Discovery of coumarin derivatives as fluorescence acceptors for intrinsic fluorescence resonance energy transfer of proteins†
Ju Hwan
Kim
ab,
Jitapa
Sumranjit
ac,
Hyo Jin
Kang
ad and
Sang J.
Chung
*ad
aBNRC, KRIBB, Yuseong, Daejeon 305-806, Korea
bNanoBio Major, UST, Yuseong, Daejeon 305-806, Korea
cNational Nanotechnology Center (NANOTEC), 111 Phahonyothin Rd., Klongluang, Pathumthani 12120, Thailand
dDepartment of Chemistry, Dongguk University, Seoul, Korea. E-mail: sjchung@dongguk.edu; Fax: +82 2 2260 8907; Tel: +82 2 2290 1523
First published on 14th October 2013
Abstract
Coumarin analogues were synthezised and evaluated as acceptors for the intrinsic fluorescence resonance energy transfer (iFRET) of tryptophan residues in target proteins. The fluorescence properties such as quantum yields, iFRET efficiencies, and Förster distances of the prepared coumarin analogs were determined in a model system, by their conjugation to biotin, utilizing streptavidin (SAV) as the iFRET donor. The coumarin derivatives reported here represent the most efficient iFRET acceptors for tryptophan, known to date.
Exploitation of fluorescent molecules, e.g. in the development of sensors and as labels for bioimaging, is mainly attributed to their high signal to noise ratio, resulting in low detection limits.1–3 Imaging of a desired biological target often requires the site specific incorporation of a fluorescent label. Unfortunately, the labelling process frequently requires engineered cell lines, resulting in prolonged (and expensive) preparation thereof. These drawbacks necessitate the development of label-free detection methods to enable the use of non-engineered cell lines.4 Label-free fluorescence methods which allow real-time imaging of complex processes offer an attractive feature and potentially form the basis for high throughput drug discovery approaches.
Fluorescence anisotropy (FA) is a well-known label-free fluorescence spectroscopic method to study carbohydrate–protein, antigen–antibody, or protein–protein interactions.5 However, the versatility of the FA method is hampered by its high sensitivity to the environment, e.g. pH and viscosity, which is problematic in the real-time sample analysis.6 The Förster (or fluorescence) resonance energy transfer (FRET),7 a well-known fluorescent method, enables real-time imaging of complex processes, e.g. protein–protein and protein–ligand interactions, as well as protein conformational changes and dynamics.8 FRET is a distance-dependent physical process by which energy is transferred non-radiatively from an excited molecular fluorophore (the donor) to another fluorophore (the acceptor) when the two fluorophores are in close proximity. Application of FRET enables real-time ratiometric detection, which can be utilized to obtain temporal information and monitor localization of the bio-analyte targets. Ratiometric detection involves the measurement of fluorescence at two distinct wavelengths, followed by the calculation of their intensity ratio. This process allows the correction of environmental effects from the sample matrix, resulting in more accurate data acquisition.9 However, application of the FRET technique in most biological systems still requires the site specific labelling of the targets.
To compensate the disadvantages associated with the aforementioned methods several research groups reported the development of the iFRET method.10 The iFRET principle enables the detection of a target protein without the labelling thereof. An iFRET probe typically comprises two structural parts, a target-specific ligand and an iFRET acceptor fluorescence motif.
Aromatic amino acids such as tryptophan, tyrosine and phenylalanine can act as fluorescence sources (iFRET donor) in proteins.11 Albeit, the strong UV absorption of proteins at 280 nm and the emission at 340–360 nm originate mostly from the indole ring of the tryptophan residue, since the quantum efficiencies of tyrosine and phenylalanine are low.12 Tryptophan is therefore the most reliable amino acid residue in proteins, which can serve as the iFRET donor. N-Biotinyl-N′-(1-naphthyl)ethylenediamine (BNEDA, IV, λabs = 340, λem = 430 nm) is reported as an iFRET probe for streptavidin detection.10a However, the reported probe was only used to detect purified target protein. The ex vivo or in cell lysate application of BNEDA may be hampered by its light emission wavelength (∼430 nm), which is close to the auto-fluorescence of intact or lysed cells (∼430 nm).13 Additionally, the short Stokes shift of BNEDA (90 nm) can induce self-absorption, which in turn affects the accuracy of the measurement. Recent studies employed the iFRET concept as a tool for monitoring protein–RNA interactions.10d Here we report the development of efficient iFRET acceptor fluorescence molecules, aimed for application in both molecular- and cell-based assays. The iFRET mechanism for our system is illustrated in Fig. 1.
Our strategy entails the conjugation of a coumarin-based fluorophore to biotin, and application of this construct in iFRET experiments with streptavidin (SAV). The coumarin-based structures were selected as the fluorophores, because their absorbance and emission properties lay close to the desired values, 350 nm and 460 nm, respectively.14 Streptavidin and its ligand, biotin, were selected as a model system due to the presence of six tryptophan amino acid residues at the ligand binding site (residues 21, 75, 79, 92, 108, and 120).15
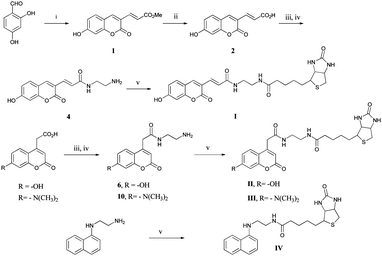
Coumarin based dyes are well-explored and numerous reports deal with their synthesis and applications.16,17 It is known that substituents at the 3- and 7-position of coumarin strongly affect the fluorescent property, by the alteration of the two lowest excited states of the fluorophore.18 Three coumarin derivatives were designed which included an electron donating group at the 7-position and either the 3- or 4-position was used for biotin conjugation. The synthesis of the coumarin derivatives was achieved either via Pechmann- or Knoevenagel condensation. The fluorescent scaffolds were subsequently conjugated to biotin via EDC mediated amide bond formation, to yield fluorescent probes I, II, III and IV (Scheme 1).
The obtained fluorescent probes I–IV are soluble in most organic solvents, however, they are insoluble in H2O. As a consequence, a mixed solvent system of DMSO and H2O was applied which is compatible with most biological substances. For the measurement of absorption and emission spectra, the fluorescent probes were diluted with PBS buffer (pH 7.4) from a DMSO stock solution. Probes I and III were diluted to a concentration of 150 nM and probe II and IV to a concentration of 50 nM. Purified streptavidin (SAV) was employed as the iFRET donor, and bovine serum albumin (BSA) served as a negative control. Although BSA contains several tryptophan residues it is expected that the absence of a biotin binding site would not allow the fluorescent probes to reach a close proximity, which is required for iFRET. The fluorescence emission spectrum of each probe was measured upon irradiation with UV light at a wavelength range from 280 to 380 nm. Quantum efficiencies were calculated using umbelliferone as a reference.19 Detailed photophysical data of all probes and tryptophan are summarized in Table 1. The emission spectrum of SAV and absorption spectra of probes I–IV are depicted in Fig. 2. From the obtained data, it became apparent that all the prepared iFRET probes surpass the known probe IV, in terms of the emission wavelength. The higher emission wavelength potentially allows clear distinction between background emission of most biological samples and the iFRET probe, a crucial factor which enables cellular imaging. When irradiated at 280 nm (protein's absorption maximum), SAV emitted strongly at 350 nm. At the same wavelength (280 nm), the probes displayed only low fluorescence, probes I–III around 460 nm and probe IV around 430 nm. Upon irradiation with near UV light, corresponding to the probe's absorption maxima, around 380 nm to 400 nm for probes I and III, 360 nm for probe II, and 340 for probe IV, strong emissions were detected at wavelengths ranging from 430 nm to 480 nm. In comparison to the measured probes, iFRET probe II possesses the longest Stokes shift (120 nm) and a comparable quantum efficiency (ΦF = 0.47) to known probe IV.
After careful examination of the photophysical data, it was decided to continue further studies with probes I–IV. iFRET probes I and III, SAV and BSA solutions were prepared at concentrations of 150 nM, in the same fashion as described for the measurement of the photophysical data. In the case of probe II and IV, a concentration of 50 nM was used for both probes and proteins. Next, for each probe, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) mixture with the SAV solution was prepared and allowed to reach their equilibrium for three minutes. Subsequently, the emission spectra were recorded with excitation at 280 nm, the protein's absorption maxima. First, the mixtures of the probes and BSA were analysed to reveal the absence of FRET characteristic, i.e., excitation at 280 nm resulted only in BSA emission at 340 nm (in the presence of probes I, II, III, or IV). The emission patterns around 460 nm resembled that of the probe only in the presence of each probe upon irradiation at 280 nm (ESI,† S3). On the other hand, the mixture of the probes and SAV showed an obvious iFRET phenomenon, even at nanomolar concentrations. When excited at 280 nm, the SAV emission at 350 nm decreased whereas the probe emission at 460 nm increased. Especially in the case of probe II (Fig. 2b), more than four fold increase in emission was detected at 460 nm.
1 (v/v) mixture with the SAV solution was prepared and allowed to reach their equilibrium for three minutes. Subsequently, the emission spectra were recorded with excitation at 280 nm, the protein's absorption maxima. First, the mixtures of the probes and BSA were analysed to reveal the absence of FRET characteristic, i.e., excitation at 280 nm resulted only in BSA emission at 340 nm (in the presence of probes I, II, III, or IV). The emission patterns around 460 nm resembled that of the probe only in the presence of each probe upon irradiation at 280 nm (ESI,† S3). On the other hand, the mixture of the probes and SAV showed an obvious iFRET phenomenon, even at nanomolar concentrations. When excited at 280 nm, the SAV emission at 350 nm decreased whereas the probe emission at 460 nm increased. Especially in the case of probe II (Fig. 2b), more than four fold increase in emission was detected at 460 nm.
To confirm the specific targeting of SAV, experiments were conducted with the most efficient probe, iFRET probe II, with denatured SAV. To this end, SAV was denatured at 100 °C, followed by the addition of probe II, and subsequent spectroscopic analysis showed the absence of iFRET characteristics (ESI,† S4). The obtained data confirmed that the tested probes selectively target SAV and that probe II is a potent iFRET acceptor for Trp-containing protein.
The calculated iFRET efficiencies of the probes and Förster distances, at which the energy transfer efficiency is 50%, are listed in Table 2 (see the details in the ESI,† S2). The Förster distances are less than 3 nm which is suitable for iFRET to occur in the model system used. Additionally, the FRET efficiency (0.14–0.36) indicates that the excited donor has a high probability to transfer its energy to a neighboring acceptor, rather than to emit energy as its own fluorescence.
| Probe | E a = (1 − Ida/Id) | R 0 b (nm) | Φ f c |
|---|---|---|---|
| a The quantum efficiency of each probe was measured in a phosphate buffer (0.1 M, pH 7.4) with umbelliferone (Φ = 0.7)19 as a standard. FRET donor = tryptophan (Φ = 0.2).20 b Förster distance. c Fluorescence quantum yields. | |||
| I | 0.22 (300–420 nm, 150 nM) | 2.56 | 0.26 |
| II | 0.14 (300–375 nm, 50 nM) | 2.04 | 0.47 |
| III | 0.36 (300–420 nm, 150 nM) | 1.79 | 0.06 |
| IV | 0.22 (300–375 nm, 50 nM) | 2.39 | 0.50 |
A striking effect was observed for probe III, which revealed the highest iFRET efficiency even though it has a relatively short Förster distance. A plausible explanation for this phenomenon lies in the correlation of the molar extinction coefficient of the probe in the calculation of the Förster distance. In comparison to the measured probes, probe III showed the smallest molar extinction coefficient (Table S1, ESI†). Due to its high iFRET efficiency it is envisioned that its fluorescence motif would locate closer to the Trp at SAV than those of the others when the probes bind to SAV.
In conclusion, the iFRET technique represents a label-free and real-time imaging method to study biomolecular events. Three fluorescent probes were prepared and conjugated to biotin. The biotinylated fluorescent probes were used in iFRET studies with purified streptavidin. Experimental data confirmed that the obtained probes are effective iFRET acceptors for tryptophan-containing proteins. Coumarin-based probe II was identified as a potent iFRET acceptor for tryptophan containing proteins, with Stokes shifts and fluorescent emission wavelengths that surpass the reported naphthyl-based probe BNEDA. Currently, we are well underway in evaluating the potential of iFRET probe II in cellular imaging. The results of our endeavours will be reported soon.
Acknowledgements
The authors express their gratitude to Amar B.T. Ghisaidoobe for revising the manuscript. This study was supported by National Research Foundation of Korea (NRF) grants (grant no.: NRF-2006-2005077, NRF-NRF-2012M3A9C4048775 and NRF-2012-0009543) funded by the Korean Ministry of Science, ICT and Future Planning (MISP).Notes and references
-
(a) M. Strianese, M. Staiano, G. Ruggiero, T. Labella, C. Pellecchia and S. D'Auria, Methods Mol. Biol., 2012, 875, 193–216 CrossRef CAS
; (b) A. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515–1566 CrossRef CAS PubMed
; (c) S. W. Thomas III, G. D. Joly and T. M. Swager, Chem. Rev., 2007, 107, 1339–1386 CrossRef PubMed
.
- C. L. Amiot, S. Xu, S. Liang, L. Pan and J. X. Zhao, Sensors, 2008, 8, 3082–3105 CrossRef CAS
.
-
(a) I. Johnson, Histochem. J., 1998, 30, 123–140 CrossRef CAS
; (b) K. M. Marks and G. P. Nolan, Nat. Methods, 2006, 3(8), 591–596 CrossRef CAS PubMed
; (c) D. Proudnikov and A. Mirzabekov, Nucleic Acids Res., 1996, 24(22), 4535–4542 CrossRef CAS PubMed
.
- P. S. Waggoner and H. G. Craighead, Lab Chip, 2007, 7, 1238–1255 RSC
.
- D. M. Jameson and J. A. Ross, Chem. Rev., 2010, 110, 2685–2708 CrossRef CAS PubMed
.
-
(a) W. A. Lea and A. Simeonov, Expert Opin. Drug Discovery, 2011, 6(1), 17–32 CrossRef CAS PubMed
; (b) J. C. Owicki, J. Biomol. Screening, 2000, 5(5), 297–306 CrossRef CAS PubMed
.
- R. M. Clegg, Curr. Opin. Biotechnol., 1995, 6, 103–110 CrossRef CAS
.
-
(a) K. E. Sapsford, L. Berti and I. L. Medintz, Angew. Chem., Int. Ed., 2006, 45, 4562–4588 CrossRef CAS PubMed
; (b) S. Zadran, S. Standley, K. Wong, E. Otiniano, A. Amighi and M. Baudry, Appl. Microbiol. Biotechnol., 2012, 96, 895–902 CrossRef CAS PubMed
.
- Y. Kurishita, T. Kohira, A. Ojida and I. Hamachi, J. Am. Chem. Soc., 2010, 132, 13290–13299 CrossRef CAS PubMed
.
-
(a) F. Liao, Y. Xie, X. Yang, P. Deng, Y. Chen, G. Xie, S. Zhu, B. Liu, H. Yuan, J. Liao, Y. Zhao and M. Yu, Biosens. Bioelectron., 2009, 25, 112–117 CrossRef CAS PubMed
; (b) Y. Xie, X. Yang, J. Pu, Y. Zhao, Y. Zhang, G. Xie, J. Zheng, H. Yuan and F. Liao, Spectrochim. Acta, Part A, 2010, 77, 869–876 CrossRef PubMed
; (c) Y. Zhang, X. Yang, L. Liu, Z. Huang, J. Pu, G. Long, L. Zhang, D. Liu, B. Xu, J. Liao and F. Liao, J. Fluoresc., 2012, 23, 147–157 CrossRef PubMed
; (d) Y. Xie, T. Maxson and Y. Tor, J. Am. Chem. Soc., 2010, 132, 11896–11897 CrossRef CAS PubMed
.
-
(a) K. Wu, W. Liu and G. Li, Spectrochim. Acta, Part A, 2013, 102, 186–193 CrossRef CAS PubMed
; (b) A. F. Petrik, M.-P. Strub and J. C. Lee, Biochemistry, 2010, 49(9), 2051–2057 CrossRef CAS PubMed
; (c) J. M. Goldberg, R. F. Wissner, A. M. Klein and E. J. Petersson, Chem. Commun., 2012, 48, 1550–1552 RSC
; (d) N. Goswami, A. Makhal and S. K. Pal, J. Phys. Chem. B, 2010, 114, 15236–15243 CrossRef CAS PubMed
.
- C. M. Yengo and C. L. Berger, Curr. Opin. Pharmacol., 2010, 10, 731–737 CrossRef CAS PubMed
.
- B. Lin, S. Urayama, R. M. Saroufeem, D. L. Matthews and S. G. Demos, Opt. Express, 2010, 18, 21074–21082 CrossRef CAS PubMed
.
- J. Donovalová, M. Cigáň, H. Stankovičová, J. Gašpar, M. Danko, A. Gáplovský and P. Hrdlovič, Molecules, 2012, 17, 3259–3276 CrossRef PubMed
.
- A. Chilkoti, P. H. Tan and P. S. Stayton, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 1754–1758 CrossRef CAS
.
- H. Hussain, J. Hussain, A. Al-Harrasi and K. Krohn, Tetrahedron, 2012, 68(12), 2553–2578 CrossRef CAS PubMed
.
- M. E. Riveiro, N. De Kimpe, A. Moglioni, R. Vazquez, F. Monczor, C. Shayo and C. Davio, Curr. Med. Chem., 2012, 17(13), 1325–1338 CrossRef
.
- J. A. Key, S. Koh, Q. K. Timerghazin, A. Brown and C. W. Cairo, Dyes Pigm., 2009, 82, 196–203 CrossRef CAS PubMed
.
- A. Shibata, H. Abe, M. Ito, Y. Kondo, S. Shimizu, K. Aikawac and Y. Ito, Chem. Commun., 2009, 6586–6588 RSC
.
- P. R. Callis and T. Liu, J. Phys. Chem., 2004, 108(14), 4248–4259 CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed synthetic procedures, photophysical data, enzyme profiling and spectroscopic data of the compounds. See DOI: 10.1039/c3mb70323a |
| This journal is © The Royal Society of Chemistry 2014 |