Cross-validation of analytical procedures for the reliable determination of Nd concentrations in nuclear fuel using ICP-OES and sector field ICP-MS
Michael
Krachler
*,
Rafael
Alvarez-Sarandes
and
Stefaan
Van Winckel
European Commission - Joint Research Centre, Institute for Transuranium Elements, ITU, P.O. Box 2340, D-76125 Karlsruhe, Germany. E-mail: michael.krachler@ec.europa.eu; Web: http://itu.jrc.ec.europa.eu Fax: +49 7247 951 588; Tel: +49 7247 951 884
First published on 2nd November 2012
Abstract
This study focussed on the development and validation of straightforward ICP-OES and sector field ICP-MS procedures for the accurate determination of the neodymium (Nd) concentration in nuclear samples. After dissolution of a spent fuel in a chemical hot cell, Nd was analysed in the acidic solution without prior separation of any fission products. In order to improve sensitivity, a high efficiency sample introduction system was coupled to the employed high resolution ICP-OES instrument. The ten most sensitive emission wavelengths of Nd were investigated systematically, all of them yielding detection limits (LODs) of less than 1 μg kg−1. The highest sensitivity and the best LOD of 0.3 μg kg−1 were obtained at the emission lines λ = 401.225 nm and λ = 430.358 nm. Compared to previous studies this LOD is more than one order of magnitude lower while sample consumption (∼0.3 mL min−1) is less than one third of the volume normally required for such analysis. In terms of radioactive samples these improved analytical figures of merit denote a considerably lower dose to the ICP-OES operator and less radioactive waste. Considering sensitivity, potential spectral interferences and stability of the spectral background, the four emission lines at λ = 401.225 nm, λ = 406.109 nm, λ = 410.946 nm and λ = 430.358 nm proved to be most useful for the reliable determination of Nd. In the absence of matrix matched reference materials having a certified Nd concentration, the accuracy of Nd ICP-OES analysis was ascertained by quantification of Nd using both external calibration and the standard addition approach. In addition, sector field ICP-MS analysis of the Nd elemental concentration was also possible without any separation step as the Nd isotopic composition of the spent fuel was well characterised in previous analyses. This new series of ICP-MS analyses was based on the interference-free isotopes 143Nd, 144Nd (a small 144Ce correction was almost negligible), 145Nd and 146Nd, which are also the four main isotopes in fission Nd. The relative isotopic results obtained using sector field ICP-MS confirmed the previously determined Nd isotopic composition. The repeated Nd concentration analysis of the dissolved spent fuel sample applying the developed ICP-OES and ICP-MS procedures gave results that overlapped within their standard deviations, underpinning the reliability of both experimental approaches.
Introduction
Part of current research in the nuclear field focuses on introducing innovative fuels dedicated to Pu management and Pu burning in existing light water reactors (LWRs) and on reducing the production of minor actinides such as Am, Cm, Np, and Pa.1,2 To this end, new experimental data are urgently needed for comparison to theoretical calculations. This knowledge will lead to a better understanding of the burn-up behaviour of such fuels in LWRs, underpinning their in-reactor fuel performance and obviously impacting safety-related aspects.As part of the radiochemical characterization of nuclear fuels, neodymium (Nd) is determined in fuels because its isotopes are useful as burn-up indicators.3 Therefore, the abundance of all seven naturally occurring Nd isotopes of such fuels is determined routinely in-house employing liquid chromatography (HPLC) coupled online to ICP-MS.4 The application of chromatography allows the online separation of other lanthanides such as Ce and Sm whose isobars (e.g.142Ce on 142Nd or 148,150Sm on 148,150Nd) would overlap with specific Nd isotopes. As such, the abundance of all Nd isotopes can be assessed reliably, while the Nd concentration is determined by employing the same analytical procedure, but following an isotope dilution step using, e.g. a 150Nd spike.4
Besides ICP-MS, ICP-OES is also considered for the analysis of trace elements, including Nd, in nuclear specimens such as non-irradiated and spent fuels.5,6 Similar to ICP-MS, however, spectral interferences of other constituents present in the analyte solution frequently hamper such analysis.5,6 To reduce the potential impact of interfering species on the reliable determination of the analyte(s), laborious separation procedures such as solvent extraction, chromatography or selective precipitation are applied prior to the actual quantification step.5,6 It is worth noting that such separation steps also include the removal of many fission products from a spent fuel. Therefore, this approach not only reduces the impact of potential interfering species but also helps to lower the radiation dose originating from the sample to the operator of the analytical instrument during analysis.
Because the well established HPLC-ICP-MS set-up routinely used in-house for Nd analysis4 became temporarily unavailable, we were urged to develop alternative analytical approaches for the determination of the Nd elemental concentration in irradiated nuclear fuel. The main purpose of this study is to highlight the development and cross-validation of these innovative, robust ICP-OES and sector field ICP-MS procedures for the reliable analysis of Nd in irradiated samples. In contrast to earlier studies,4 our aim was to analyze Nd directly in solutions of dissolved spent fuel, i.e. without a chemical separation prior to analysis. This was only possible because the isotopic composition of the fission Nd in the sample (needed for this ICP-MS analysis) was already well characterised in previous analyses. Avoiding the laborious separation step, the speed of Nd analysis in nuclear fuel is therefore accelerated considerably.
Experimental
Reagents and samples
High purity water (18.2 MΩ cm) from a MilliQ (Millipore) water purification system and sub-boiled nitric acid were used for the preparation of all solutions. Spikes for standard addition experiments (45 μg kg−1 and 90 μg kg−1) as well as external calibration solutions for both ICP-OES (0, 2, 5, 10, 20, 50, 100 μg kg−1) and sector field ICP-MS (0, 0.6, 1, 5, 20 μg kg−1) were prepared in 0.14 M and 1 M nitric acid, respectively, from high purity Nd stock solutions.A 2 mm slice of an irradiated (Th,Pu)O2 fuel rod was dissolved by boiling under reflux in a so-called “Thorex solution” containing Al(NO3)3·9H2O, NaF and 14.4 M HNO3 in a chemical hot cell at JRC-ITU. From this “mother solution” an intermediate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 dilution was prepared with 0.14 M nitric acid from which aliquots have been further diluted to 1
100 dilution was prepared with 0.14 M nitric acid from which aliquots have been further diluted to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 with 0.14 M HNO3 for ICP-OES and 1
20 with 0.14 M HNO3 for ICP-OES and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 with 1 M HNO3 for sector field ICP-MS analysis. Reported results refer to the concentration of the intermediate dilution as it was the first common solution for the comparison of both techniques.
50 with 1 M HNO3 for sector field ICP-MS analysis. Reported results refer to the concentration of the intermediate dilution as it was the first common solution for the comparison of both techniques.
Instrumentation
The sample introduction systems of the employed ICP-OES and ICP-MS instruments have been installed in glove boxes to allow the analysis of radioactive samples. This complex modification of the original instrumental design is necessary to protect the operator from α and β radiation and possible radioactive contamination originating from the handling of radioactive sample solutions.All ICP-OES measurements were carried out with a commercial high resolution ICP-OES instrument (Ultima2, HORIBA Jobin Yvon, Longjumeau, France). To further improve the stability and intensity of the ICP-OES signals, a high efficiency sample introduction system (Apex E, Elemental Scientific, Inc., Omaha, NE, USA) was employed. Detailed operating and data acquisition parameters of the sequentially working ICP-OES are summarised in Table 1.
| Plasma parameters | |
| Radio frequency | 40.68 MHz |
| Forward power | 1000 W |
| Reflected power | <1 W |
| Argon gas flow rates | |
| Plasma gas | 12 L min−1 |
| Nebulizer gas | 2.5 L min−1 |
| Sheath gas | 0.2 L min−1 |
| Acquisition parameters | |
| Sample introduction | Apex Q, ESI, USA |
| Nebuliser | PolyPro ST, polypropylene, ESI |
| Sample uptake | 0.30 mL min−1, pumped |
| Gain | 3 |
| Entrance slit | 20 μm |
| Exit slit | 15 μm |
| Measurement | Peak top, background |
| Integration time | 8 s per measurement point |
| Number of replicates | 3 |
For ICP-MS measurements, a sector field ICP-MS (Element 2, Thermo Fisher, Bremen, Germany) equipped with Ni cones, a cyclonic spray chamber (Tracey, Glass Expansion, West Melbourne Vic, Australia) and a self-aspirating PFA nebulizer (Elemental Scientific, 100 μL min−1 sample uptake rate) were employed. The RF generator was operated at 1150 W, the Ar gas flows were as follows: plasma gas: 16 L min−1; auxiliary gas: 0.8 L min−1; sample gas: 0.9–1.1 L min−1 (daily optimized).
Results and discussion
While no certified reference material for the assessment of the accuracy of Nd analysis in spent fuel is commercially available, it is essential to provide a self-consistent dataset that considers various instrumental techniques. Because the detection of Nd using ICP-OES and ICP-MS is based on different physical principles, comparable Nd results obtained via both analytical techniques and using different suppliers of Nd stock solutions for the preparation of calibration solutions help to support the reliability of the measured Nd concentrations.Analytical figures of merit for ICP-OES
The ten most sensitive Nd wavelengths from the ICP-OES instrument software database were initially evaluated for optimum analytical performance (Table 2).| Wavelength [nm] | Relative intensitya | LODb |
|---|---|---|
| a Normalized to the emission intensity at λ = 529.317 nm. b Limit of detection in μg kg−1 based on the 3 σ criterion. | ||
| 529.317 | 1.0 | 0.8 |
| 395.220 | 1.1 | 0.8 |
| 380.536 | 1.4 | 0.6 |
| 417.732 | 1.5 | 0.9 |
| 378.425 | 1.6 | 0.8 |
| 395.115 | 1.7 | 0.6 |
| 410.946 | 2.1 | 0.5 |
| 406.109 | 2.2 | 0.4 |
| 401.225 | 2.4 | 0.3 |
| 430.358 | 4.8 | 0.3 |
After optimizing the detector voltage for each emission wavelength, aspirating a 100 μg kg−1 Nd standard solution and employing the auto-attenuate function of the instrument software, individual calibration curves have been established. Correlation factors of regression lines of signal intensity versus Nd concentration were always higher than 0.999; in the case of the four most sensitive emission lines the correlation was always better than 0.9999 (Table 2). Typical Nd calibration curves are shown in Fig. 1 for three selected wavelengths. The relative signal intensities of the ten investigated Nd emission lines indicate a ∼5-fold spread in sensitivity at most (Table 2). Unsurprisingly, the three most intensive emission lines at λ = 401.225 nm, λ = 406.109 nm, and λ = 430.358 nm highlighted in Table 2 have also been employed for Nd analysis in the past.5–10 It is interesting to note, though, that the majority of the published studies5,7–10 employed the most intensive emission lines at λ = 430.358 nm while the signal at λ = 401.225 nm provides the identical detection limit (LOD) for Nd (see below for details).
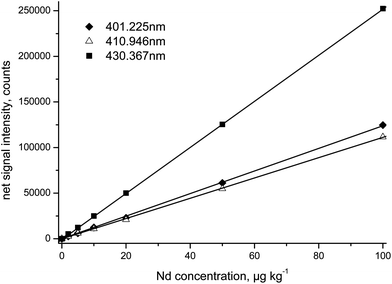 | ||
| Fig. 1 Calibration curves (0, 2, 5, 10, 20, 50, 100 μg kg−1) for Nd established at three emission wavelengths. Please note that replicate ICP-OES measurements generally give relative standard deviations of <1% for individual calibration points. | ||
In addition, Table 2 summarizes the LOD for the ten investigated emission wavelengths that were calculated by considering 17 blank measurements and the 3 σ criterion. These data clearly show the tendency of decreasing LOD with increasing signal intensity, thus indicating the impact of counting statistics on the LOD. Besides counting statistics, however, also the stability of the spectral background at the left and right side of the Nd signal influences the achievable LOD. This fact explains, for example, the ∼30% lower LOD at λ = 380.536 nm compared to that at λ = 430.358 nm, even though the signal intensity is slightly higher at the latter wavelength.
It is interesting to note, however, that all LODs established here are significantly lower than previously reported values, even those LODs calculated from the least sensitive emission lines reported in Table 2. Premadas and Srivastava, for example, reported an instrumental ICP-OES LOD for Nd as high as 45 μg L−1 assessed at λ = 430.358 nm in a study dealing with uranium hydrometallurgical products.9 Using the same ICP-OES model as employed in the current study and a conventional pneumatic nebulizer, a LOD of 26.1 μg L−1 for λ = 430.358 nm was reported in a recent study focussing on the determination of trace impurities in nuclear grade uranium oxide.5 In contrast to this earlier investigation,5 the LOD obtained at this emission line in the present study using a low flow nebulizer and a desolvating sample introduction system is almost two orders of magnitude lower. In their ICP-OES analysis of Nd in simulated complex nuclear waste solutions, Huff and Horwitz11 reported a LOD of 16 μg L−1 at λ = 406.109 nm. Employing the same Nd emission line, another more recent study on the ICP-OES determination of trace elements in basil powder came up with a LOD of 4.8 μg kg−1.7
Using an ultrasonic nebulizer (USN) and the Nd wavelength at λ = 430.358 nm, Navarro and co-workers8 reported a LOD of 4.1 μg kg−1. While this value is still almost an order of magnitude higher than our LOD at λ = 406.109 nm, this approach has another distinct drawback when analysing radioactive samples: the extensive sample uptake rate of 3 mL min−1 required for optimum USN performance in that investigation8 is ∼10 times higher than that in the present study. Compared to our experimental set-up the increased sample volume needed for Nd analysis in the study of Navarro et al.8 gives rise to an approximately 10 times higher radiation dose to the operator of the ICP-OES instrument. At the same time, using the USN, the volume of radioactive waste is largely increased. However, the amount of this radioactive waste should be limited to the absolute minimum because it represents a radiation dose to the analyst and, in addition, is exceedingly expensive to dispose of.
The Apex high efficiency sample introduction system used in this study, in turn, allows for optimum performance at low sample uptake rates (0.3 mL min−1). As such, both the radiation dose and the amount of radioactive waste are kept as low as possible while the sensitivity is enhanced largely. Taken together, the Apex is the preferred sample introduction system for the analysis of radioactive samples combining the advantages of high sensitivity, low sample consumption and the production of only small amounts of radioactive waste.
Selection of optimum Nd wavelengths for spent fuel ICP-OES analysis
When comparing the Nd emission spectra of both the calibration standards (representing a natural Nd isotopic composition) and the spent fuel (fission Nd isotopic composition, decay product as a consequence of Pu and U fissile isotopes), it was evident that out of the 10 investigated Nd wavelengths listed in Table 2, the maxima of the emission signals at λ = 380.536 nm and λ = 395.115 nm were shifted towards lower wavelengths in the spent fuel solutions by about two and three picometers, respectively (Fig. 2A and B). A closer look at these spectra revealed that this shift, however, is not caused by the well known isotopic shift of emission lines that is most pronounced for light (e.g. Li (ref. 12)) and heavy elements (e.g. U (ref. 13 and 14)). In contrast, the ICP-OES signal centred around λ = 380.534 nm reflects a spectral interference from Th that originates from the sample matrix (Th,Pu)O2 and overlaps with the Nd signal at λ = 380.536 nm (Fig. 2A). While the actual Nd concentration in the dissolved fuel solution is around 18 μg kg−1, visual inspection of the ICP-OES spectrum indicates a Nd concentration of more than 50 μg kg−1, which is pretended by the interfering emission signal of Th. In other words, the presence of substantial amounts of Th in the analyte solution yielded an emission signal that overlapped with the Nd ICP-OES signal resulting in an overestimation of the actual Nd concentration in the nuclear fuel sample. Besides, the ICP-OES signal at λ = 380.536 nm suffers from a general bad spectral background and a distinct shoulder that is possibly related to Nd (Fig. 2A). A similar scenario holds true for the Nd emission line at λ = 395.115 nm whose reliable quantification is hampered by a Nd unspecific peak at the right side of the Nd signal as well as by the occurrence of a predominant Th signal at λ = 395.112 nm (Fig. 2B).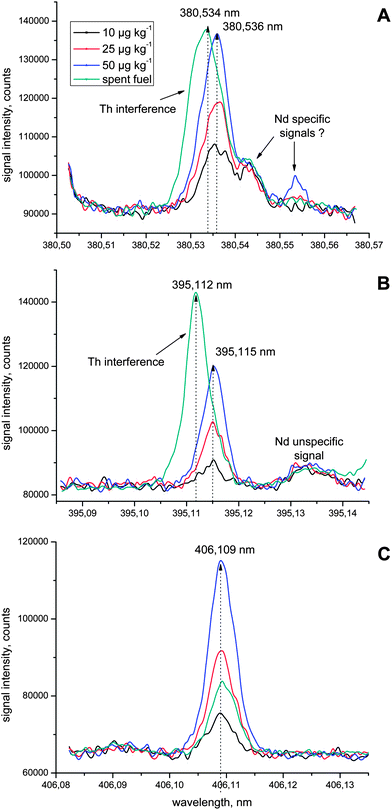 | ||
| Fig. 2 Smoothed high resolution ICP-OES spectra of the analysis of Nd standard solutions containing 10, 25 and 50 μg kg−1 each as well as a spent nuclear fuel at (A) λ = 380.536 nm and (B) λ = 395.115 nm highlighting spectral interferences that hamper the reliable determination of Nd (see text for details). Note that no such spectral interference is evident at the other investigated Nd emission lines such as (C) λ = 406.109 nm, for example, which is highlighted for comparison. | ||
Taken together, the use of both above mentioned Nd wavelengths would largely falsify the obtained Nd concentration in the case of a (Th,Pu)O2 fuel (Fig. 2A and B). Therefore, both Nd wavelengths were not considered any further, also because more sensitive and adequate Nd emission lines were identified (see below).
All other eight investigated Nd wavelengths (Table 2) did not reveal a measurable spectral overlap with emission signals of other elements during the ICP-OES analysis of the (Th,Pu)O2 fuel. However, after considering the sensitivity of these remaining emission lines, the stability of the spectral background around the Nd emission signals as well as the occurrence of potential spectral interferences, two other wavelengths (λ =395.220 nm and λ = 529.317 nm) were excluded from further in-depth investigation.
Quantification of Nd using ICP-OES
At an initial stage of this study, three aliquots of the spent fuel were analyzed on four individual days using newly established calibration curves each time. To this end, the six Nd emission wavelengths λ = 378.425 nm, λ = 401.225 nm, λ = 406.109 nm, λ = 410.946 nm, λ = 417.732 nm, and λ = 430.358 nm remaining after the previous evaluation stage were tested in more detail. These experiments clearly indicated that three (λ =378.425 nm, λ = 417.732 nm, and λ = 430.358 nm) out of these six wavelengths gave systematically lower and/or less reproducible Nd concentrations compared to the results obtained considering the emission lines at λ = 401.225 nm, λ = 406.109 nm, and λ = 410.946 nm (Fig. 3). In fact, the overall reproducibility of the Nd concentrations (3 sample aliquots × 3 wavelengths × 4 replicate measurements = 36 individual Nd concentration values) assessed via the latter three wavelengths was better than 1%. While the precision of the Nd measurements at these three wavelengths was certainly satisfactory, no statement on the accuracy of these results was possible at this stage. As a consequence and because no matrix matched radioactive reference material with a certified Nd concentration is available, another sample aliquot of this nuclear fuel (having a slightly different dilution factor compared to the previous aliquot) was prepared in the institute's chemical hot cells with the aim of verifying the accuracy of the Nd results by repeating the above measurement series, but this time involving both external calibration and standard addition.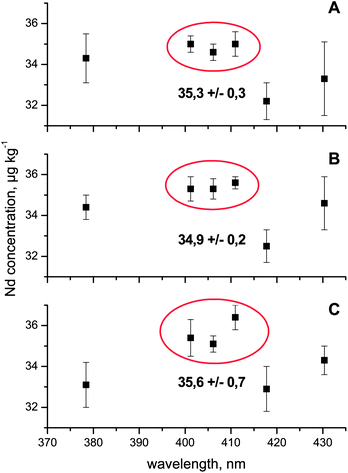 | ||
| Fig. 3 Neodymium concentration (μg kg−1) in three individual aliquots (A–C) of a dissolved spent fuel assessed at the six most promising emission wavelengths. Each individual data point represents the average and standard deviation of four independent analyses carried out on four different days. The mean and standard deviation displayed in each panel considers the mean Nd concentration obtained at λ = 401.225 nm, λ = 406.109 nm, and λ = 410.946 nm (red circle). See the text for further details. | ||
For this accuracy assessment, the four most sensitive emission wavelengths of Nd, listed in Table 2, were considered. The results of this experiment, summarized in Table 3, clearly revealed that the average concentration values calculated from both external calibration and standard addition were matching well within their standard deviations. No systematic offset was identified for any of the investigated four wavelengths. In this respect, the applied standard addition approach largely confirmed the accuracy of the Nd results obtained by external calibration. As such, each of the four above mentioned emission lines can be potentially used for the quantification of Nd without requiring the use of the laborious standard addition approach.
| Wavelength, nm | 401.225 | 406.109 | 410.946 | 430.358 |
|---|---|---|---|---|
| a Six individual aliquots measured on three different days. b Three individual aliquots measured on three different days. | ||||
| External calibration (N = 18)a | 32.4 ± 0.7 | 32.2 ± 0.9 | 32.0 ± 1.3 | 32.6 ± 1.5 |
| Standard addition (N = 9)b | 31.9 ± 0.3 | 31.7 ± 1.3 | 32.0 ± 0.8 | 32.0 ± 1.2 |
Quantification of Nd in a nuclear fuel using sector field ICP-MS
The determination of several Nd isotopes in a dissolved spent nuclear fuel sample suffers from isobaric interferences that cannot be resolved spectroscopically with sector field ICP-MS. For example, 142Nd is interfered by 142Ce, 148Nd by 148Sm, and 150Nd by 150Sm. In addition, the small isobaric 144Ce contribution, known from in-house γ-spectrometry results, to the 144Nd ICP-MS signal needs to be considered to obtain reliable 144Nd data. To overcome the analytical problems described above, normally a chromatographic separation of the other lanthanides from the Nd is performed.4In order to determine accurately the total Nd concentration using ICP-MS without any elemental separation technique via external calibration, only the interference-free 143Nd, 144Nd (a small 144Ce correction to be applied), 145Nd, and 146Nd nuclides have been considered. This approach would not work, however, if the isotopic Nd composition of the investigated sample would not be known from previous in-house ion chromatography (IC)-ICP-MS measurements4 and be considered appropriate for the calculation of the Nd concentration.
In this context it is important to note that the Nd isotopic composition in a nuclear fuel varies distinctly from the natural Nd isotopic abundances reported in the literature.15 According to the measured isotopic composition using IC-ICP-MS, the sum of the four Nd isotopes 143Nd, 144Nd, 145Nd, and 146Nd in the dissolved fuel sample measured in the present study represents ∼84.7 wt% of the total Nd. In contrast, the natural abundance of these four Nd isotopes amounts to only ∼61.5 wt%. The reason for this difference is the fact that fission of Pu and U produces fission products, such as various Nd isotopes, according to the fission yields of the fissionable actinide. Fig. 4 highlights the different isotopic compositions of natural Nd compared to a typical spent fuel sample containing fission Nd.
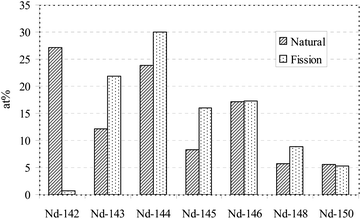 | ||
| Fig. 4 Isotopic composition (at%) of natural Nd compared to fission Nd. Note that the fission Nd isotopic composition depends on many parameters such as, e.g., the type of nuclear fuel, irradiation history, burn-up and cooling time. | ||
During irradiation in a nuclear reactor the Nd isotopic composition in the nuclear fuel is not constant but changes with the composition of fissionable isotopes in the fuel. For example, in this (Th,Pu)O2 fuel, the initial fissions come predominantly from 239Pu resulting in a Nd isotopic composition according to the fission yields of 239Pu. With irradiation time, more and more 233U is being produced and the Nd isotopic composition (slightly) changes as the fission yields of 233U are (slightly) different. After irradiation and some cooling time, the Nd isotopic composition only changes due to the decay of longer-living radioactive predecessors and that is specifically the case for mass 144 where radioactive 144Ce decays to 144Nd (via144Pr). Results for the Nd isotopic composition of previous IC-ICP-MS analyses have been corrected for the 144Ce decay between the previous analysis date and the actual date of analysis of the Nd concentration.
The ICP-MS measurements have been performed on three independent dilutions of the dissolved fuel solution. To all blank solutions, standard solutions and sample dilutions for ICP-MS measurements, Ho was added as an internal standard at a concentration level of 1 μg kg−1. Three replicate measurements of each standard solution have been conducted before and after the samples giving a total of 6 blank and 24 standard solution measurements. Each sample has also been measured three times. It should be noted that due to the different isotopic compositions of standards versus samples, the calibration is based on the isotopic concentration in the standard solutions, presuming a natural isotopic composition of Nd in the commercial Nd standard solution. The average Nd concentration of the nine measurements (three repeats of three independent dilutions) has been calculated for the four main ‘fission Nd’ isotopes. The results of the four isotopes have been summed up and knowing the isotopic composition, the total elemental concentration could be calculated. For comparison with the ICP-OES results, the concentrations have been calculated back to the concentration in the intermediate dilution of the dissolved nuclear fuel. The reported uncertainties include the standard deviation on the mean of the 9 results, and the uncertainties on the calibration, the isotopic composition, the dilutions and the 144Ce correction. The whole procedure, including calibration, has been repeated twice, leading to the results as reported in Table 4. It can be noted as well that the isotopic results of the four Nd isotopes of interest confirmed the relative isotopic composition as measured previously using IC-ICP-MS, within the experimental uncertainties (data not shown).
Comparison of ICP-OES and ICP-MS
In order to test the developed methodologies, a dissolved spent fuel sample was analysed for its Nd concentration using both ICP-OES and sector field ICP-MS. The ICP-OES result of 664 ± 24 μg kg−1 (Table 5) compared well to the corresponding ICP-MS concentration of 688 ± 18 μg kg−1 (Table 4). The mean Nd concentrations assessed via both instrumental approaches differed by only approximately 3.5%. Considering the uncertainty of the certified Nd concentration of the individual stock standard solutions used for calibrating the analytical instruments as well as all difficulties associated with the experimental work in hot cells and glove boxes, both datasets are in excellent agreement with each other. Therefore, the methodology applied here underpins the reliability of the obtained Nd concentration data. While frequently no certified matrix matched reference materials are available, such analyses using instrumentation with different physical detection principles are especially needed and useful when dealing with radioactive samples.| Wavelength, nm | 401.225 | 406.109 | 410.946 | 430.358 |
|---|---|---|---|---|
| a Six individual aliquots measured on three different days. b Calculated as the grand average and standard deviation of the averages obtained at the four individual wavelengths. | ||||
| (N = 18)a | 666 ± 16 | 661 ± 21 | 658 ± 26 | 670 ± 31 |
| Final ICP-OES resultb | 664 ± 24 | |||
Acknowledgements
We gratefully acknowledge the support of M. Cardinale and B. Lynch of ITU in the preparation of the spent fuel solutions in the hot cell facilities of ITU and L. Aldave de las Heras for measurement of the isotopic composition of fission Nd.References
- International Atomic Energy Agency (IAEA), Thorium Fuel Cycle — Potential Benefits and Challenges, IAEA, IAEA-TECDOC-1450, Vienna, Austria, May 2005, ISBN 92–0–103405–9, ISSN 1011–4289 Search PubMed.
- K. Ikeda and H. Sekimoto, Prog. Nucl. Energy, 2011, 53, 902–908 CrossRef CAS.
- ASTM International, Standard Test Method for Atom Percent fission in Uranium and Plutonium Fuel (Neodymium-148 Method), ASTM, 2005, pp. E321–E396 Search PubMed.
- L. Perna, F. Bocci, L. Aldave de las Heras, J. de Pablo and M. Betti, J. Anal. At. Spectrom., 2002, 17, 1166–1171 RSC.
- K. Satyanarayana and S. Durani, J. Radioanal. Nucl. Chem., 2010, 285(3), 659–665 CrossRef CAS.
- C. H. Lee, M. Y. Suh, K. S. Choi, J. S. Kim, B. C. Song, K. Y. Jee and W. H. Kim, Anal. Chim. Acta, 2001, 428, 133–142 CrossRef CAS.
- M. E. Ghanjaoui, M. L. Cervera, M. El Razi and M. de la Guardia, Food Chem., 2011, 125, 1309–1313 CrossRef CAS.
- M. S. Navarro, H. H. G. J. Ulbrich, S. Andrade and V. A. Janasi, J. Alloys Compd., 2002, 344, 40–45 CrossRef CAS.
- A. Premadas and P. K. Srivastava, J. Radioanal. Nucl. Chem., 2002, 251, 233–239 CrossRef CAS.
- J. A. Gásquez, E. DeLima, R. A. Olsina, L. D. Martinez and M. de la Guardia, Talanta, 2005, 67, 824–828 CrossRef.
- E. A. Huff and E. P. Horwitz, Spectrochim. Acta, Part B, 1985, 40, 279–286 CrossRef.
- M. Krachler, S. Van Winckel, M. Cardinale, B. Lynch and T. Murakami, Microchem. J., 2012, 105, 9–14 CrossRef CAS.
- M. Krachler and P. Carbol, J. Anal. At. Spectrom., 2011, 26, 293–299 RSC.
- M. Krachler and D. H. Wegen, J. Anal. At. Spectrom., 2012, 27, 335–339 RSC.
- J. R. De Laeter, J. K. Böhlke, P. De Bièvre, H. Hidaka, H. S. Peiser, K. J. R. Rosman and P. D. P. Taylor, Pure Appl. Chem., 2003, 75, 683–800 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
