Separation of phenol from model oils with quaternary ammonium salts via forming deep eutectic solvents†
Wujie
Guo
a,
Yucui
Hou
b,
Weize
Wu
*a,
Shuhang
Ren
a,
Shidong
Tian
a and
Kenneth N.
Marsh
*c
aState Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing, 100029, China. E-mail: wzwu@mail.buct.edu.cn; Tel: +86 10 64427603
bDepartment of Chemistry, Taiyuan Normal University, Taiyuan, 030031, China
cCentre for Energy, School of Mechanical and Chemical Engineering, The University of Western Australia, Crawley, WA 6009, Australia. E-mail: ken.marsh@uwa.edu.au
First published on 8th November 2012
Abstract
A variety of quaternary ammonium salts have been used to efficiently separate phenols from model oils, which avoids the use of mineral alkali and acids that produce phenol-containing waste water. It was found that some quaternary ammonium salts showed high phenol-removal efficiencies, which could reach as high as 99.9%. The separation mechanism was also discussed.
Phenol, one of the major industrial organic chemicals, is currently used in processes to produce phenolic resins, bisphenol A, caprolactam, alkylphenols, adipic acid, and other products.1 Phenolic compounds are mainly derived from coal liquefaction oil, coal tar and petroleum, and also from biomass via pyrolysis as documented in recent reports.2 These processes provide mixtures containing phenols and oils (called neutral oil compared with phenols). Therefore, it is necessary to separate phenols from oils before further refining.
The present method to separate phenols from oil mixtures is to chemically extract phenols using aqueous alkaline solutions (such as aqueous NaOH) and then to acidify the extract using mineral acids (such as aqueous H2SO4) to recover the phenols. The disadvantages of the method are the use of large amounts of both strong alkalis and acids, and the production of excessive amounts of waste water containing phenols. Therefore, a method to separate phenols from oil mixtures using a non-aqueous method to avoid the above problems would be environmentally advantageous.
In recent years, ionic liquids (ILs) have been successfully applied in a large diversity of separation processes.3 However, there are disadvantages of using them in that they can be expensive and difficult to handle. An alternative is to use deep eutectic solvents (DESs) which are to some extent similar to ionic liquids.4–6 DESs have a eutectic temperature very much lower than would normally be expected and are usually formed from mixtures of organic halide salts with hydrogen-bond donors (HBDs), where a strong hydrogen bond is formed.7 Currently, DESs are being used in research as well as in industry because they are non-toxic, non-reactive with water, and biodegradable.8,9
As early as 2003, Abbott et al. have shown that mixtures of amides with quaternary ammonium salts form low melting point eutectics that have unusual solvent properties.10 A publication by Abbott et al. described DESs based on mixtures of quaternary ammonium salts with a wide variety of other HBD, such as acids, amines and alcohols.8,11 DESs are good media for the deposition of Ag, Cr, Cu, Sn, and Zn metals, which could have potential applications in the electro-plating of metals.12,13 In addition, DESs have been tested as a reaction media for the synthesis of lanthanide–organic frameworks.14
Industrial applications of DESs are very promising. The use of DESs as extraction media for glycerol from biodiesel based on rapeseed and soybeans was disclosed for the first time in 2007 by Abbott et al.11 Hayyan et al.15 reported that a quaternary ammonium salt–glycerol-based DES could be used as a solvent for extracting total glycerol from palm-oil-based biodiesel. Recently, new DESs have been synthesized by the reaction of phosphonium-based salts with different HBDs.16
In our previous work, we demonstrated that choline chloride (ChCl) could form DES with phenol and cresols, which could be used to separate phenols from oils and there were no ChCl detected in the extracted oils, which provides an efficient way to separate phenols from oils with removal efficiencies from 90% to 95%.17 However, ChCl has a relatively low phenol-removal efficiency. More importantly, the effect of the structure of quaternary ammonium salts on the removal efficiency and the mechanism of DES separation remain unclear.
In this work, a variety of quaternary ammonium salts were used to separate phenols from model oils. The model oil was toluene as it is a typical component of coal tar or coal liquefaction oil. Mixtures were prepared by dissolving a known mass of phenol in toluene. The quaternary ammonium salts studied include tetramethylammonium chloride (TMAC), tetramethylammonium bromide (TMAB), tetraethylammonium chloride (TEAC), methyltriethylammonium chloride (MTAC), tetraethylammonium bromide (TEAB), tetrapropylammonium chloride (TPAC), tetrabutylammonium chloride (TBAC), ammonium chloride (NH4Cl), and choline bromide (ChBr). ChCl, which was proven to be successful in separating phenols from the model oils, was used for comparison.
In a typical experiment, taking TEAC as an example, a known mass of TEAC was added to the model oil in a 25 cm3 glass tube. The glass tube was partly immersed in a water bath that was set at the desired temperature and was magnetically stirred. After stirring for a known amount of time, the two phases were allowed to settle for more than 30 min. A small amount of sample from the upper oil phase was taken and analysed by GC (Shimadzu 2014) to determine its composition.
The separation of phenol by NH4Cl, TMAC, TEAC, TPAC, TBAC were carried out at 303.2 K and the results are shown in Fig. 1. It can be seen that the cation of the quaternary ammonium salts has a significant influence on the phenol-removal efficiency. The effect of the cation of the quaternary ammonium salts on the phenol-removal efficiency follows the order: [TEA]+ > [TPA]+ > [TMA]+ > NH4+ = [TBA]+. NH4Cl remained as a solid, which means it cannot absorb phenol from the toluene phase to form a eutectic solvent. This is probably due to the low enthalpy of formation of the eutectic mixture and no hydrogen bond formation between the Cl− ion and the OH group on phenol. When TMAC was added to the model oil, a eutectic phase was observed immediately, the phenol concentration in the oil phase decreased greatly, and the phenol extraction efficiency was 95.7%. TEAC showed a better performance than TMAC, with the phenol extraction efficiency by TEAC being better than 99.9%. TPAC showed a lower phenol-removal efficiency than TEAC, which could be due to steric hindrance of the longer alkyl chain length on the cation leading to weaker hydrogen-bonding interactions. When TBAC was added to the model oil solution, it was completely miscible with the model oil because the increase of the alkyl chain length on the cation enhances the interaction between the salt and oil and the higher miscibility.
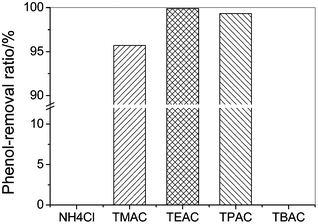 | ||
| Fig. 1 The phenol-removal efficiency of the quaternary ammonium salts from model oil (initial phenol contents, 200.6 g L−1; temperature, 303.2 K; mole ratio of salt to phenol, 1; extraction time, 30 min). | ||
The results show that at a certain linear alkyl chain length on the quaternary ammonium cation, the phenol-removal efficiency reaches a maximum. The reason may be that as the length of the alkyl chain increases so does the volume of the salt, which results in an increase in the distance between the charge centers of the cations and anions, and consequently reduces their interactions. Hence there is an optimum distance between the anion and cation for hydrogen bond formation that results in a high phenol-removal efficiency. NaCl and NH4Cl do not form DESs with phenols, probably because the distance between the cations and anions is too close and the interaction between the cations and anions is too strong for the phenol to form a DES. TMAC, TEAC and TPAC have large cations, and the distance between the cations and anions is sufficient to result in a lower interaction between the cations and anions, sufficient for the phenols to interact with anions to form a DES. TBAC has alkyl chain lengths that are too long, which increases the interaction between toluene and TBAC, so that TBAC was also completely miscible with toluene and phenol.
Fig. 2 shows the effect of symmetry of the quaternary ammonium cations on the phenol-removal efficiency which follows the order: TMAC > ChCl and TEAC > MTAC. The phenol-removal efficiency of salts with symmetric cations is greater than that with asymmetric cations, probably because the symmetric cations see a stiffer intermolecular potential than the asymmetric cations.18 For example ChCl has a lower symmetry than TMAC. In addition ChCl exhibits anisotropic motion, which leads to an uneven distribution of electric charge and a weaker interaction between the cation and anion than TMAC. The results indicate that these weaker interactions are unfavourable for its enhanced phenol-removal efficiency due to the low entropy changes arising from forming a DES.
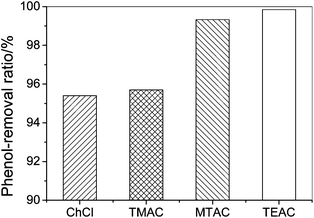 | ||
| Fig. 2 The phenol-removal efficiency by quaternary ammonium salts with asymmetric and symmetric structures from model oil (initial phenol contents, 200.6 g L−1; temperature, 303.2 K; mole ratio of salt to phenol, 1; extraction time, 30 min). | ||
The effect of the anion on the phenol-removal efficiency is shown in Fig. 3. Chloride-based quaternary ammonium salts show greater phenol-removal efficiency than bromine-based quaternary ammonium salts because the chloride ion has larger electronegativity and a stronger interaction with the phenol hydroxyl group than that of bromine ion. For instance, phenol-removal efficiency is 95.4% for ChCl, while it is 90.4% for ChBr.
 | ||
| Fig. 3 The phenol-removal efficiency by quaternary ammonium salts with different anions from model oil (initial phenol contents, 200.6 g L−1; temperature, 303.2 K; mole ratio of salt to phenol, 1; extraction time, 30 min). | ||
Interestingly, there was no ammonium salt found in the oil phase determined by AgNO3 titration. For a given quaternary ammonium salt, the energy of the hydrogen bond will be related to the anion–HBD interaction and hence the entropy change will be a measure of the phenol-removal efficiency. In addition, the charge distribution on the ammonium salts cations is relatively diffuse, which results in weak interactions between the cations and anions.19 Thus, the coordinating role of the cations and anions of quaternary ammonium salts is the main factor that influences the phenol-removal efficiency.
Remarkably, the chloride-based quaternary ammonium salts also play a significant role in the migration of phenol between the coexisting phases. Since the hydroxyl group in the molecular structure of phenol favours the hydrogen-bonding between the halide anion and phenol, hydrogen-bonding interactions can be expected. Hence, additional information on hydrogen-bonding was investigated. Fourier-transform infrared spectroscopy (FT-IR) is an attractive method to obtain hydrogen bonding information.20 Hydrogen bonding has a considerable influence on the ν-OH stretching vibration in phenols, which is observed at wave numbers between 3200 cm−1 and 3600 cm−1. Consider for example the FT-IR (Fig. 4) for phenol and the DES formed with TMAC. The ν-OH stretching vibration of pure phenol was observed at 3331 cm−1, which shifted to 3218 cm−1 in the DES. Absorption bands in the FT-IR spectra are mainly caused by changes between different vibrational states of bonds in molecules.20 The IR spectra of phenol in the DES undergoes a change in the vibrational states because a portion of the cloud of electrons of the oxygen atom transfers to the hydrogen bonding, resulting in a smaller force constant. Thus, the shift of the ν-OH stretching vibration suggests the existance of hydrogen-bonding between phenol and TMAC when the DES is formed.
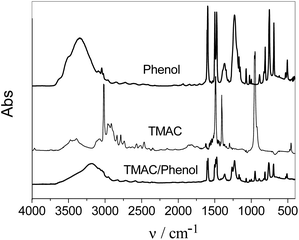 | ||
| Fig. 4 FT-IR spectra of phenol, TMAC, and DES. | ||
Considering the cost of quaternary ammonium salts, it is preferable to use less expensive quaternary ammonium salts in any industrial operation. Hence, this study investigated the effect of the salt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phenol mole ratio on phenol separation. The extraction ratios tested were 0.2
phenol mole ratio on phenol separation. The extraction ratios tested were 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.4
1, 0.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.6
1, 0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.8
1, 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (mol/mol) salt
1 (mol/mol) salt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phenol. Each extraction process took place at 303.2 K under atmospheric pressure for 30 min; the results are shown in Fig. S1.† The mole ratio of salts to phenol affects the phenol separation efficiency. When TEAC with amounts >0.8 mole ratio of ammonium salt to phenol was added to toluene and phenol mixtures, the phenol content in the toluene rich phase did not decrease further, and a homogeneous transparent liquid was observed at the bottom of the glass tube.
phenol. Each extraction process took place at 303.2 K under atmospheric pressure for 30 min; the results are shown in Fig. S1.† The mole ratio of salts to phenol affects the phenol separation efficiency. When TEAC with amounts >0.8 mole ratio of ammonium salt to phenol was added to toluene and phenol mixtures, the phenol content in the toluene rich phase did not decrease further, and a homogeneous transparent liquid was observed at the bottom of the glass tube.
Fig. S2† shows the effect of separation time on the phenol-removal efficiency from toluene. When a 0.8 mole ratio of TEAC to phenol was added to toluene with a phenol mixture of 200.6 g L−1, the phenol content was rapidly reduced with time, to 0.6 g L−1 in toluene. We conclude that TEAC forms a stable eutectic mixture with phenol that can be used for the removal of phenol from the model oil (toluene). The equilibrium time for a high removal efficiency of 99.8% is less than 3 min. In fact, the time taken to reach a complete equilibrium composition of the eutectic was less than 30 min. This short equilibrium time means that there is a rapid mass transfer for phenol. In the following work, the eutectic time was fixed at 30 min, a contact time more than sufficient to establish equilibrium.
With the above criteria the effect of temperature on the phenol-removal efficiency from toluene was investigated at temperatures from 293.2 K to 313.2 K with the results shown in Fig. S3.† As the temperature was increased, the phenol-removal efficiencies were found to be constant at 99.9%. These results indicate that the separation is not sensitive to temperature.
To recycle the ammonium salts, it has to be recovered from the eutectic mixture. In this work, dibutyl ether (DBE) and diethyl ether (DEE) were efficient solvents to recover TMAC from the eutectic mixture. The typical recovery process for DBE is shown as follows. 2.34 g of TMAC was added to 25 cm3 of toluene solution with an initial phenol content of 200.6 g L−1 at 303.2 K, where the mole ratio of TMAC to phenol was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The mixture was magnetically stirred for 30 min, and settled for 30 min. Then 25 cm3 of DBE was added as an anti-solvent to the DES, and the mixture was shaken for separation. During the process, TMAC was re-crystallized quickly and precipitated at the bottom of the separator glass tube. The phenol content in the DBE phase was measured by GC. The re-crystallized TMAC was separated, and dried under vacuum at 373.2 K for at least 2 h to remove the small amount of dissolved toluene and DBE until the TMAC mass was constant. Then, the regenerated TMAC was identified by FT-IR and 1H NMR (see Fig. S4 and S5†) and was reused for the next cycle. The phenol removal efficiency was 95.7% for the original sample of TMAC and those using regenerated TMAC were sequentially 95.5%, 95.4% and 95.4%. The mass of original TMAC was 2.34 g, and the masses of regenerated TMAC were sequentially 2.37 g, 2.41 g, 2.35 g and 2.36 g which were the same mass as the original within experimental uncertainty. The results show that TMAC can be reused and the phenol-removal performance remains constant after four cycles. Importantly, the amount of phenol in the DBE phase was equal to the reduction in the amount of phenol in the oil, which indicates that DBE could extract phenol from TMAC-based DES completely. However, no TEAC was re-crystallized from the TEAC-based DES using DBE as anti-solvent, and DEE showed a better performance than DBE. 21% of the phenol could be recovered after exhaustive extraction from TEAC-based DES using DEE (see Fig. S6†). While still unoptimised, the result shows that an anti-solvent could be added to recover TEAC.
1. The mixture was magnetically stirred for 30 min, and settled for 30 min. Then 25 cm3 of DBE was added as an anti-solvent to the DES, and the mixture was shaken for separation. During the process, TMAC was re-crystallized quickly and precipitated at the bottom of the separator glass tube. The phenol content in the DBE phase was measured by GC. The re-crystallized TMAC was separated, and dried under vacuum at 373.2 K for at least 2 h to remove the small amount of dissolved toluene and DBE until the TMAC mass was constant. Then, the regenerated TMAC was identified by FT-IR and 1H NMR (see Fig. S4 and S5†) and was reused for the next cycle. The phenol removal efficiency was 95.7% for the original sample of TMAC and those using regenerated TMAC were sequentially 95.5%, 95.4% and 95.4%. The mass of original TMAC was 2.34 g, and the masses of regenerated TMAC were sequentially 2.37 g, 2.41 g, 2.35 g and 2.36 g which were the same mass as the original within experimental uncertainty. The results show that TMAC can be reused and the phenol-removal performance remains constant after four cycles. Importantly, the amount of phenol in the DBE phase was equal to the reduction in the amount of phenol in the oil, which indicates that DBE could extract phenol from TMAC-based DES completely. However, no TEAC was re-crystallized from the TEAC-based DES using DBE as anti-solvent, and DEE showed a better performance than DBE. 21% of the phenol could be recovered after exhaustive extraction from TEAC-based DES using DEE (see Fig. S6†). While still unoptimised, the result shows that an anti-solvent could be added to recover TEAC.
In summary, a variety of quaternary ammonium salts, i.e., TMAC, TMAB, TEAC, TEAB, TPAC, TBAC, ChCl, ChBr, were successful in separating phenols from model oils. The results show that quaternary ammonium salts that are composed of cations with appropriate chain lengths as well as high symmetry and anions with higher electronegativity were conducive to separate phenols from model oil mixtures. A maximum removal efficiency of phenol was attained by TEAC at a mole ratio of 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (TEAC
1 (TEAC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phenol), with 99.9% removal. Moreover, the extraction equilibrium was very fast and the extraction separation efficiency was not sensitive to temperature. Therefore, it can be effectively performed at room temperature. Significantly, TMAC can be easily recovered by DBE or DEE as an anti-solvent and reused without mass loss and reduction in separation efficiency. While still unoptimised, the process shows that an anti-solvent could be used to recover the quaternary ammonium salts. In contrast with the traditional methods to separate phenol compounds from oil, this proposed method involving the formation of eutectic solvents with ammonium salts avoids the use of alkalis and acids and the production of phenol containing waste water.
phenol), with 99.9% removal. Moreover, the extraction equilibrium was very fast and the extraction separation efficiency was not sensitive to temperature. Therefore, it can be effectively performed at room temperature. Significantly, TMAC can be easily recovered by DBE or DEE as an anti-solvent and reused without mass loss and reduction in separation efficiency. While still unoptimised, the process shows that an anti-solvent could be used to recover the quaternary ammonium salts. In contrast with the traditional methods to separate phenol compounds from oil, this proposed method involving the formation of eutectic solvents with ammonium salts avoids the use of alkalis and acids and the production of phenol containing waste water.
We thank Professors Zhenyu Liu and Qingya Liu for their help. This work is financially supported by Shanxi Scholarship Council of China (2011-086) and the National Basic Research Program of China (2011CB201303).
Notes and references
- H. H. Schobert and C. Song, Fuel, 2002, 81, 15–32 CrossRef CAS.
- C. Song, L. Hou, A. K. Saini, P. G. Hatcher and H. H. Schobert, Fuel Process. Technol., 1993, 34, 249–276 CrossRef CAS.
- A. Berthod, M. J. Ruiz-Angel and S. Carda-Broch, J. Chromatogr., A, 2008, 1184, 6–18 CrossRef CAS.
- A. P. Abbott, G. Capper, D. L. Davies and R. K. Rasheed, Chem.–Eur. J., 2004, 10, 3769–3774 CrossRef CAS.
- A. P. Abbott, G. Capper, D. L. Davies, H. L. Munro, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2001, 2010–2011 RSC.
- A. P. Abbott, G. Capper, D. L. Davies and R. Rasheed, Inorg. Chem., 2004, 43, 3447–3452 CrossRef CAS.
- K. Shahbaz, F. S. Mjalli, M. A. Hashim and I. M. AlNashef, Energy Fuels, 2011, 25, 2671–2678 CrossRef CAS.
- A. P. Abbott, D. Boothby, G. Capper, D. L. Davies and R. K. Rasheed, J. Am. Chem. Soc., 2004, 126, 9142–9147 CrossRef CAS.
- F. H. Hurley, J. Thomas and P. WIer, J. Electrochem. Soc., 1951, 98, 203–206 CrossRef CAS.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 70–71 RSC.
- A. P. Abbott, P. M. Cullis, M. J. Gibson, R. C. Harris and E. Raven, Green Chem., 2007, 9, 868–872 RSC.
- A. P. Abbott, J. Griffith, S. Nandhra, C. O'Connor, S. Postlethwaite, K. S. Ryder and E. L. Smith, Surf. Coat. Technol., 2008, 202, 2033–2039 CrossRef CAS.
- A. P. Abbott, G. Capper, K. J. McKenzie and K. S. Ryder, J. Electroanal. Chem., 2007, 599, 288–294 CrossRef CAS.
- F. Himeur, I. Stein, D. S. Wragg, A. M. Z. Slawin, P. Lightfoot and R. E. Morris, Solid State Sci., 2010, 12, 418–421 CrossRef CAS.
- M. Hayyan, F. S. Mjalli, M. A. Hashim and I. M. AlNashef, Fuel Process. Technol., 2010, 91, 116–120 CrossRef CAS.
- M. A. Kareem, F. S. Mjall, M. A. Hashim and I. M. AlNashef, J. Chem. Eng. Data, 2010, 55, 4632–4637 CrossRef CAS.
- K. Pang, Y. C. Hou, W. Z. Wu, W. J. Guo, W. Peng and K. N. Marsh, Green Chem., 2012, 14, 2398–2401 RSC.
- D. Xiao, L. G. Hines Jr., S. Li, R. A. Bartsch and E. L. Quitevis, J. Phys. Chem. B, 2009, 113, 6426–6433 CrossRef CAS.
- J. Golding, N. Hamid, D. R. MacFarlane, M. Forsyth, C. Forsyth, C. Collins and J. Huang, Chem. Mater., 2001, 13, 558–564 CrossRef CAS.
- N. Asprion, H. Hasse and G. Maurer, Fluid Phase Equilib., 2001, 186, 1–25 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Spectroscopic data and further details. See DOI: 10.1039/c2gc36602a |
| This journal is © The Royal Society of Chemistry 2013 |
