Magneto-capillary valve for integrated purification and enrichment of nucleic acids and proteins†
Remco C.
den Dulk
ab,
Kristiane A.
Schmidt
a,
Gwénola
Sabatté
a,
Susana
Liébana
ac and
Menno W. J.
Prins
*ab
aPhilips Research, High Tech Campus, Eindhoven, The Netherlands. E-mail: menno.prins@philips.com; Tel: +31 40 27 48497
bEindhoven University of Technology, Department of Applied Physics, Eindhoven, The Netherlands
cUniversitat Autònoma de Barcelona, Departament de Química, Bellaterra, Spain
First published on 21st September 2012
Abstract
We describe the magneto-capillary valve (MCV) technology, a flexible approach for integrated biological sample preparation within the concept of stationary microfluidics. Rather than moving liquids in a microfluidic device, discrete units of liquid are present at fixed positions in the device and magnetic particles are actuated between the fluids. The MCV concept is characterized by the use of two planar surfaces at a capillary mutual distance, with specific features to confine the fluids by capillary forces, and the use of a gas or a phase-change material separating the stationary aqueous liquids. We have studied the physics of magneto-capillary valving by quantifying the magnetic force as a function of time and position, which reveals the balance of magnetic, capillary and frictional forces in the system. By purification experiments with a fluorescent tracer we have measured the amount of co-transported liquid, which is a key parameter for efficient purification. To demonstrate the versatility of the technology, several MCV device architectures were tested in a series of biological assays, showing the purification and enrichment of nucleic acids and proteins. Target recovery comparable to non-miniaturized commercial kits was observed for the extraction of DNA from human cells in buffer, using a device architecture with patterned air valves. Experiments using an enrichment module and patterned air valves demonstrate a 40-fold effective enrichment of DNA in buffer. DNA was also successfully purified from blood plasma using paraffin phase-change valves. Finally, the enrichment of a protein biomarker (prostate-specific antigen) using geometrical air valves resulted in a 7-fold increase of detection signal. The MCV technology is versatile, offers extensive freedom for the design of fully integrated systems, and is expected to be manufacturable in a cost-effective way. We conclude that the MCV technology can become an important enabling technology for point-of-care systems with sample in–result out performance.
Introduction
The integration and miniaturization of laboratory procedures into lab-on-chip devices is an important technological trend in in vitro diagnostics. The aim is to achieve a seamless use of diagnostics in the medical workflow by providing compact systems that can analyze patient samples at the point-of-care, close to the patient.1 Ease-of-use is an important characteristic of point-of-care diagnostics, ideally with sample in–result out performance. An important problem is that raw biological samples are often not directly suitable for analysis and that elaborate multi-step sample preparation processes are required before actual analysis of the sample can take place.2–4 Several detection technologies have been successfully miniaturized and integrated into lab-on-chip formats, but the integration of sample preparation has been more problematic. Sample preparation often still demands a substantial amount of additional manual handling by a trained operator and is an important bottleneck in the process from sample to result. Sample handling can be automated using pipetting robots, however, pipetting mechanisms are fundamentally difficult to integrate and miniaturize in a lab-on-chip format. One approach is to use pressure-driven microfluidic flows, but a disadvantage of pressure-driven microfluidics is that the external equipment required to operate a miniaturized lab-on-chip device is often large and complex. Such systems may be suited for decentralized laboratory settings, but for real point-of-care diagnostics novel solutions are necessary that miniaturize the total system and make it rapid, easy-to-use and cost-effective.A novel approach in the search for less complicated methods for integrated sample preparation is the concept of stationary microfluidics.5–12 Instead of moving liquids in a microfluidic device, discrete units of liquid are present at fixed positions in a device and magnetic particles are displaced between the liquids. Stationary microfluidics offers a great potential for integration and miniaturization, since no bulky external equipment is required to operate the miniaturized lab-on-chip device. The fluids are stationary and for the actuation of the magnetic particles a small movable permanent magnet is sufficient. The literature on the moving of magnetic particles between stationary fluids describes different approaches. Pipper et al.5 have demonstrated an open device with droplets on a hydrophobic surface. Lehmann et al.6 have used aqueous liquids immobilized on hydrophilic spots surrounded by a bulk volume of oil. Shikida et al.7 have shown a device with aqueous droplets separated by a small constriction and surrounded by a bulk volume of oil. Berry et al.8 have used the same concept and applied it in a miniaturized well plate format to eliminate the gravity dependence. Sur et al.9 have used a layer of oil floating on top of the aqueous liquids. These systems have been applied for various biological sample preparation processes, such as the purification of nucleic acids from cells in buffer,6,8 blood5,9 or urine,9 and also for an enzymatic immunoassay.7
A common element in the approaches listed above is that all use an oil phase between the aqueous phases. The oil has two functions: (i) it forms an immiscible barrier between the aqueous phases and (ii) the oil lowers the interfacial tension and makes it possible to extract the magnetic particles from the aqueous phases with a relatively small force. However, the presence of an oil phase has important drawbacks. A first drawback lies in the complications for system integration. The goal of a completely integrated system is to require only the insertion of the sample and no other fluids. Pipetting steps should be eliminated as much as possible. In the reported devices, the oil is inserted by an additional pipetting step and moreover, the sequence of sample pipetting versus oil pipetting steps is subtle and needs to be carefully obeyed to achieve successful operation. In the system of Lehmann the aqueous fluids need to be pipetted through the volume of oil, and in the systems of Sur and Berry the oil needs to be pipetted after the aqueous fluids have been applied. The oil application processes thus put constraints on the system and limit the number of suitable technical solutions, which is an important drawback from the perspective of full system integration. A second drawback of using an oil phase is that bio-assay incompatibilities arise. Some assay processes are compatible with oil, e.g. nucleic acid purification by the Boom method,2 but other processes like the purification and enrichment of proteins can be strongly disturbed by the presence of an oil phase. Non-specific interactions are promoted at the aqueous/oil interface, the aqueous/oil interface can become unstable, and the interface may change the properties of the biomaterials. Surfactants may help to reduce such non-specific interactions, but surfactants have a strong influence on the physical properties of the aqueous/oil interface and thereby decrease the window of stable and reproducible operation. Recently, the first oil-free devices have been reported.10,11 Bordelon et al.10 used a one-dimensional tube with a millimeter-sized inner diameter. Disadvantages of this large-diameter tube concept are that the miniaturization possibilities are limited, and that the concept lacks the fabrication and scaling advantages of planar lab-on-a-chip technologies. Building on earlier developed open-surface droplet concepts,5,6 Zhang et al.11 have demonstrated a technology with surface elevations which improves the positional control of the droplets. However, a general disadvantage from an application perspective is that open droplet technologies require accurate pipetting steps and have risks of contamination and evaporation.
In this paper we describe the magneto-capillary valve (MCV) technology, a stationary microfluidic concept that allows enrichment and purification of nucleic acids and proteins without the need for an oil phase. The uniqueness of the MCV approach lies in the use of two planar surfaces at a capillary mutual distance, with specific features to confine the fluids by capillary forces, and the use of a gas or a phase-change material separating the stationary aqueous liquids. The designs intrinsically have high liquid confinement forces and low amounts of co-transported liquid upon transfer of particles through the magneto-capillary valve. Fig. 1A depicts how magnetic particles are transported from one stationary liquid to another by magnetic forces originating from a small movable permanent magnet. Since the operation of the device is based on the balance between magnetic and capillary forces, we have named it the magneto-capillary valve.12 A number of device architectures are depicted in Fig. 1B. The first one is the patterned air valve, in which the aqueous solutions are confined in well-separated volumes by a pattern of hydrophilic and hydrophobic regions. The second is the geometrical air valve, in which the aqueous solutions are confined by sharp geometrical transitions. The third is the patterned paraffin valve, in which the aqueous solutions are separated by a plug of solid paraffin that can be briefly melted when magnetic particles need to cross the valve. Fig. 1B also shows the concept of an enrichment module, a device with fluid volumes of different sizes in order to allow enrichment of the sample. The enrichment module provides a practical and cost-effective way to accommodate a sample volume in the milliliter range combined with an elution volume in the microliter range.
 | ||
| Fig. 1 Overview of the magneto-capillary valve technology. (A) Principle of the magneto-capillary valve: (i) magnetic particles are dispersed in the liquid in chamber 1. (ii) The particles are collected above the magnet in chamber 1 and transported towards the valve region. (iii) The cloud of particles is pulled into the valve region by deforming the meniscus. (iv) The particles arrive in chamber 2 and the magneto-capillary valve closes by capillary forces. (B) Schematic drawings of MCV device architectures: three distinct valve types and an enrichment module. (C) Schematic drawing of a patterned air valve cartridge in exploded view (top) and assembled view (bottom), showing double-sided tape (red) that joins the transparant planar top and bottom parts. Aqueous liquids (blue) with a typical volume of 15 μl are confined in four chambers by a pattern of hydrophilic and hydrophobic regions. (D) Top: photo of an MCV cartridge in the experimental setup, showing the permanent magnet (blue, 4 mm Ø) embedded in a white background, gently touching the bottom of the cartridge. Bottom: top view microscope image, showing two translucent aqueous chambers and the magnet in the valve region between the chambers. The magnet (blue) draws a cloud of magnetic particles (black/brown) from the left chamber to the right chamber. | ||
The MCV technology provides a platform for solid phase extraction, which is a common type of sample preparation.2,13,14 Analytes are coupled to magnetic particles in the sample matrix and are transported through one or more washing buffers to be finally eluted from the particles in a buffer that is appropriate for detection of the analyte. Intrinsic differences exist between sample preparation methods for nucleic acids, cells and proteins. Therefore, it is challenging to conceive a platform that suits such widely different purposes. In this paper we will demonstrate that the MCV technology is able to handle nucleic acids, cells and proteins. First, a physical characterization of the MCV technology is presented. We describe the behavior of the valve in a quasi-static model that balances magnetic forces, capillary forces and friction forces, we quantify the amount of co-transported liquid during the magnetic valving process, and we describe a number of parameters that define the window of operation for the device. Subsequently, we demonstrate a wide range of sample preparation processes, namely the extraction of DNA from cells in buffer, the enrichment of DNA in buffer, the purification of DNA from blood plasma and the enrichment of a biomarker protein (prostate-specific antigen, PSA).
Experimental methods
Cartridge fabrication
As depicted in Fig. 1C, the MCV cartridges studied in this paper consist of a planar bottom part, a planar top part, and a layer of double-sided adhesive tape that joins the two parts together. The top part is a standard microscope glass slide (25 × 75 mm2) of 1 mm thickness, in which small filling holes have been fabricated by laser machining. The bottom part is a thin microscope glass slide of 0.5 mm thickness. The two glass slides are joined together by a double-sided adhesive tape with a thickness in the order of 100 μm, in order to form a planar capillary microfluidic device. The experiments were performed with MCV devices of various architectures, as illustrated in Fig. 1B. In all cases the bottom part is homogeneously hydrophobic and the top part has specific features. In the case of patterned air valves, the top part is patterned into hydrophilic and hydrophobic regions, which confines the liquid into separate chambers with a volume of typically 15 μl each. The double-sided adhesive tape is used in this case only as a spacer and is not in direct contact with the liquids. The glass slides are rendered hydrophobic by depositing a self-assembled monolayer (SAM) of ‘fluorosilane’ (1H,1H,2H,2H-perfluorodecyldimethylchlorosilane 97%, ABCR) by chemical vapor deposition. The pattern of hydrophilic and hydrophobic regions is created by locally removing the SAM with an atmospheric oxygen plasma applied through a metal mask. In the case of geometrical air valves, the top part is homogeneously hydrophilic, and the liquids are confined by the double-sided adhesive tape and by holes in the top part that are located at the valve region. In the case of paraffin valves, the top part is patterned into hydrophilic and hydrophobic regions, and the liquid is confined by the double-sided adhesive tape and by a plug of paraffin wax (docosane, C22H46, Tm = 44.4 °C) that is located at the valve region. The paraffin is deposited into the valve region after joining the top and bottom parts together with the double-sided adhesive tape. The cartridge is heated to approximately 50 °C and melted paraffin is injected into the cartridge. By the design of the double-sided adhesive tape and the pattern of hydrophilic and hydrophobic regions, the paraffin fills exclusively the valve region. The bottom part of a paraffin cartridge is equipped with thin-film resistive heaters of Indium Tin Oxide (ITO) to be able to locally melt the paraffin.The enrichment of target molecules requires a sample volume at the input that is significantly larger than the sample volume at the output. Since the typical volume for real-time PCR detection is in the order of 10 μl, an input volume in the order of a milliliter is required to realize a volumetric enrichment of 100 times. To keep the footprint of the MCV cartridge small, the large input volume is implemented by placing an extension module of acrylic glass on top of the cartridge. The height of the module is 10 mm, which results in a maximum input volume of 2.88 ml. With a typical elution volume of 15 μl in the MCV cartridges, this results in a volume ratio of 190 times.
Quantification of the magnetic force
The magnetic force that is applied to the cloud of particles is determined by three parameters: 1) the magnetic field of the permanent magnet, 2) the magnetic response of the superparamagnetic particles to the magnetic field, and 3) the position of the particles relative to the magnetic field. The magnetic flux density of the permanent magnet was calculated numerically (Comsol) using the parameters of the actual magnet used for the experiments: a neodynium iron boron cylinder of 4 mm diameter, 5 mm length and a remanent magnetization of 1200 mT. The magnetic response of the superparamagnetic particles was determined by measuring their magnetic susceptibility in a Vibrating Sample Magnetometer (VSM). The VSM data was fitted to a Langevin function and corrected for demagnetization effects that arise due to the large aspect ratio of the cloud of particles in an MCV cartridge. In every experiment the time evolution of the position of the particles relative to the magnet was determined by a series of top-view microscopic images (as in the bottom panel of Fig. 1D). An image processing algorithm (Matlab) processes the recorded movie and stores for each movie frame the position of the particle cloud in a Boolean matrix. Subsequently, the magnetic force that is applied to the cloud of particles is determined by numerically evaluating, | (1) |
![[H with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0048_20d1.gif) represents the magnetic field of the permanent magnet,
represents the magnetic field of the permanent magnet, ![[M with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_004d_20d1.gif) the magnetic response of the superparamagnetic particles to the magnetic field, and dV the position of the particles relative to the magnetic field. In this approach it is assumed that the number of particles in the cloud is known and constant during an experiment and that the particles are homogeneously dispersed in the cloud. Under these assumptions, the numerical evaluation yields the magnetic force that is applied to the cloud of particles as a function of time.
the magnetic response of the superparamagnetic particles to the magnetic field, and dV the position of the particles relative to the magnetic field. In this approach it is assumed that the number of particles in the cloud is known and constant during an experiment and that the particles are homogeneously dispersed in the cloud. Under these assumptions, the numerical evaluation yields the magnetic force that is applied to the cloud of particles as a function of time.
In the numerical evaluation, the magnetic force is decomposed into the x-, y- and z-component. The largest force is in the z-direction, which is directed perpendicular to the bottom surface of the cartridge. The force in the y-direction is nearly zero, since the movement of the magnet is linear in the x-direction. Most interesting is therefore the force in the x-direction, because this is the driving force of the cloud which is counteracted by capillary force and friction.
Buffers and particles
The following buffers2,15 were used in the biological model assays: lysis/binding buffer LB1 (100 mM Tris-HCl, 5 M GuSCN, 100 mM Triton X-100, pH 7.6), lysis/binding buffer LB2 (100 mM Tris-HCl, 5 M GuSCN, pH 6.4), wash buffer WB (100 mM Glycine-HCl, pH 3.5), and elution buffer EB (10 mM Tris-HCl, pH 8.5).The following superparamagnetic particles were used in the experiments: magnetic particles (Dynabeads M-270 Carboxylic Acid, Invitrogen) with a diameter of 2.8 μm at a concentration of 30 mg ml−1 (2 × 109 particles ml−1) and magnetic silica particles (Dynabeads MyOne Silane, Invitrogen) with a diameter of 1 μm at a concentration of 40 mg ml−1.
Measurement of co-transported liquid
Magnetic particles (5 μl of M-270 particles) were washed in water (Milli-Q purified). The particles were separated from their storage buffer using a magnetic rack, the supernatant was replaced by water, the solution was vortexed to mix well, and the same steps were repeated again once. Subsequently, 9 μl fluorescent dye solution (9.25 μM ATTO-532 stock) was added to the 5 μl of particles, resulting in 14 μl solution of 5.95 μM fluorescent dye containing 107 magnetic particles. The solution was injected into the first chamber of an MCV cartridge and the other chambers were filled with pure water. The particles were magnetically transferred from chamber 1 to chamber 4 at a velocity of 0.5 mm s−1, with a 30 s linear mixing motion (50 mm s−1) in each of the four chambers, all effectuated by an automatic stage. For each chamber, the concentration of the fluorescent dye was measured off-chip in a Raman Systems R-3000 spectrometer in fluorescence mode, using 532 nm laser excitation. By diluting the highest concentrations and by using a longer integration time for the lower concentrations, it was possible to measure over a range of five orders of magnitude, from 10 μM down to 1 nM.Extraction of DNA from human cells in buffer
Human genomic DNA (gDNA) was purified from samples with THP-1 cells in MCV devices with patterned air valves. The THP-1 cells, which originate from a human acute monocytic leukemia cell line, were cultured in RPMI growth medium (RPMI 1640, Pen-Strep, Glutamax and fetal bovine serum) and transferred into phosphate buffered saline (PBS) buffer. The number of cells was determined by cell counting under a microscope using a Burker Turk counting chamber. A volume of 5.6 μl sample, containing on average 5.6, 56 or 560 cells, was incubated for 10 min in a tube with 3.4 μl of binding buffer LB2 and 5 μl of magnetic silica particles washed in LB2. THP-1 cells lyse easily in the LB2 buffer due to the high concentration of GuSCN, which at the same time inactivates nucleases to protect the DNA from degradation. During incubation of the lysis/binding step, the cartridge was filled with 14 μl wash buffer WB in chamber 2 and 14 μl elution buffer EB in chamber 3. After the lysis/binding step, the liquid with the DNA binding magnetic silica particles was injected into chamber 1 of the cartridge. The particles were magnetically transferred to WB and to EB. The elution in EB was enhanced by a magnetic mixing motion for 5 min. Finally, the particles were removed from the eluate by transporting them to chamber 4. The complete process was performed at room temperature within a total time of 16 min (10 min binding, 1 min magnetic particle transport and 5 min magnetically actuated elution). The eluate was pipetted out of the cartridge and a volume of 5 μl was analyzed by real-time PCR (polymerase chain reaction), using a commercial assay targeting RNase P (TaqMan RNase P detection reagents, Applied Biosystems). A reference assay was performed using the QIAamp Blood Mini kit (Qiagen). The QIA kit provides extraction of nucleic acids using a silica filter membrane in a standard Eppendorf tube (spin column). The instructions of the manufacturer were followed, which involves a sample volume of 200 μl (containing on average 5.6, 56 or 560 cells) incubated with 200 μl lysis/binding buffer and an elution volume of 100 μl, of which 5 μl was analyzed by real-time PCR.Enrichment of DNA in buffer
Integrated enrichment of DNA was performed in MCV devices with patterned air valves and an enrichment module. 800 μl of Milli-Q water was spiked with plasmid DNA containing a Staphylococcus aureus gene fragment (SA pDNA) and incubated for 10 min in a tube with 1200 μl binding buffer LB2 and 5 μl of magnetic silica particles washed in LB2. The quantity of DNA was 104 or 105 copies per sample, corresponding to a sample concentration of 12.5 or 125 cps μl−1, respectively. During incubation of the binding step, the cartridge was filled with 14 μl WB in chamber 2 and 14 μl EB in chamber 3. After the binding step, the 2 ml of liquid with the DNA binding magnetic silica particles were injected into chamber 1 of the cartridge and a large magnet (8 mm diameter, 10 mm length) was performing an automated motion under the cartridge for about 1 min to collect the particles at the bottom of the chamber. Subsequently, the magnet was exchanged for a smaller one (4 mm diameter, 5 mm length) and the particles were magnetically transferred to WB and to EB. In EB the elution was enhanced by a magnetic mixing motion of 5 min. Finally, the particles were removed from the eluate by transporting them into the hydrophobic valve region. The complete process was performed at room temperature within a total time of 17 min (10 min binding, 2 min magnetic particle transport and 5 min magnetically actuated elution). The eluate was pipetted out of the cartridge and analyzed by real-time PCR, using a Taqman16 assay targeting the 442 gene.17Purification of DNA from blood plasma
Plasmid DNA containing a Staphylococcus aureus gene fragment (SA pDNA) was purified from plasma samples in MCV devices with patterned paraffin valves. A volume of 3.4 μl binding buffer LB1 was spiked with SA pDNA and incubated for 10 min in a tube with the sample matrix (5.6 μl of defibrinated plasma) and 5 μl of magnetic silica particles washed in LB1. During incubation of the binding step, the cartridge was filled with 14 μl of LB2 in chamber 2 and 14 μl of EB in chamber 3. After the binding step, the liquid with the DNA binding magnetic silica particles was injected into chamber 1 of the cartridge. The particles were magnetically transferred to LB2 and to EB. Just before each transfer, the paraffin valve was heated just above the melting temperature (44 °C) by an integrated thin film heater. Right after each transfer, the heater was switched off and the paraffin resolidified, thus closing the valve. In EB the elution was enhanced by a magnetic mixing motion of 5 min. Finally, the particles were removed from the eluate by transporting them to chamber 4. The complete process was performed at room temperature within a total time of 16 min (10 min binding, 1 min magnetic particle transport and 5 min magnetically actuated elution). The eluate was pipetted out of the cartridge and analyzed by real-time PCR, using a Taqman16 assay targeting the 442 gene.17Protein enrichment in buffer
The protein enrichment assay was performed in MCV devices with geometrical air valves using an assay for prostate-specific antigen (PSA), a protein that is associated with prostate disorders. The details of this assay have been described previously by Sabatte et al.18 5 pM PSA was prepared in 1200 μl of 5% bovine serum albumin (BSA) in PBS. PSA was captured by magnetic particles coated with αPSA-10 via a DNA linker for 15 min in a tube. After the capture step, the particles were magnetically separated from the liquid, resuspended in 15 μl of 0.05% Triton X-100 in PBS, and injected into chamber 1 of an MCV cartridge with geometrical air valves. The particles were magnetically transferred to chamber 2, containing 15 μl of NEB EcoR1 buffer (New England Biolabs) with DNase, in which elution was performed by degrading the DNA linker between particle and antibody. The elution was enhanced by a magnetic mixing motion of 10 min. Finally, the particles were removed from the eluate by transporting them to chamber 3. The complete process was performed at room temperature within a total time of 26 min (15 min capture, 1 min magnetic particle transport and 10 min magnetically actuated elution). The eluate was pipetted out of the cartridge and analyzed directly in a sandwich immunoassay (IA) detection system based on frustrated total internal reflection.19 As a reference, the same enrichment assay was also performed in tubes.Results and discussion
1. Physical characterization of the MCV technology
To understand the working principles of the MCV technology, we investigated the physical mechanisms that determine the behavior of the magneto-capillary valve by (1) measuring the magnetic force during a crossing of particles through the valve, (2) measuring the amount of co-transported liquid and (3) defining a window of operation.| Fcap = w γlv (cos θt + cos θb) + 2 t γlv | (2) |
Fig. 2 shows the magnetic force of an ensemble of 107 superparamagnetic particles of 2.8 μm diameter in an MCV cartridge. The cartridge has a capillary thickness of 100 μm and contains two chambers with aqueous fluid, separated by a 4 mm hydrophobic region. The ensemble of magnetic particles is concentrated into a dense cloud by a small neodynium permanent magnet, resulting in a cloud of particles with a diameter of about 1 to 1.5 mm. The particles were magnetically transferred at a velocity of 0.5 mm s−1 between the two chambers. By combining recorded images of the magnetic particle cloud with the measured susceptibility of the particles and the calculated magnetic field of the magnet, the magnetic force that is applied to the particles during the transfer process can be determined. The results are shown in Fig. 2.
 | ||
| Fig. 2 Magnetic force profile during an MCV crossing. The force has been determined from video images recorded during the crossing of an ensemble of magnetic particles through a magneto-capillary valve (see Experimental methods for details). The force reflects the variations in capillary and frictional forces that the ensemble of particles experiences. The different phases of the crossing process (A to G) correspond to clearly distinguishable features in the magnetic force profile. The small discontinuities in the magnetic force profile between phase A and B and between phase G and A are due to an artifact in the image processing. | ||
The particle transfer process can be characterized by several phases. Phase A represents intra-chamber transport, where the cloud of particles moves within the liquid of a chamber. In phase B, the cloud is compressed against the meniscus, leading to a smaller cloud with a larger particle density. In phase C, the cloud moves into the hydrophobic valve region. In phase D, necking of the liquid thread occurs until pinch-off separates the dense cloud of particles from the bulk of the liquid in the chamber. In phase F, the cloud of particles is transported through the valve region towards the next chamber. In phase G, the cloud touches the liquid in the next chamber and the particles flow into the chamber, which concludes the crossing of particles through the valve.
The forces in these different phases are as follows. Before phase A, the system is at rest and the magnetic force equals the static surface friction. In phase A, the magnet is set into motion and the cloud accelerates to follow the movement of the magnet. The force of 19 μN thus represents the sum of surface and viscous friction. In phase B, the cloud is compressed against the meniscus in the protrusion. The magnetic force increases until it equals the sum of capillary and frictional forces of 157 μN, marked by phase C. The cloud then moves into the hydrophobic region and almost immediately necking (phase D) starts to occur. Necking lowers the force to 121 μN at which point the liquid neck breaks and pinch-off occurs (phase E). In phase F, the cloud moves as a separate droplet through the hydrophobic region, which apparently requires a slightly increasing force as the droplet travels. At the transition between phase F and G, the droplet with particles first touches the hydrophilic region and shortly thereafter (at t = 40 s) the droplet touches the liquid in the next chamber. This pulls the droplet forward and lowers the magnetic force to −9 μN. When the cloud is moving into the liquid of the second chamber, the system is again in phase A, experiencing a friction force of 15 μN during the intra-chamber transport. At the end of phase A, the magnet stops moving and after deceleration of the cloud the system is at rest again.
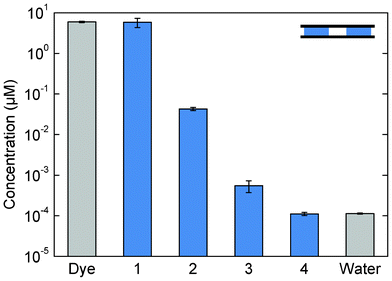 | ||
| Fig. 3 Concentration of a fluorescent tracer dye in the four chambers of an MCV cartridge after a purification procedure. Each chamber contained a volume of 14 μl. The dye concentration decreases by two orders of magnitude for each crossing of magnetic particles through a magneto-capillary valve. The concentration in chamber 4 was not distinguishable from the background fluorescence of water. Each bar represents the average of three independent experiments. The error bars indicate the standard deviation. | ||
In the cloud density method, the co-transported liquid volume was estimated from the recorded top view images. Using the observed cloud area, the known capillary thickness of the device, and the known total amount of magnetic particles in the cloud (i.e. the volume occupied by the particles), the volume of interstitial liquid can be determined. An average co-transported volume of 0.14 ± 0.01 μl was found, which corresponds very well to the results of the fluorescence measurements. The results demonstrate a very low amount of co-transported liquid, which is a key requirement for efficient purification.
The compression of the cloud, as described in phase B of Fig. 2, is an important parameter, since it determines how much interstitial liquid will be co-transported with the cloud. A typical cloud of particles that is magnetically confined, but not compressed (phase A) has a total volume of 0.89 μl, which consists of 0.12 μl solid particle material and 0.77 μl interstitial liquid. A compressed cloud (phase C) has a total volume of 0.27 μl, which means that typically only 0.15 μl of interstitial liquid is present. This points to the importance of having high capillary forces in the device. A high capillary force strongly confines the liquid and demands a high magnetic force for the particle cloud to exit from the fluid, which results in strong compression of the cloud and a low amount of co-transported liquid.
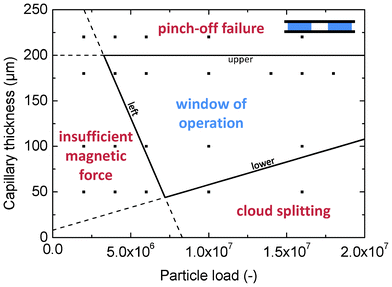 | ||
| Fig. 4 Parameter space of capillary thickness and particle load. The squares indicate the experiments that were performed. The areas in the diagram describe the behavior of the magneto-capillary valve. In the central region successful MCV crossing is observed. In the three outer regions non-ideal operation is observed. | ||
The lower boundary indicates the occurrence of cloud splitting, which means that only a part of the magnetic particle ensemble is pulled into the second fluid chamber. Cloud splitting occurs when the necking and pinch-off processes (phases D and E in Fig. 2) divide the magnetic particle cloud into two parts. Cloud splitting is observed for large cloud diameters in combination with strong pinch-off forces, which is the case for a large particle load in combination with a small capillary thickness.
The upper boundary indicates the limit for successful pinch-off, an important event in the process of crossing, which closes the valve after the cloud of particles has crossed the hydrophobic valve region. Pinch-off is driven by the capillary pressure difference between the hydrophobic valve region and the hydrophilic chamber. The capillary pressure originates from the curvature of the meniscus, so the distance t between the top and bottom part of the MCV device is a critical parameter. The capillary pressure difference that drives the pinch-off can be described by
 | (3) |
Since pinch-off is a very important process in the MCV, several other parameters that influence the processes of necking and pinch-off were investigated: (i) shape of the protrusion, (ii) distance between the chambers, and (iii) hydrostatic pressure in the chamber. The parameters of surface tension and surface energy were not varied.
Two different protrusion shapes were investigated in particular: a triangular protrusion with a sharp tip (as in Fig. 1 and Fig. 2) and a straight protrusion with a rounded tip. The rounded tip resulted in successful pinch-off for a capillary thickness of 100 μm or below, but not with 100% success rate. The sharp tip showed successful pinch-off in all cases for a capillary thickness of 180 μm, which has considerably enlarged the window of operation. From visual observations it appears that the triangular shape of the protrusion allows the contact line to flow smoothly along the boundary between the hydrophilic and hydrophobic region. In this way, the necking process is very reproducible and creates a pinch-off always at the same place, exactly at the sharp tip. An additional advantage of the triangular protrusion is that the compression of the cloud against the meniscus is focused, resulting in a smaller width of the cloud and thus minimizing the force required to cross. The focused compression is quite insensitive to the diameter of the cloud, which makes the design very robust for varying particle load.
When the distance between the chambers is too small, pinch-off occurs after the cloud has reached the other chamber. This means that a temporary fluid connection is established between the chambers, which may decrease the purification efficiency. It has been experimentally determined that for typical MCV operation (within the window of operation) a minimal distance of 2 mm is required between the chambers to ensure reliable and reproducible operation.
Finally, also the hydrostatic pressure in the chamber is of influence to the pinch-off process, since pinch-off is driven by the pressure difference between the valve region and the chamber. It has been observed that an underpressure in the chamber enhances the process of necking and pinch-off considerably (see Suplementary Information, Movie 3†). In our experiments, such underpressure was realized by slightly underfilling the hydrophilic chamber.
2. Purification and enrichment of DNA and proteins
To demonstrate the versatility of the MCV technology, four examples of biological sample preparation are presented using the various MCV device architectures as shown in Fig. 1B: (1) extraction of DNA from human cells in buffer using a device with patterned air valves, (2) enrichment of DNA in buffer using an enrichment module and patterned air valves, (3) purification of DNA from blood plasma using patterned paraffin valves, and (4) enrichment of a biomarker protein (prostate-specific antigen, PSA) in buffer using geometrical air valves. The results are valued on the basis of target recovery, which covers the combined effect of purification efficiency (indicating the suppression of inhibitors) and yield (accounting for capture and elution efficiency and other losses that may occur in the process).Fig. 5 shows the real-time PCR results after DNA extraction from three different quantities of THP-1 cells. The cycle threshold (Ct) values are shown for extraction in the MCV cartridge and for extraction by the QIAamp Blood Mini kit (see Experimental methods for details). The elution volume is 14 μl in the MCV cartridge and 100 μl in the QIA kit. In both cases a 5 μl aliquot of the eluate was analyzed in the PCR reaction. We observe that the Ct values from the MCV purification (‘MCV raw’) are much smaller than the Ct values from the QIA kit (‘QIA raw’). This is mainly caused by the much smaller elution volume in the MCV cartridge. Ct values have also been calculated for the total target quantities in the full elution volumes, i.e. respectively shifted by a factor of log2(14/5) for ‘MCV corr’ data and a factor of log2(100/5) for ‘QIA corr’ data. The resulting Ct values are very similar for the two methods, showing that the overall process (i.e. the binding and elution of DNA and the suppression of PCR inhibitors) is very similar for both methods. The line represents a linear fit through the MCV data points, showing a reproducible target recovery. Finally, the results show that low numbers of cells can be detected with both purification methods. One should however be careful in interpreting the absolute cell numbers, because some free DNA from accidentally lysed cells (not detected in the cell counting procedure) may have contributed to the real-time PCR signal.
 | ||
| Fig. 5 DNA extraction assay. The top panel shows the assay format, the bottom panel shows the real-time PCR results for the extraction of genomic DNA from THP-1 cells in buffer. The cycle threshold (Ct) value is shown as a function of the average number of input cells. Extraction was performed by MCV cartridge (MCV) or by QIAamp Blood Mini kit (QIA). The open symbols represent the raw Ct data, the closed symbols represent the Ct values after correction for the different volumes of the eluates. The line represents a linear fit through the ‘MCV corr’ data. For the MCV purifications, each data point represents the average of three independent experiments. The error bars indicate the standard deviation. | ||
DNA enrichment was performed in MCV devices with patterned air valves and an enrichment module. To enable enrichment, the capture volume needs to be significantly larger than the elution volume. Since the cartridge is a planar capillary device, a much larger volume would require a much larger footprint. Therefore, the enlarged capture volume is extended perpendicular to the surface of the substrates, as illustrated in Fig. 1B. The height of the extension is limited due to the rapid non-linear decrease of the magnetic force over the distance between magnet and particles. The maximum volume is therefore a trade-off between the footprint of the capture chamber and the waiting time before all particles are collected at the bottom of the capture chamber. The height of the module was chosen 10 mm, which results in a maximum capture volume of 2.88 ml. With a typical elution volume of 15 μl, a volumetric enrichment of 190 times can be obtained. Although the enrichment module is combined with patterned air valves in this particular experiment, the enrichment concept can be used in combination with any type of magneto-capillary valve.
Fig. 6 shows the PCR results of the enriched purifications compared to the PCR results of 5 μl aliquots that were taken directly from the 800 μl samples. The Ct values of the direct aliquots are high due to the low DNA concentrations, and as expected the Ct values in the enriched samples are much lower. The volumetric enrichment factor in the MCV device is 57, since the volume is reduced from a sample volume of 800 μl to an elution volume of 14 μl. The total starting volume, however, is 2 ml due to the 1200 μl of lysis/binding buffer that is added to the sample to achieve reliable DNA capture. In the best case, target recovery is 100% and the DNA concentration in the eluate would be 57 times higher than the DNA concentration in the 800 μl sample. In theory, the enrichment would therefore result in a ΔCt of 5.8 compared to the direct aliquots. For the enriched purifications, an average ΔCt of 5.3 was found, indicating a target recovery of about 2−0.5 = 70%. As a result, the effective enrichment factor is 40. Table 1 summarizes the performance of the integrated enrichment in terms of ΔCt and enrichment factor.
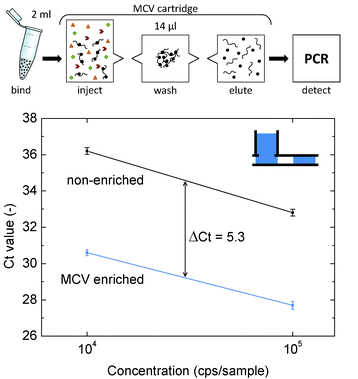 | ||
| Fig. 6 DNA enrichment assay. The top panel shows the assay format, the bottom panel shows the real-time PCR results for the enrichment of two concentrations of plasmid DNA in buffer. The cycle threshold (Ct) value is shown as a function of the DNA concentration. Data marked ‘MCV enriched’ refers to MCV cartridges with an enrichment module (2 ml input volume) and patterned air valves. Data marked ‘non-enriched’ represents PCR results of 5 μl aliquots taken directly from the input sample. Each data point represents the average of three independent experiments. The error bars indicate the standard deviation. | ||
| ΔCt | Enrichment factor | ||
|---|---|---|---|
| No enrichment | (direct) | 0.0 | 20.0 = 1 |
| Volumetric enrichment | (theory) | −5.8 | 25.8 = 57 |
| Target recovery | +0.5 | 2−0.5 = 0.7 | |
| Effective enrichment | (enriched) | −5.3 | 25.3 = 40 |
The recovery of 70% indicates that the binding step is not negatively influenced by the large volume in which the binding takes place. Since the amount of particles is the same as used for binding in small volumes, we conclude that the capture and binding process is very efficient and apparently not limited by diffusion.
 | ||
| Fig. 7 DNA purification assay. The top panel shows the assay format, the bottom panel shows the real-time PCR results for the purification of different concentrations of plasmid DNA from plasma samples in MCV cartridges with patterned paraffin valves. The cycle threshold (Ct) value is shown as a function of the DNA concentration. The solid line is a linear curve fit to the reference samples for the PCR. The dashed line indicates a recovery of 50%. Each data point represents the average of three independent experiments. The error bars indicate the standard deviation. | ||
The main challenge of integrating the protein enrichment assay into an MCV device was reliable actuation of antibody-coated particles in the cartridge. In various experiments, antibody-coated particles were observed to stick to the surface of the device. It appeared to be important to add a detergent to the solution in order to avoid non-specific sticking of the particles. By using an MCV device with geometrical air valves, the valve operation was not negatively influenced by the reagents used in the protein enrichment assay. In Fig. 8 the result of the protein enrichment assay performed in MCV cartridges is compared to the result of the same enrichment assay performed manually in tubes, showing a comparable performance. Moreover, the results of the enriched samples are compared to direct immunoassay detection of a non-enriched sample of 5 pM PSA, showing that the enrichment assay provides a 7-fold increase in signal. The immunoassay detection of a blank sample (a sample without PSA) gave for all three methods an optical signal of about 1%. The results demonstrate that proteins can be successfully enriched using MCV devices, leading to a significant increase of signal in sandwich immunoassay detection.
 | ||
| Fig. 8 Protein enrichment assay. The top panel shows the assay format, the bottom panel shows the sandwich immunoassay (IA) data measured after the enrichment of a protein biomarker (PSA) in buffer. The 5 pM PSA samples were either enriched in MCV cartridges with geometrical air valves (MCV), or enriched manually in tubes (tube) or not enriched (non-enriched). Each bar represents the average of three independent experiments. The error bars indicate the standard deviation. | ||
Conclusions
We have presented the magneto-capillary valve (MCV) technology, a novel microfluidic concept for enrichment and purification of nucleic acids and proteins. The technology is based on stationary microfluidics, i.e. discrete units of aqueous liquid are present at fixed positions in a microfluidic device and magnetic particles are actuated between the fluids. The uniqueness of the MCV approach lies in the use of two planar surfaces at a capillary mutual distance, with specific features to confine the fluids by capillary forces, and the use of a gas or a phase-change material to separate the stationary aqueous liquids (see Fig. 1). We have demonstrated MCV devices with patterned air valves, paraffin phase-change valves and geometrical air valves. The physics of magneto-capillary valving was investigated by quantifying the magnetic force as a function of time and position from video recordings. The data reveals the magneto-capillary force balance and provides a detailed understanding of the magnetic, capillary and frictional forces that determine the behavior of the system. The results show a large window in which the MCV can be operated successfully, thus providing ample freedom in system design.A key requirement for efficient purification is a low amount of co-transported liquid. The amount of co-transported liquid was experimentally determined in a device architecture with patterned air valves. An average co-transported volume of 0.14 μl per magnetic transfer was found, which indicates the high potential for efficient purification.
We have demonstrated the wide applicability of the MCV technology in four biological model assays, showing purification and enrichment of nucleic acids and proteins from various sample types. The extraction of DNA from human cells in buffer was demonstrated in MCV devices with patterned air valves. Good target recovery was observed, comparable to the performance of a commercial kit (Qiagen). Enrichment of DNA in buffer using MCV devices with patterned air valves and an enrichment module resulted in a 40-fold effective enrichment, corresponding to a 40 times increase in sensitivity. Successful purification of DNA from blood plasma was demonstrated in MCV devices with patterned paraffin valves. With a target recovery ranging from 35 to 70%, the performance is on a par with commercial solutions, but now in a microtechnology format dedicated for miniaturization and integration. Finally, successful enrichment of a protein biomarker (PSA) was demonstrated using MCV devices with geometrical air valves. A 7-fold increase of detection signal was observed as a result of the enrichment procedure. We can therefore conclude that the MCV technology is very versatile, allowing purification and enrichment of nucleic acids, proteins and potentially also cells. With its ample freedom in system design, which is essential for further system development, the MCV technology is a valuable building block for integrated point-of-care devices.
Earlier publications on stationary microfluidics have shown efficient purification in various model assays, varying in complexity, analyte and sample matrix.5–11 However, none of the reports was based on a co-planar capillary device technology, and nearly all used liquid oil as a medium to separate the different stationary fluids.5–9 In our work we have focused on a concept suited for miniaturization, integration and industrial manufacturing. This has resulted in the MCV technology which is based on the use of two planar surfaces at a capillary mutual distance, with specific features to confine the fluids by capillary forces, and the use of a gas or a robust phase-change material to separate the stationary aqueous liquids in the device. The designs intrinsically have high liquid confinement forces and low amounts of co-transported liquid upon transfer of particles through the magneto-capillary valve.
The future perspective of the MCV technology involves scientific, engineering and integration topics. An interesting scientific topic concerns the structure and fluid mechanics of the cloud of magnetic particles. In this study, we have considered the cloud of particles as a magnetizable body without internal structure. However, in reality the ensemble of magnetized particles contains strings of particles aligned with the magnetic field lines. The magnetic displacement of the particle cloud thus creates flow patterns in which strings are broken and reformed. A detailed study of the magneto-hydrodynamics inside the cloud might provide new insights into phenomena such as viscous friction, surface friction, cloud stability and cloud relaxation. This will also help to understand the pinch-off process and the resulting efficiency of particle transfer for different valve parameters. An interesting engineering topic is to evaluate different base materials and valve designs. The current MCV devices were fabricated from glass slides. It is interesting to also study injection molded plastic parts and the effect of various coatings on particle-surface interactions. In this paper we have reported the patterned air valve, the paraffin phase-change valve, and the geometrical air valve. The paraffin valve has the advantage of a robust solid structure, but disadvantages are that the pinch-off is less strong due to the reduced interfacial tension, and that biomaterials may interact with the paraffin in liquid state. The device architecture with geometrical air valves is very attractive due to its low complexity and good pinch-off properties, so it merits further device and assay studies. Finally, the development of an integrated point-of-care instrument requires a clear application focus.22 It will be particularly interesting to further develop the technology with focus on applications that will benefit from the MCV enrichment function and thereby enable rapid and highly sensitive bio-assays that are fully integrated.
In summary, the MCV technology adds a new and promising building block to the library of lab-on-a-chip technologies. It offers ample freedom in design, is versatile in terms of biological application, and is expected to be manufacturable in a cost-effective way. We therefore conclude that the MCV technology has the potential to become an important enabling technology for point-of-care devices with sample in–result out performance.
Acknowledgements
The authors want to thank Sigi Neerken, Roel Penterman, Paul van de Wiel and Herman Beijerinck for many stimulating discussions. We are grateful to Ron Gill for his assistance in the measurement of co-transported liquid. We further like to thank Eveline den Biezen, Astrid Provoost and Irene Dobbelaer for their contributions to the biological model assays, Wim Talen, Michel Bruijninckx and others in Philips Innovation Services for technical realization of cartridges and experimental setups, and the colleagues and students in the department of Molecular Diagnostics at Philips Research for the enjoyable collaboration.References
- M. A. Burns, Science, 2002, 296, 1818–1819 CrossRef CAS.
- R. Boom, C. J. Sol, M. M. Salimans, C. L. Jansen, P. M. Wertheim-van Dillen and J. van der Noordaa, J. Clin. Microbiol., 1990, 28, 495–503 CAS.
- P. Radstrom, R. Knutsson, P. Wolffs, M. Lovenklev and C. Lofstrom, Mol. Biotechnol., 2004, 26, 133–146 CrossRef.
- J. Lichtenberg, N. F. de Rooij and E. Verpoorte, Talanta, 2002, 56, 233–266 CrossRef CAS.
- J. Pipper, Y. Zhang, P. Neuzil and T. M. Hsieh, Angew. Chem., Int. Ed., 2008, 47, 3900–3904 CrossRef CAS.
- U. Lehmann, C. Vandevyver, V. K. Parashar and M. A. Gijs, Angew. Chem., Int. Ed., 2006, 45, 3062–3067 CrossRef CAS.
- M. Shikida, K. Takayanagi, H. Honda, H. Ito and K. Sato, J. Micromech. Microeng., 2006, 16, 1875–1883 CrossRef.
- S. M. Berry, E. T. Alaridb and D. J. Beebe, Lab Chip, 2011, 11, 1747–1753 RSC.
- K. Sur, S. M. McFall, E. T. Yeh, S. R. Jangam, M. A. Hayden, S. D. Stroupe and D. M. Kelso, J. Mol. Diagn., 2010, 12, 620–628 CrossRef CAS.
- H. Bordelon, N. M. Adams, A. S. Klemm, P. K. Russ, J. V. Williams, H. K. Talbot, D. W. Wright and F. R. Haselton, ACS Appl. Mater. Interfaces, 2011, 3, 2161–2168 CAS.
- Y. Zhang, S. Park, K. Liu, J. Tsuan, S. Yang and T.-H. Wang, Lab Chip, 2011, 11, 398–406 RSC.
- R. C. den Dulk, K. A. Schmidt, R. Gill, J. C. B. Jongen and M. W. J. Prins, Proc. MicroTAS 2010 Int. Conf., 665–667 Search PubMed.
- J. Wen, L. A. Legendre, J. M. Bienvenue and J. P. Landers, Anal. Chem., 2008, 80, 6472–6479 CrossRef CAS.
- C. W. Price, D. C. Leslie and J. P. Landers, Lab Chip, 2009, 9, 2484–2494 RSC.
- R. Gonzalez, B. Masquelier, H. Fleury, B. Lacroix, A. Troesch, G. Vernet and J. N. Telles, J. Clin. Microbiol., 2004, 42, 2907–2912 CrossRef CAS.
- P. M. Holland, R. D. Abramson, R. Watson and D. H. Gelfand, Proc. Natl. Acad. Sci. U. S. A., 1991, 88, 7276–7280 CrossRef CAS.
- F. Martineau, F. J. Picard, D. Ke, S. Paradis, P. H. Roy, M. Ouellette and M. G. Bergeron, J. Clin. Microbiol., 2001, 39, 2541–2547 CrossRef CAS.
- G. Sabatte, H. Feitsma, T. H. Evers and M. W. J. Prins, Biosens. Bioelectron., 2011, 29, 18–22 CrossRef CAS.
- D. M. Bruls, T. H. Evers, J. A. H. Kahlman, P. J. W. van Lankvelt, M. Ovsyanko, E. G. M. Pelssers, J. J. H. B. Schleipen, F. K. de Theije, C. A. Verschuren, T. van der Wijk, J. B. A. van Zon, W. U. Dittmer, A. H. J. Immink, J. H. Nieuwenhuis and M. W. J. Prins, Lab Chip, 2009, 9, 3504–3510 RSC.
- Z. C. Long, A. M. Shetty, M. J. Solomon and R. G. Larson, Lab Chip, 2009, 9, 1567–1575 RSC.
- J. Berthier, “Microdrops and digital microfluidics”, William Andrew Inc. ( 2008) Search PubMed.
- C. D. Chin, V. Linder and S. K. Sia, Lab Chip, 2012, 12, 2118–2134 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Three movies of magnetic particles crossing a magneto-capillary valve in various device architectures. See DOI: 10.1039/c2lc40929a |
| This journal is © The Royal Society of Chemistry 2013 |
