmission XRD images were taken for open liquid-crystalline samples of 1a,b on the polyimide film (Kapton) substrate to determine the liquid-crystalline structures. The 2D XRD image of 1b (Fig. 3a) shows diffraction spots with a six fold symmetry from the (100) plane, which is the signature of a hexagonal geometry for the homeotropically aligned columnar sample. A hexagonal arrangement for 1a in the liquid-crystalline state is also suggested from the 2D XRD image (Fig 3b), although the angular spread of the diffraction pattern was seen due to the randomized columnar orientation.
 | ||
| Fig. 3 2D transmission XRD patterns of (a) 1b (n = 12) and (b) 1a (n = 10) at 29 °C. | ||
On the basis of these results, the intercolumnar distance of 1a is about 37 Å assuming a hexagonal columnar structure is formed. The fully extended molecular length of 1a is estimated to be 45 Å as shown in Fig. 4. Compound 1a adopts a distorted roof-shaped conformation, resulting in an intrinsic dipole moment perpendicular to the long molecular axis. The number of molecules inside a stratum of each column is estimated to be approximately 2 molecules on average assuming the density is 1.0 g cm−3 (see ESI‡). Fig. 5 shows the proposed molecular packing of 1a,b in the columnar liquid-crystalline structures. One explanation of possible assembled structures is that the rigid bisphenylsulfone moieties form 1D columns surrounded by the molten alkoxy chains, as suggested for hexacatenar mesogenic compounds.13d,22 The intermolecular dipole–dipole interactions16 between the sulfonyl moieties and π–π interactions between the phenyl rings as well as nanosegregation of the aromatic part from the surrounding alkoxy moieties play a key role in the columnar organization.
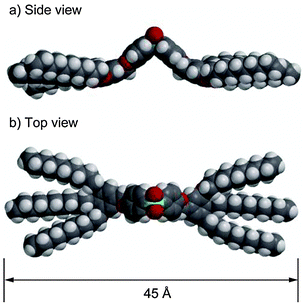 | ||
| Fig. 4 CPK model of 1a (n = 10) calculated using Wavefunction Spartan'04. | ||
 | ||
| Fig. 5 Proposed self-assembled structure of 1a,b (R = –CnH2n+1) in the columnar liquid-crystalline phase. The arrows indicate the dipole moment. The bisphenyl sulfone moieties stack linearly through the dipole–dipole interactions and nanosegregation between rigid aromatic (red) and flexible alkyl chain (blue) parts. | ||
Uniaxial orientation of the columnar phases has been achieved by applying alternating current (AC) electric fields (Fig. 6). The dark image in the top area in Fig. 6 shows the homeotropic alignment of 1a filled between two patterned indium tin oxide (ITO) electrodes with an applied AC electric field. The thickness of the sample is 9 μm. The vertical alignment of the columns is obtained when the AC electric fields of 8 V μm−1 in the frequency range of 10 Hz–1 kHz are applied to 1a in the liquid-crystalline phase at 25 °C. The alignment change from polydomains to monodomain is seen within 15 min under the electric field (8 V μm−1, 10 Hz). The uniaxial alignment of the columns is maintained when the applied electric field is turned off. Application of direct current electric fields to the liquid-crystalline samples also results in the vertical alignment of the column. The ability to orient the columns under the electric fields may be due to the polarization of the sulfonyl groups along the columnar axis. However, it is not clear if these sulfone-based liquid crystals exhibit ferroelectric properties or form the polarization compensated structures. In order to confirm the polar order, second-harmonic generation measurements are needed.23
 | ||
| Fig. 6 Polarized optical microscopic image of 1a in the columnar state at 25 °C. The sample is filled in a sandwiched glass cell (thickness, 9 μm) with a patterned ITO electrode. An AC electric field (8 V μm−1, 10 Hz) is applied to the sample between the ITO from a vertical direction. Dashed lines denote the border between the electric field applied area and non-applied area. | ||
The gelation abilities of compounds 1a,b and 2a,b were examined in various organic solvents at a concentration of 5 wt% of sulfone derivatives. Only compound 1b exhibits gelation abilities in dodecylbenzene, dodecane, and 1-octanol at room temperature, whereas no gelation is induced for 1a and 2a,b in these solvents. A translucent gel of 1b (Fig. 7a) is formed in dodecylbenzene when the heated solution is cooled quickly under 20 °C. The sol–gel transition temperature is 24–25 °C (see ESI‡). The morphology of the gel was examined by scanning electron microscopy (SEM). The SEM image of 1b (Fig. 7b) shows the formation of three-dimensional networks composed of entangled fibrous aggregates. The approximate diameter of the fibers is 200–800 nm. The length is more than 70 μm. The X-ray diffraction pattern of the dodecylbenzene gel of 1b at room temperature gives a weak peak of 34 Å in the small-angle region (see ESI‡), while compound 1b in the columnar liquid-crystalline state shows diffraction peaks of 36 and 17 Å. On the basis of these results, it is assumed that compound 1b in the solvents forms the fibrous aggregates with the ordered structure similar to the columnar phase by the dipole–dipole interactions of sulfonyl moieties.
 | ||
| Fig. 7 (a) Photograph of dodecylbenzene gel of 1b (5 wt%) at room temperature and (b) scanning electron micrograph of the xerogel of 1b obtained after removal of the solvent. | ||
Conclusions
We have developed a new type of polar columnar liquid crystals by employing aromatic sulfone moieties as mesogens. The vertical alignment of the columns on the surface of ITO substrates has been achieved by applying electric fields. These compounds also form fibrous solid aggregates in solution. The dipole–dipole interactions of sulfonyl groups may be a useful tool for the construction of novel supramolecular architectures, leading to functional materials.Experimental
Synthesis of bisphenylsulfone ether 1a
A mixture of bis(4-hydroxyphenyl)sulfone (0.18 g, 0.72 mmol), 3,4,5-tris(decyloxy)benzyl chloride (0.96 g, 1.61 mmol), and K2CO3 (0.50 g, 3.62 mmol) in N,N-dimethylformamide (15 mL) was stirred at 80 °C for 10 h in an argon atmosphere. The reaction mixture was extracted with ethyl acetate and washed by a saturated NH4Cl aqueous solution. The organic phase was dried with anhydrous MgSO4, filtered, and concentrated. The residue was purified by silica gel column chromatography (eluent: hexane–ethyl acetate = 10/1) to give 0.83 g (84%) of 1a as a white solid. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.86 (d, J = 8.8 Hz, 4H), 7.03 (d, J = 8.8 Hz, 4H), 6.57 (s, 4H), 4.96 (s, 4H), 3.97 (m, 12H), 1.82–1.70 (m, 12H), 1.47–1.27 (m, 84H), 0.88 (t, J = 6.8 Hz, 18H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 162.24, 153.37, 134.07, 130.58, 129.50, 115.19, 106.12, 73.43, 70.75, 69.13, 31.92, 31.89, 30.31, 29.72, 29.62, 29.57, 29.38, 29.36, 26.09, 26.07, 22.66, 14.10. Elemental analysis calcd (%) for C86H142O10S: C 75.56, H 10.47; found: C 75.86, H 10.82.Electric field alignment of columnar liquid crystal 1a
A glass cell with patterned ITO electrodes was obtained from EHC Co. Ltd. Compound 1a was filled between two pieces of the ITO electrodes. The thickness of the sample is 9 μm. Electric fields were applied to the sample in the columnar liquid-crystalline state at a temperature close to the isotropic-columnar transition. They were applied by an nF WF1943A function generator and a bipolar amplifier HSA4011.Sample preparation for the SEM observation
The dodecylbenzene gel of 1b (5 wt%) coated on a glass plate was immersed in methanol at room temperature to remove the dodecylbenzene solvent from the network of the fibers of 1b. The resultant xerogel was dried in air at room temperature and shaded with platinum. SEM measurements of the xerogel were performed on a Keyence VE-9800.Acknowledgements
This work was partially supported by a Grant-in-Aid for Scientific Research (A) (No. 23245030) from the Japan Society for the Promotion of Science and Grant-in-Aid for Scientific Research (No. 22107003) on Innovative Areas: “Fusion Materials: Creative Development of Materials and Exploration of Their Function through Molecular Control” (Area no. 2206) from the Ministry of Education, Culture, Sports, Science and Technology. M.Y. is thankful for financial support from The Murata Science Foundation and Kurita Water and Environment Foundation.Notes and references
- (a) Handbook of Liquid Crystals, ed. D. Demus, J. W. Goodby, G. W. Gray, H.-W. Spiess and V. Vill, Wiley-VCH, Weinheim, 1998 Search PubMed; (b) J. W. Goodby, Liq. Cryst., 2011, 38, 1363 Search PubMed.
- (a) T. Kato, Science, 2002, 295, 2414 CrossRef CAS; (b) T. Ikeda, J. Mamiya and Y. Yu, Angew. Chem., Int. Ed., 2007, 46, 506 CrossRef CAS; (c) T. Kato, N. Mizoshita and K. Kishimoto, Angew. Chem., Int. Ed., 2006, 45, 38 CrossRef CAS; (d) B. R. Wiesenauer and D. L. Gin, Polym. J., 2012, 44, 461 Search PubMed; (e) A. P. H. J. Schenning, Y. C. Gonzalez-Lemus, I. K. Shishmanova and D. J. Broer, Liq. Cryst., 2011, 38, 1627 Search PubMed; (f) Y. Sagara and T. Kato, Nat. Chem., 2009, 1, 605 CrossRef CAS.
- (a) C. D. Simpson, J. Wu, M. D. Watson and K. Müllen, J. Mater. Chem., 2004, 14, 494 RSC; (b) R. Deschenaux, B. Donnio and D. Guillon, New J. Chem., 2007, 31, 1064 RSC; (c) T. Kato, T. Yasuda, Y. Kamikawa and M. Yoshio, Chem. Commun., 2009, 729 RSC; (d) B. M. Rosen, C. J. Wilson, D. A. Wilson, M. Peterca, M. R. Iman and V. Percec, Chem. Rev., 2009, 109, 6275 CrossRef CAS.
- (a) T. Kato, Angew. Chem., Int. Ed., 2010, 49, 7847 CrossRef CAS; (b) M. Yoshio, T. Mukai, H. Ohno and T. Kato, J. Am. Chem. Soc., 2004, 126, 994 CrossRef CAS; (c) M. Yoshio, T. Kagata, K. Hoshino, T. Mukai, H. Ohno and T. Kato, J. Am. Chem. Soc., 2006, 128, 5570 CrossRef CAS; (d) T. Ichikawa, M. Yoshio, A. Hamasaki, J. Kagimoto, H. Ohno and T. Kato, J. Am. Chem. Soc., 2011, 133, 2163 CrossRef CAS; (e) T. Ichikawa, M. Yoshio, A. Hamasaki, S. Taguchi, F. Liu, X. Zeng, G. Ungar, H. Ohno and T. Kato, J. Am. Chem. Soc., 2012, 134, 2634 Search PubMed; (f) V. Percec, G. Johansson, J. Heck, G. Ungar and S. V. Batty, J. Chem. Soc., Perkin Trans. 1, 1993, 1411 RSC; (g) B.-K. Cho, A. Jain, S. M. Gruner and U. Wiesner, Science, 2004, 305, 1598 CrossRef CAS.
- (a) R. J. Bushby and O. R. Lozman, Curr. Opin. Solid State Mater. Sci., 2002, 6, 569 CrossRef CAS; (b) A. Tracz, J. K. Jeszka, M. D. Watson, W. Pisula, K. Müllen and T. Pakula, J. Am. Chem. Soc., 2003, 125, 1682 CrossRef CAS; (c) T. Yasuda, T. Shimizu, F. Liu, G. Ungar and T. Kato, J. Am. Chem. Soc., 2011, 133, 13437 CrossRef CAS.
- (a) D. L. Gin and R. D. Noble, Science, 2011, 332, 674 CrossRef; (b) J. E. Bara, A. K. Kaminski, R. D. Noble and D. L. Gin, J. Membr. Sci., 2007, 288, 13 CrossRef CAS; (c) X. Lu, V. Nguyen, M. Zhou, X. Zeng, J. Jin, B. J. Elliott and D. L. Gin, Adv. Mater., 2006, 18, 3294 CrossRef CAS.
- (a) M. Zhou, T. J. Kidd, R. D. Noble and D. L. Gin, Adv. Mater., 2005, 17, 1850 CrossRef CAS; (b) M. Zhou, P. R. Nemade, X. Lu, X. Zeng, E. S. Hatakeyama, R. D. Noble and D. L. Gin, J. Am. Chem. Soc., 2007, 129, 9574 CrossRef CAS; (c) M. Henmi, K. Nakatsuji, T. Ichikawa, H. Tomioka, T. Sakamoto, M. Yoshio and T. Kato, Adv. Mater., 2012, 24, 2238 Search PubMed.
- (a) V. Percec, M. R. Imam, T. K. Bera, V. S. K. Balagurusamy, M. Peterca and P. A. Heiney, Angew. Chem., Int. Ed., 2005, 44, 4739 CrossRef CAS; (b) H. Yu, A. Shishido, J. Li, K. Kamata, T. Iyoda and T. Ikdeda, J. Mater. Chem., 2007, 17, 3485 RSC; (c) F. J. M. Hoeben, P. Jonkheijm, E. W. Meijer and A. P. H. J. Schenning, Chem. Rev., 2005, 105, 1491 CrossRef CAS; (d) T. Kato, N. Mizoshita and K. Kanie, Macromol. Rapid Commun., 2001, 22, 797 CrossRef CAS.
- (a) C. Tschierske, J. Mater. Chem., 1998, 8, 1485 RSC; (b) T. Kato and N. Mizoshita, Curr. Opin. Solid State Mater. Sci., 2002, 6, 579 CrossRef CAS; (c) I. M. Saez and J. W. Goodby, J. Mater. Chem., 2005, 15, 26 RSC; (d) M. Lee, B.-K. Cho and W.-C. Zin, Chem. Rev., 2001, 101, 3869 CrossRef CAS; (e) J. Barberá, B. Donnio, L. Gehringer, D. Guillon, M. Marcos, A. Omenat and J. L. Serrano, J. Mater. Chem., 2005, 15, 4093 RSC; (f) S. I. Stupp, V. LeBonheur, K. Walker, L. S. Li, K. E. Huggins, M. Keser and A. Amstutz, Science, 1997, 276, 384 CrossRef.
- (a) M. Sawamura, K. Kawai, Y. Matsuo, K. Kanie, T. Kato and E. Nakamura, Nature, 2002, 419, 702 CrossRef CAS; (b) A. Serrette, P. J. Carroll and T. M. Swager, J. Am. Chem. Soc., 1992, 114, 1887 CrossRef CAS; (c) B. Xu and T. M. Swager, J. Am. Chem. Soc., 1995, 117, 5011 CrossRef CAS; (d) G. Pelzl, S. Diele and W. Weissflog, Adv. Mater., 1999, 11, 707 CrossRef CAS.
- (a) Low Molecular Mass Gelators Design, Self-Assembly, Function, Topics in Current Chemistry, ed. F. Fages, Springer, Berlin, 2005, vol. 256 Search PubMed; (b) A. Ajayaghosh and V. K. Praveen, Acc. Chem. Res., 2007, 40, 644 CrossRef CAS; (c) J. H. van Esch and B. L. Feringa, Angew. Chem., Int. Ed., 2000, 39, 2263 CrossRef; (d) T. Kato, Y. Hirai, S. Nakaso and M. Moriyama, Chem. Soc. Rev., 2007, 36, 1857 RSC.
- (a) T. Kitamura, S. Nakaso, N. Mizoshita, Y. Tochigi, T. Shimomura, M. Moriyama, K. Ito and T. Kato, J. Am. Chem. Soc., 2005, 127, 14769 CrossRef CAS; (b) Y. Hirai, S. S. Babu, V. K. Praveen, T. Yasuda, A. Ajayaghosh and T. Kato, Adv. Mater., 2009, 21, 4029 CrossRef CAS; (c) S. Kume, K. Kuroiwa and N. Kimizuka, Chem. Commun., 2006, 2442 RSC; (d) H. Eimura, M. Yoshio, Y. Shoji, K. Hanabusa and T. Kato, Polym. J., 2012, 44, 594 Search PubMed; (e) T. Seki, T. Karatsu, A. Kitamura and S. Yagai, Polym. J., 2012, 44, 600 Search PubMed; (f) T. Sawada, M. Tsuchiya, T. Takahashi, H. Tsutsumi and H. Mihara, Polym. J., 2012, 44, 651 Search PubMed.
- (a) M. Hashimoto, S. Ujiie and A. Mori, Adv. Mater., 2003, 15, 797 CrossRef CAS; (b) K. Isoda, T. Yasuda and T. Kato, J. Mater. Chem., 2008, 18, 4522 RSC; (c) J. Mamiya, K. Kanie, T. Hiyama, T. Ikeda and T. Kato, Chem. Commun., 2002, 1870 RSC; (d) S. Yagai, S. Kubota, T. Iwashima, K. Kishikawa, T. Nakanishi, T. Karatsu and A. Kitamura, Chem.–Eur. J., 2008, 14, 5246 CrossRef CAS; (e) F. Camerel, L. Bonardi, G. Ulrich, L. Charbonnière, B. Donnio, C. Bourgogne, D. Guillon, P. Retailleau and R. Ziessel, Chem. Mater., 2006, 18, 5009 CrossRef CAS.
- (a) T. D. Shaffer and V. Percec, Makromol. Chem., 1986, 187, 1431 Search PubMed; (b) Y.-M. Chang, C.-S. Hsu and M.-C. Liu, Mater. Chem. Phys., 1996, 43, 250 Search PubMed.
- S. Wang and J. E. McGrath, in Synthetic Methods in Step-Growth Polymers, ed. M. E. Rogers and T. E. Long, Wiley-Interscience, New York, 2003, pp. 327–374 Search PubMed.
- (a) S. D. Evans, E. Urankar, A. Ulman and N. Ferris, J. Am. Chem. Soc., 1991, 113, 4121 CrossRef; (b) N. Tillman, A. Ulman and J. F. Elman, Langmuir, 1990, 6, 1512 Search PubMed; (c) S. D. Evans, K. E. Goppert-Berarducci, E. Urankar, L. J. Gerenser and A. Ulman, Langmuir, 1991, 7, 2700 Search PubMed.
- K. Xu and C. A. Angell, J. Electrochem. Soc., 2002, 149, A920 CrossRef CAS.
- (a) E. L. Dane, S. B. King and T. M. Swager, J. Am. Chem. Soc., 2010, 132, 7758 CrossRef CAS; (b) H. Detert and E. Sugiono, Synth. Met., 2001, 122, 15 Search PubMed.
- W. Xie, G. M. Geise, B. D. Freeman, C. H. Lee and J. E. McGrath, Polymer, 2012, 53, 1581 Search PubMed.
- (a) M. L. Bushey, T.-Q. Nguyen and C. Nuckolls, J. Am. Chem. Soc., 2003, 125, 8264 CrossRef; (b) H. Shimura, M. Yoshio, A. Hamasaki, T. Mukai, H. Ohno and T. Kato, Adv. Mater., 2009, 21, 1591 CrossRef CAS; (c) K. Sato, Y. Itoh and T. Aida, J. Am. Chem. Soc., 2011, 133, 13767 Search PubMed; (d) A. R. A. Palmans, J. A. J. M. Vekemans, R. A. Hikmet, H. Fischer and E. W. Meijer, Adv. Mater., 1998, 10, 873 CrossRef CAS; (e) H. Takezoe, K. Kishikawa and E. Gorecka, J. Mater. Chem., 2006, 16, 2412 RSC; (f) D. Kilian, D. Knawby, M. A. Athanassopoulou, S. T. Trzaska, T. M. Swager, S. Wróbel and W. Hasse, Liq. Cryst., 2000, 27, 509 CrossRef CAS; (g) E. Gorecka, D. Pociecha, J. Mieczkowski, J. Matraszek, D. Guillon and B. Donnio, J. Am. Chem. Soc., 2004, 126, 15946 CrossRef CAS; (h) K. Kishikawa, S. Nakahara, Y. Nishikawa, S. Kohmoto and M. Yamamoto, J. Am. Chem. Soc., 2005, 127, 2565 CrossRef CAS; (i) D. Miyajima, F. Araoka, H. Takezoe, J. Kim, K. Kato, M. Takata and T. Aida, Science, 2012, 336, 209 Search PubMed.
- (a) B. W. Messmore, J. F. Hulvat, E. D. Sone and S. I. Stupp, J. Am. Chem. Soc., 2004, 126, 14452 CrossRef CAS; (b) M. Yoshio, Y. Shoji, Y. Tochigi, Y. Nishikawa and T. Kato, J. Am. Chem. Soc., 2009, 131, 6763 CrossRef CAS; (c) Y. Shoji, M. Yoshio, T. Yasuda, M. Funahashi and T. Kato, J. Mater. Chem., 2010, 20, 173 RSC.
- (a) T. Yasuda, H. Ooi, J. Morita, Y. Akama, K. Minoura, M. Funahashi, T. Shimomura and T. Kato, Adv. Funct. Mater., 2009, 19, 411 CrossRef CAS; (b) D. Fazio, C. Mongin, B. Donnio, Y. Galerne, D. Guillon and D. W. Bruce, J. Mater. Chem., 2001, 11, 2852 RSC.
- (a) Y. Okada, S. Matsumoto, Y. Takanishi, K. Ishikawa, S. Nakahara, K. Kishikawa and H. Takezoe, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2005, 72, 020701 CrossRef; (b) E. Gorecka, D. Pociecha, J. Matraszek, J. Mieczkowski, Y. Shimbo, Y. Takanishi and T. Hakezoe, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2006, 73, 031704.
Footnotes |
| † This article is included in the All Aboard 2013 themed issue. |
| ‡ Electronic supplementary information (ESI) available. See DOI: 10.1039/c2nj40681k |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2013 |
