Microalgae as human food: chemical and nutritional characteristics of the thermo-acidophilic microalga Galdieria sulphuraria
Giulia Grazianib, Simona Schiavoc, Maria Adalgisa Nicolaib, Silvia Buonoa, Vincenzo Fogliano*a, Gabriele Pintoac and Antonino Pollioac
aCRIAcq, University of Naples Federico II, Parco Gussone Ed 77, I-80055 Portici, Italy
bDepartment of Food Science University of Naples Federico II, Parco Gussone Ed 77, I-80055 Portici, Italy
cDepartment of Plant Biology University of Naples Federico II, Parco Gussone Ed 77, I-80055 Portici, Italy
First published on 11th October 2012
Abstract
The use of microalgae as a food source is still poorly developed because of the technical difficulties related to their cultivation and the limited knowledge about their chemical composition and nutritional value. The unicellular red microalga Galdieria sulphuraria has a very high daily productivity and its cultivation under acidic conditions avoided any bacterial contamination. G. sulphuraria can be cultured under autotrophic and heterotrophic conditions: in this study a screening of 43 strains showed that in the latter case a duplication of biomass production was obtained. The proximate composition (protein, carbohydrates, fiber and lipids), the micronutrient content (carotenoids, phycobiliproteins, chlorophylls and vitamins) together with the antioxidant activity of the biomass produced by a selected strain of G. sulphuraria under both cultivation conditions were determined. Results showed that the material is rich in proteins (26–32%) and polysaccharides (63–69%) and poor in lipids. Under heterotrophic cultivation conditions, the lipid moiety mainly contained monounsaturated fatty acids. Among micronutrients, some B group vitamins are present, beta-carotene is the main carotenoid and phycobiliproteins are present under both cultivating conditions. G. sulphuraria proteins are strictly associated with polysaccharide components and therefore not digestible. In the second part of the work, an extraction protocol using Viscozyme L, a commercial enzymatic preparation containing a mixture of polysaccharidases, was developed which made G. sulphuraria proteins a good substrate for human gastrointestinal enzymes. All in all, the data suggested that G. sulphuraria biomass has a potential use as food ingredients both for protein-rich or insoluble dietary fibre-rich applications. The low concentration of lipids and the absence of green color make this microalgae source particularly useful for the addition to many food preparations.
Introduction
Low pH environments derived from the oxidation of sulphur compounds are diffused worldwide. They are often associated with secondary volcanism and, in this case, their temperatures range from 50 to 80 °C.1 In these very selective environments, the dominant photosynthetic eukaryotes belong to the class Cyanidiophyceae,2 a class encompassing three genera: Cyanidium, Galdieria and Cyanidioschyzon.3Galdieria is the only genus of this class able to grow heterotrophically, using numerous carbon sources.4 It has been hypothesized that the aptitude of growing under heterotrophic conditions is related to the ability of Galdieria to thrive also in habitats where light is absent.5 The trophic flexibility of Galdieria makes this microalga a promising candidate for the exploitation of heterotrophic mass cultivations.Large-scale microalgal cultures are presently used in fish feeding and production of fine chemicals, whereas the production of biodiesel from microalgae is still in its infancy.6,7 The use of microalgae as a food source for humans and animals has been increasing since the early 1950's.8,9
Microalgae can represent a valuable source of vitamins and fatty acids. Because of the high protein content of some species it might be useful to use them for manufacturing healthy foods both for humans and animals.10
Microalgae are usually photosynthetic organisms, but a few species are able to grow under heterotrophic conditions. This possibility opens a promising field of research, through the use of cheap fermentors instead of expensive photobioreactors, allowing us to obtain high densities of microalgae cells and therefore a high yield.11
In this respect, Galdieria species are of particular interest as it grows at pH values between 1.5 and 2.0, thus preventing bacterial contamination, one of the major problems of large scale microalgae cultures. Heterotrophic cultures of Galdieria sulphuraria contain high levels of phycocianin, a pigment used as a fluorescent marker in diagnostics, and as a dye in food and cosmetics.12 In addition to the production of fine chemicals Galdieria species also have significant potential as a source of protein and other macronutrients. One of the unique characteristics of this alga is the high protein content of the cell wall, as reported in the pioneering work of Bailey and Staehelin,13 who first suggested that microalgae from Galdieria genera could have a nutritional application. The difficulties in introducing microalgae based ingredients in many foods are due to the strong green color and the oxidability of the lipid moiety14 but the peculiar features of G. sulphuraria have the potential to overcome these bottlenecks.
In this study, the biochemical composition of a selected G. sulphuraria strain has been evaluated by comparing the chemical constituents of cultures grown in autotrophy or heterotrophy, on a medium supplemented with 3% glycerol, putting particular emphasis on the potential nutritional value of the alga. The main problem related to the adoption of microalgae proteins as human food is due to their poor digestibility. In this paper, an enzyme-based extraction method is proposed which made protein a good substrate for human digestive enzymes.
Materials and methods
Algal strains and medium
The Galdieria strains investigated belong to the species G. sulphuraria and are maintained in the ACUF collection of the Biological Science Department of University ‘Federico II’, Naples, Italy (http://www.biologiavegetale.unina.it/acuf.html). The strain numbers are indicated in Table 1. A modified Allen Medium15 with (NH4)2SO4 as a nitrogen source was adopted in all the experiments, and the nitrogen content in the medium was fixed at 40 mg L−1. Carbon dioxide was supplemented by sparging filtered air into the medium. In the strains grown under artificial light no organic carbon source was supplemented to the medium. In heterotrophy experiments, organic carbon was supplemented with 3% glycerol. The pH was set at 1.5 and was controlled daily, whereas temperature was maintained at 36 ± 1 °C. The axenic conditions of the cultures were checked by spreading 100 μL samples of each culture on the surface of agar plates containing Bacto Yeast Extract (DIFCO 212 750) or Malt Extract (DIFCO 211 320). Test plates were incubated at 37 °C for 3 days and at 25 °C for 1 week.| G. sulphuraria ACUF strain number | Autotrophy doubling time (hours) | Heterotrophy doubling time (hours) | G. sulphuraria ACUF strain number | Autotrophy doubling time (hours) | Heterotrophy doubling time (hours) |
|---|---|---|---|---|---|
| 077/300 | 70 ± 3.5c | 41 ± 5.5d | 006/326 | 45 ± 7.3a | 26 ± 2.5b |
| 075/301 | 57 ± 3.8b | 55 ± 6.1d | 005/327 | 59 ± 5.1b | 42 ± 3.3d |
| 074/302 | 47 ± 7.6b | 33 ± 4.3c | 004/328 | 45 ± 5.1a | 35 ± 4.0b |
| 073/303 | 48 ± 4.0b | 41 ± 1.8d | 162/330 | 40 ± 1.3a | 58 ± 6.0e |
| 072/304 | 65 ± 6.5c | 31 ± 4.5c | 139/331 | 67 ± 3.0b | 20 ± 1.8a |
| 071/305 | 53 ± 6.1b | 34 ± 2.9c | 140/332 | 74 ± 3.5c | 48 ± 4.9d |
| 070/306 | 50 ± 5.1b | 17 ± 0.3b | 141/333 | 60 ± 4.2b | 18 ± 2.0a |
| 068/307 | 43 ± 1.9b | 41 ± 4.8d | 142/334 | 64 ± 3.9b | 18 ± 0.9a |
| 067/308 | 44 ± 4.2b | 29 ± 3.1c | 134/338 | 75 ± 4.5c | 35 ± 3.9c |
| 064/309 | 39 ± 2.3a | 16 ± 0.5a | 135/339 | 42 ± 3.5a | 20 ± 2.1a |
| 022/312 | 45 ± 2.1b | 21 ± 4.7b | 136/340 | 63 ± 6.8b | 34 ± 2.4c |
| 021/313 | 56 ± 8.5b | 18 ± 2.2b | 137/341 | 53 ± 3.7b | 27 ± 3.0b |
| 018/316 | 119 ± 4.3e | 20 ± 3.0b | 138/342 | 56 ± 2.9b | 22 ± 1.2a |
| 017/317 | 84 ± 8.3d | 27 ± 3.1c | 214/343 | 61 ± 7.4b | 52 ± 5.5e |
| 016/318 | 118 ± 8.1e | 82 ± 6.5e | 101/345 | 130 ± 1.8f | 95 ± 8.1f |
| 014/319 | 65 ± 8.5c | 43 ± 2.8d | 216/346 | 78 ± 6.2c | 47 ± 3.9d |
| 013/320 | 115 ± 9.7e | 95 ± 7.7e | 192/347 | 95 ± 8.0e | 31 ± 0.5b |
| 011/322 | 69 ± 7.8c | 19 ± 0.7b | 166/348 | 74 ± 4.4c | 29 ± 1.3b |
| 010/323 | 48 ± 5.5b | 30 ± 3.9c | 231/349 | 56 ± 4.9b | 38 ± 3.7c |
| 009/324 | 52 ± 8.1b | 35 ± 2.4c | 080/350 | 64 ± 6.6b | 33 ± 1.2c |
| 007/325 | 55 ± 3.2b | 41 ± 4.9d | 079/351 | 67 ± 3.7b | 59 ± 6.3e |
| 078/352 | 67 ± 3.0b | 47 ± 5.5d |
Selection of the strain for mass culture
Tests to assess strain doubling times in autotrophy and heterotrophy were performed in batch cultures carried out in 100 mL Erlenmeyer flasks, containing 50 mL of Allen medium inoculated with samples from agar slants of each strain. Batch culture tests lasted 9 days, and the initial algal concentration was 5 × 104 cells per mL.Typically, the flasks were placed on a Plexiglas shaking apparatus under continuous irradiance (150 μE m−2 s−1) provided by daylight fluorescent Philips lamps (TLD 30W/55). In heterotrophy experiments, each flask was wrapped with an aluminum foil. Growth experiments were carried out in triplicate and repeated two times.
The cultures were sampled daily and the growth of the cultures was followed by measuring their optical density at 550 nm with a Secomam250 spectrophotometer. Doubling times were calculated according to the formula: K = log2 (N1 − N0)/ΔT, in which K is doublings per day, and N1 and N0 denote the population size at the beginning and the end of the time interval. At the beginning and end of the tests, the ammonium concentration in the culture medium was measured using an ammonium selective electrode (Mettler ion meter SG8), and the dissolved oxygen with a HI 9143 Dissolved Oxygen Meter (Hanna Instruments).
Operating conditions for autotrophic and heterotrophic mass cultures
For autotrophic and heterotrophic mass cultures, a gradual increase of the concentration of the selected G. sulphuraria strain 064/309 was performed to reach the required volumes. Dense algal cultures (0.25 g L−1 of dry weight) were transferred from 100 mL flasks to 500 mL Erlenmeyer flasks containing 250 mL of Allen Medium. The flasks were placed in a Climatic Chamber, under continuous illumination of 100 μE m−2 s−1 and mixing provided by air bubble sparging, until microalgae concentration reached a value of about 0.5 g L−1 of dry weight.Mass cultures were carried out according to the procedures described elsewhere in detail.16 In brief, glass cylindrical bioreactors having 5 L of internal volume were inoculated with the selected culture from the 500 mL Erlenmeyer flasks. In the heterotrophy experiments, the bioreactors were covered with aluminum foils. The working volume was set at 4 L. Air was sparged at the photobioreactor bottom by means of a porous ceramic diffuser, at a volumetric flow rate of 40 NL h−1. Filters of 0.2 μm were used to sterilize air flow inlet and outlet. The bioreactors were housed in a thermostated chamber equipped with the same kind of lamps as previously indicated. In the autotrophic cultures, the light irradiance (IL) was set at 150 μE m−2 s−1. A concentrated Allen medium (2×) was supplemented to the culture two times a week during the test to restore the initial nitrogen concentration. The nitrogen integration did not dilute the broth since the added liquid volume balanced the periodic culture sampling. In the heterotrophy experiments, the concentrated medium added to bioreactors contained also 3% glycerol.
The experiments under the above described fed-batch conditions lasted 30 days. Cell dry weight determination was made with duplicate samples of cultures. Cells were centrifuged in an ALC pK121centrifuge at 4000 rpm for 10 minutes, and washed one time with a 0.5 NaCl solution and two times with distilled water, to remove culture medium constituents. Then, the algae were collected on pre-weighted fiber glass filters (Whatman GF/A), dried at 105 °C for 24 hours, and weighted.
Characterization of lipids
Lipids were extracted from algal biomass using the chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol mixture. Briefly: 20 mL of a mixture chloroform
methanol mixture. Briefly: 20 mL of a mixture chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (2
methanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) were added to 5 g of the freeze dried sample followed by 20 min of mixing. This mixing was repeated for 10 min both after adding the second portion of 10 mL of chloroform and after addition of 20 mL of water. After centrifugation, the organic phase was evaporated to dryness and the weight of the residue was determined after 30 min at 105 °C. The lipid extract prepared for total lipid determination was suspended in hexane and used for the fatty acid methylation. Fatty acids were converted to their methyl esters before analysis. Hexane extract (1 mL) was added with 200 μL of KOH 2 N in methanol for 30 s at room temperature, and 1 μL was injected directly in the GC apparatus. The analysis of fatty acid methyl esters was carried out using a Shimadzu 17A gas chromatograph equipped with a fused silica capillary column (Phenomenex ZB-WAX, 0.50 μm film thickness, 60 m × 0.32 mm i.d.) and a FID detector. Helium was used as carrier gas at 2 mL min−1. The temperature program was 80 °C × 2 min, rate 15 °C min−1 until 180 °C, constant at 180 °C for 6 min, a second rate of 5 °C min−1 until 240 °C, constant at 240 °C for 20 min. The injector and FID temperatures were 250 and 270 °C, respectively. Identification of fatty acid was made using reference fatty acids methyl esters (FAME) from Merck (Merck, Darmstadt, Germany).
1, v/v) were added to 5 g of the freeze dried sample followed by 20 min of mixing. This mixing was repeated for 10 min both after adding the second portion of 10 mL of chloroform and after addition of 20 mL of water. After centrifugation, the organic phase was evaporated to dryness and the weight of the residue was determined after 30 min at 105 °C. The lipid extract prepared for total lipid determination was suspended in hexane and used for the fatty acid methylation. Fatty acids were converted to their methyl esters before analysis. Hexane extract (1 mL) was added with 200 μL of KOH 2 N in methanol for 30 s at room temperature, and 1 μL was injected directly in the GC apparatus. The analysis of fatty acid methyl esters was carried out using a Shimadzu 17A gas chromatograph equipped with a fused silica capillary column (Phenomenex ZB-WAX, 0.50 μm film thickness, 60 m × 0.32 mm i.d.) and a FID detector. Helium was used as carrier gas at 2 mL min−1. The temperature program was 80 °C × 2 min, rate 15 °C min−1 until 180 °C, constant at 180 °C for 6 min, a second rate of 5 °C min−1 until 240 °C, constant at 240 °C for 20 min. The injector and FID temperatures were 250 and 270 °C, respectively. Identification of fatty acid was made using reference fatty acids methyl esters (FAME) from Merck (Merck, Darmstadt, Germany).Carotenoid extraction and determination
Carotenoids were extracted from 100 mg of lyophilized microalgae with 100 mL of dichloromethane (25%) and methanol (75%) according to ref. 17. The extract solution, mixed with the algal cells, was then separated by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 5 min, and the supernatant, containing pigments, was collected. After the extraction procedure was repeated three times, the pigments were almost completely extracted. The combined pigment extracts were re-centrifuged at 10
000g for 5 min, and the supernatant, containing pigments, was collected. After the extraction procedure was repeated three times, the pigments were almost completely extracted. The combined pigment extracts were re-centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 5 min and kept in the dark at −20 °C for HPLC analysis. Saponification of carotenoid esters was carried out according to ref. 18. Six mL of 0.107 M NaOH dissolved in methanol, which was freshly prepared, was added to 30 mL of the pigment extract solution under a nitrogen atmosphere. The mixture (36 mL) was evaporated and concentrated to 30 mL under nitrogen during the process of adding NaOH, and then kept at 5 °C in darkness under nitrogen for 12 h in a water bath for complete hydrolysis of carotenoid esters. The saponified pigment extract solution was directly separated and determined by HPLC as described in ref. 19.
000g for 5 min and kept in the dark at −20 °C for HPLC analysis. Saponification of carotenoid esters was carried out according to ref. 18. Six mL of 0.107 M NaOH dissolved in methanol, which was freshly prepared, was added to 30 mL of the pigment extract solution under a nitrogen atmosphere. The mixture (36 mL) was evaporated and concentrated to 30 mL under nitrogen during the process of adding NaOH, and then kept at 5 °C in darkness under nitrogen for 12 h in a water bath for complete hydrolysis of carotenoid esters. The saponified pigment extract solution was directly separated and determined by HPLC as described in ref. 19.Determination of protein, carbohydrates and dietary fibre content
Samples were analyzed for protein concentration by the Kjeldahl method20 using a nitrogen conversion factor of 6.25. Carbohydrate determination was performed on 2 g of freeze dried samples treated with hydrochloric acid (0.2 M) at 85 °C for 1 h. After neutralization by sodium hydroxide, reducing sugars were determined using the Fehling test. Total fiber content was determined by the AOAC 985.29 gravimetric method.Determination of vitamin content
The procedure described in ref. 21 was employed for water-soluble vitamins. Two g of freeze dried sample was weighed in a 100 mL volumetric amber flask, 40 mL of water and 4 mL of NaOH 2 M were added and the suspension was vigorously shaken, and then 50 mL of phosphate buffer 1 M (pH 5.5) were added in order to lower the pH of the final solution at 7. The suspension was sonicated for 10 min in an ultrasonic bath and filtered through a 0.22 mm Millipore syringe MillexTM GP filter (Bedford, MA, USA) before the HPLC analysis. HPLC separation was carried out at a flow rate of 0.8 mL min using an HPLC (Model LC 10, Shimadzu, Osaka, Japan), with a diode array detector and a Prodigy ODS3 100 Å column (250 × 4.6 mm, particle size 5 μm) (Phenomenex, CA, USA). The mobile phases were (A) consisting of an aqueous solution of TFA 0.025% pH 2.6, and (B) acetonitrile. Vitamin elution was achieved using the following linear gradient: initial isocratic step 100% A for 5 min, followed by a linear gradient to solvent (B)–solvent (A) (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75, v/v) in 6 min. Then a second linear gradient to solvent (B)–solvent (A) (40
75, v/v) in 6 min. Then a second linear gradient to solvent (B)–solvent (A) (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60, v/v) in 8 min, this mixture was held for 1 min. Finally, the initial conditions were reestablished in 1 min and held for 4 min. Chromatographic detection was performed at 210 nm for vitamins B5, B8 and B12 and at 275 nm for vitamins B1, C, PP, B6, B9 and B2. Three injections were performed for each sample.
60, v/v) in 8 min, this mixture was held for 1 min. Finally, the initial conditions were reestablished in 1 min and held for 4 min. Chromatographic detection was performed at 210 nm for vitamins B5, B8 and B12 and at 275 nm for vitamins B1, C, PP, B6, B9 and B2. Three injections were performed for each sample.Vitamin E extraction and analysis was performed as previously described.22
Phycobiliprotein determination
Phycobiliproteins were quantitatively extracted using the procedure reported in ref. 23. The concentrations of phycocyanin and phycoerythrin were calculated from the measured absorbances using the Kursar and Alberte equation.24Antioxidant activity
Total antioxidant activity was determined using a direct measurement of ABTS˙+ (2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid) called the ‘QUENCHER’ approach.25 ABTS radical cation (ABTS˙+) was produced by reacting ABTS stock solution (ABTS dissolved in water to a 7 mM concentration) with 2.45 mM potassium persulfate (final concentration) and allowing the mixture to stand in the dark at 4 °C for 12–16 h before use until the radical was stable. The ABTS˙+ solution was diluted in a hydroalcoholic mixture ethanol–water (50/50) to an absorbance of 0.70 (±0.02) at 734 nm. The direct measurement of ABTS is done on a finely ground lyophilized algae sample sieved at 500 μM. Samples were diluted with cellulose (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9), and 10 mg of the diluted sample was used for the determination. After being suspended in 6 mL of ABTS˙+ diluted solution, the mixture was stirred in the dark for 25 min and then centrifuged at 4000 rpm for 2 min. The absorbance was read at 734 nm using as blank the same quantity of the ABTS˙+ diluted chromophore in which had been suspended only cellulose. The antioxidant capability (AC) was expressed, using Trolox as standard, as mmol of Trolox Equivalent (TE) per kg−1 of dried weight, therefore TEAC notation was used.
9), and 10 mg of the diluted sample was used for the determination. After being suspended in 6 mL of ABTS˙+ diluted solution, the mixture was stirred in the dark for 25 min and then centrifuged at 4000 rpm for 2 min. The absorbance was read at 734 nm using as blank the same quantity of the ABTS˙+ diluted chromophore in which had been suspended only cellulose. The antioxidant capability (AC) was expressed, using Trolox as standard, as mmol of Trolox Equivalent (TE) per kg−1 of dried weight, therefore TEAC notation was used.Protein extraction by enzymatic treatment
Preliminary extraction of G. sulphuraria fresh and freeze dried material were performed in water, hydroalcoholic solutions, detergents (2% Triton X-100 and 1% SDS) and 9 M urea also in the presence of a reducing agent (1% β-mercaptoethanol). The extraction rate was monitored by colorimetric protein assay (Bio-Rad, Hercules, CA, USA).The commercial enzymes used in this study included Viscozyme L (a multi-enzyme complex containing a wide range of carbohydrases, including arabanase, cellulase, b-glucanase, hemicellulase and xylanase), pepsin and trypsin. Enzymatic digestion of the freeze dried sample was performed dissolving 3 g of powdered sample in 120 mL of water and treating with 4 mL Viscozyme L at pH 4, 37 °C for 24 h. After digestion, the tube was centrifuged at 4000g for 15 min, and Viscozyme digested material was taken for further digestion with pepsin and tripsin. Algal biomass was also digested with proteases pepsin and trypsin with or without a pre treatment with Viscozyme digestion. 3 g of powdered sample was treated first with 2 mL pepsin solution (800–2500 U mg−1, 100 mg mL−1, pH 2, 37 °C for 2 h) and then with 2 mL tripsin solution (Type II-S; 100 mg mL−1 in phosphate buffer pH 7.6, 37 °C for 2 h). The same enzymatic digestions were applied also to A. platensis.
The protein profile of freeze-dried algal powder, after enzymatic treatments, was determined by SDS-PAGE on 4 % and 12.0% acrylamide slab gels. A total of 10 mg of protein samples were dissolved in 1 mL of sample buffer (0.5 M Tris–HCl, pH 6.8, containing 10% (w/v) SDS, 10% (v/v) glycerol, 5% β-mercaptoethanol and 0.1% (w/v) bromophenol blue) and heated at 100 °C for 5 min. Every sample (10 μL) was applied to each lane of gel. The separation was performed at 120 V constant voltage for 1.5 h. The polypeptides used as molecular mass markers were: phophorylase b (94 kDa), albumin (67 kDa), ovalbumin (43 kDa), carbonic anhydrase (30 kDa), trypsin inhibitor (20.1 kDa) and lactalbumin (14.4 kDa). The separated protein were visualised by staining the gel with 0.1% (w/v) Coomassie Brilliant Blue R-250 in 10% (v/v) acetic acid: 40% (v/v) methanol and destained with 10% (v/v) acetic acid containing 40% (v/v) methanol.
Results and discussion
Selection of the G. sulphuraria strain for mass cultures
Forty-three G. sulphuraria strains, coming from either acidic or thermo-acidic environments isolated in different Italian regions, were grown under autotrophic or heterotrophic conditions. In the latter case, preliminary experiments indicated that the growth-rate of G. sulphuraria is not modified using glycerol as a carbon source instead of glucose or sucrose. Therefore, glycerol was used in this screening as it is a cheap byproduct that is widely available from biodiesel production. The growth performance of the G. sulphuraria strains in batch culture experiments is summarized in Table 1, reporting doubling times of each strains under the two cultivating conditions: the lower the doubling time, the higher the daily biomass produced. The cultures maintained the exponential phase of growth during the course of the experiment (nine days). Ammonium concentration and dissolved oxygen were measured at the beginning and end of the tests. Also in the case of strain 064/309, which showed the lowest doubling times, only a 10% reduction of ammonium concentration was observed (not shown), whereas the dissolved oxygen remained unchanged, indicating that both nitrogen and oxygen were not limiting during the course of the experiments.As expected, all the G. sulphuraria strains growing experiments were characterized by a complete absence of bacterial contaminations.
It is worth noticing that most strains strongly decreased their doubling times when grown in heterotrophy. G. sulphuraria 064/309, isolated in Sicily from a sulfur mine, showed the highest doubling time under heterotrophic conditions and, based on these results, it was chosen for mass cultivation. In the 5 L reactors, under the fed-batch conditions above described, G. sulphuraria 064/309 reached after 30 days a biomass of 5.7 g L−1 dry weight under autotrophic conditions and 29 g L−1 dry weight in heterotrophy.
Macronutrient composition
Macronutrient composition of G. sulphuraria cultivated under the two conditions is summarized in Table 2. The protein content was higher than that of vegetables, which is a common feature for microalgae. The protein content of heterotrophic and autotrophic biomass was 27% and 33%, respectively. The difference is mainly due to different amounts of phycobiliproteins, which in autotrophic algae is 8.2%, while in heterotrophic algae, due to the absence of the photosynthetic process, it is only 1.1%. The protein concentration was comparable to that of other microalgae which have been proposed as a source of proteins to integrate into the diet in developing countries.26| Nutrients | G. sulphuraria heterotrophic mg g−1 | G. sulphuraria autotrophic mg g−1 |
|---|---|---|
| Proteins | 265 ± 25 | 325 ± 32 |
| Lipids | 11.4 ± 3.0 | 17.7 ± 3.2 |
| Total carbohydrates | 691 ± 31 | 629 ± 16 |
| Dietary fibre | 541 ± 38 | 540 ± 25 |
The lipid concentration was very low under both culture conditions: as shown in Table 2, the percentage of lipids was 1.1% in autotrophic and 1.4% under heterotrophic conditions, respectively. Lipid content was much lower than that reported for other microalgae.27,28 It is known that the concentration of lipid in microalgae is extremely variable, reaching productive peaks of 70–80% for those used in oil production.29
Qualitative profile of the lipid fraction of G. sulphuraria is reported in Table 3. In this case, there were significant differences between the two growing conditions. Under heterotrophic conditions, the unsaturated/saturated ratio was about five, while it decreased nearly to one in the material grown autotrophically; on the other hand, palmitic acid represents, for autotrophic algae, about 40% of lipid fraction while it was only 20% in heterotrophic algae. Linoleic acid was the major fatty acid in autotrophic algae while oleic acid was the most represented in heterotrophic algae. These results confirmed that microalgae are metabolically very flexible and their total lipid concentration as well as fatty acid composition can change significantly depending on the strain and on the growth conditions used.19
| Fatty acids | G. sulphuraria heterotrophic% | G. sulphuraria autotrophic% |
|---|---|---|
| C14:0 | 2.7 ± 0.4 | 0.9 ± 0.1 |
| C16:0 | 14.7 ± 2.0 | 39.4 ± 2.5 |
| C16:1 | 2.4 ± 0.8 | n.d. |
| C18:0 | n.d. | 4.7 ± 1.8 |
| C18:1 | 57.5 ± 3.7 | 8.6 ± 1.9 |
| C18:2 | 19.5 ± 2.6 | 45.2 ± 2.3 |
| C18:3 | 2.7 ± 0.0 | 1.1 ± 0.1 |
The high polyunsaturated fatty percentage with respect to the total fatty acid content, typical of microalgae, is observed in G. sulphuraria biomass obtained in autotrophic cultivation. The high prevalence of monounsaturated fats is a response to heterotrophic growth reported also in Chlorella zofingiensis,30 and probably related to a reduction of thylakoid structural lipids, occurring in the algae grown in the dark.
Regarding the possible use of the whole G. sulphuraria biomass, the low amount of total lipids and the limited percentage of polyunsaturated fatty acids can positively influence the storage time limiting the oxidative degradation, which is usually an important problem when microalgae are incorporated in foods.31
Carbohydrates are the main macronutrients present in G. sulphuraria. The transition to heterotrophic cultivation did not modify the carbohydrate content, which was 69.1%, of which 54.1% was insoluble dietary fiber, whereas in autotrophic algae, carbohydrates represented 62.9%, of which 54.0% was insoluble dietary fiber. The content of insoluble dietary fiber was higher than that reported in the literature for other microalgae: the carbohydrate fraction was mainly represented by heterogeneous soluble polymers, and the cellulosic cell wall represents on average about 10% of the algal dry matter.14 In most marine red algae high-molecular-weight, sulfated galactans is the major cell wall component.32,33 The red unicellular alga Porphyridium cruentum also have a carbohydrate content ranging from 40 to 57% of dry weight, with a prevalence of storage amylopectine- and amilose-polyglucans,27 whereas the Cyanidiophycean Cyanidium caldarium synthesizes large amounts of storage glycogen polyglucans, but not amylose.34 The species Galdieria maxima grown heterotrophically on glucose increases its carbohydrates content by a factor of 4, synthesizing a storage polysaccharide with a very high branching degree, which is accumulated in cytoplasm.35 In G. sulphuraria, the cellulosic-like polymers are more represented than storage polysaccharides, with an average amount of about 35% of dried biomass.
The presence of insoluble dietary fiber can be regarded as a positive feature of G. sulphuraria biomass. Insoluble dietary fiber is able to reach the colon and to stimulate the growth of positive microflora, thus contributing to the improvement of the human intestinal ecosystem. The addition of fiber-rich algal products to the diet could increase overall dietary fiber intake, helping people to reach the recommended dietary allowance (RDA).36
All in all, G. sulphuraria showed an unusual compositional profile among microalgae, with a large prevalence of carbohydrates, a good content of proteins and a very low level of lipids. This pattern is similar to that found in some brown and red macroalgae, which have been considered as good sources of functional ingredients.37
Micronutrient and pigment composition
Microalgae are a valuable source of many essential vitamins (e.g., A, B1, B2, B6, B12, C, E, nicotinate, biotin, folic acid and pantothenic acid).14As shown in Table 4 the concentration of vitamin E was 9 and 15 mg kg−1 of dry weight for autotrophic and heterotrophic algae, respectively. The content of water soluble vitamins changed under the different culture conditions. In fact, heterotrophic algae contain vitamin B2 (riboflavin) and B3 (niacin) respectively at a level of 30 mg kg−1 and 32 mg kg−1, respectively. However, autotrophic algae only contain 20 mg kg−1 vitamin B3 and no vitamin B2.
| G. sulphuraria heterotrophic | G. sulphuraria autotrophic | |
|---|---|---|
| Vitamins (mg kg−1) | ||
| B2 | 30 ± 2 | n.d. |
| B3 | 32 ± 3 | 20 ± 2 |
| E | 9 ± 1 | 15 ± 3 |
| Carotenoids (mg kg−1) | ||
| β-Carotene | n.d. | n.d. |
| Cryptoxanthin | n.d. | n.d. |
| Astaxanthin | n.d. | 575 ± 123 |
| Lutein | n.d. | 387 ± 112 |
| Chlorophills (mg kg−1) | ||
| Chlorophyll a | n.d. | 12.020 ± 541 |
| Phycobiliproteins (g kg−1) | ||
| Allophycocyanin | 4.5 ± 1.2 | 79.0 ± 3.1 |
| Phycocyanin | 0.5 ± 0.0 | 0.2 ± 0.0 |
| Phycoerytrin | 6.5 ± 1.1 | 3.3 ± 0.8 |
The vitamin content of microalgae is of fundamental nutritional importance for their use as food, however limited and conflicting data, with huge variations among the studies, are present in the literature. Some of the differences may be due to variations in culture conditions and harvesting and storage procedure. Many studies on the vitamin content of different microalgae used in aquacultures were performed;38,39 the vitamin E content of G. sulphuraria, both autotrophic and heterotrophic, was 10 to 20 times lower than the literature data, while water soluble vitamin contents were in accordance with literature reports. De Roeck-Holtzhauer et al.40 measured the content of ten vitamins in five microalgae; thiamine, riboflavin, ascorbic acid, pyridoxyl phosphate and the fat-soluble vitamins A, D, E and K. The authors concluded that despite variability, microalgae can represent a valuable source of B group vitamins, particularly riboflavin.
Microalgae are also a source of pigments like chlorophyll (0.5% to 1% of dry weight), carotenoids (0.1% to 0.2% of dry weight on average and up to 14% of dry weight for β-carotene of Dunaliella) and phycobiliproteins.26
Data reported in Table 4 indicated that carotenoids are only a minor pigment in G. sulphuraria: they were detected only in the autotrophic algae, the main ones being astaxanthin and lutein. The absence of carotenoids in heterotrophic algae was due to the lack of photosynthesis under these growth culture conditions. Furthermore, under the autotrophic conditions the presence of chlorophyll at 1.21%, which is in the standard range for microalgae, was detected by chromatographic analysis.
Phycobiliproteins constitute the main pigments of the analysed biomass and they have been proposed for commercial use as a natural dye. In G. sulphuraria, allophycocyanin is the dominant form in the autotrophic algae, while phycoerytrin was the main phycobiliprotein in the heterotrophic algae. The occurrence of phycobiliproteins in the algal biomass under heterotrophic conditions (phycocyanin and phycoerytrin) was in agreement with literature studies reporting that some substances involved in the photosynthetic apparatus continue to be synthesized in the dark, for example, the light-harvesting pigment phycocyanin from G. sulphuraria.41 In the literature, it was reported that phycocyanin and allophycocyanin are always present in Cyanophyceae and Rhodophyceae.42 Moreover, the expression of genes encoding phycobiliproteins has been shown to vary in response to chemical and physical stimuli including nutrient availability, temperature, light intensity and in some cases light wavelength. The different growing conditions used in this study might have stimulated the phycoerythrin production by G. sulphuraria.43 Data obtained on G. sulphuraria shown in Table 4 are in agreement with those reported in the literature for Rhodophyta in which the red color is due to the presence of high amounts of the biliprotein phycoerythrin in addition to the blue phycocyanin and chlorophyll a.44
Antioxidant capacity
Antioxidant capacity (TEAC) of the dried biomass from G. sulphuraria cultivated under heterotrophic and autotrophic conditions was of 5.6 mmol kg−1 and of 29.6 mmol kg−1 dry weight, respectively. The high value under the autotrophic conditions paralleled the higher concentrations of carotenoids and pigments well known in the literature for their antioxidant activity. The absolute value of antioxidant activity is comparable to that reported for Porphyridium cruentum.27 The comparison of these data with those reported in the literature for plant foods indicated that TEAC of G. sulphuraria biomass are comparable with those of some vegetables.45 These results suggest that G. sulphuraria (particularly under autotrophic conditions) can be used as ingredients in food preparations to improve the nutritional value.Extraction, bioaccessibility and digestibility of proteins from G. sulphuraria
The benefit derived from the consumption of protein-rich foods depends on protein digestibility which can be affected by the low bioaccessibility of the proteins because of their folding and/or the strict association with other food components.46 In the case of G. sulphuraria, most of the proteins are embedded in the polysaccharide components and the treatment with different procedures usually adopted for protein extraction (urea, solvent or detergents) do not allow us to achieve a satisfactory protein solubilization.In Fig. 1 panel A, the electrophoretic pattern of proteins extracted from microalgae G. sulphuraria and A. platensis with 9 M urea and 1% β-mercaptoethanol is shown. While the proteins from A. platensis (extracted as control) are well resolved in distinct bands, the extract from G. sulphuraria gave a long smear, indicating that polysaccharide cell walls are still bound to the proteins which cannot be separated on the SDS gel.
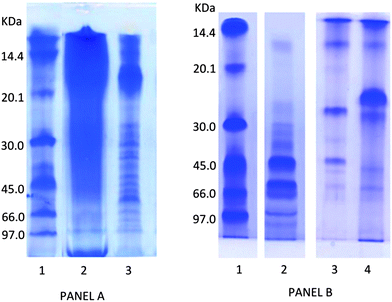 | ||
| Fig. 1 SDS-PAGE analysis of proteins extracted from freeze-dried biomass of G. sulphuraria and A. platensis panel A: proteins extracted from microalgae with 9 M urea and 1% b-mercaptoethanol. Lanes: 1, molecular mass markers; 2, G. sulphuraria extract (9 M urea and 1% β-mercaptoethanol); A platensis extract (9 M urea and 1% β-mercaptoethanol). Panel B: lanes: 1, molecular mass markers; 2, G. sulphuraria extract (Viscozyme digestion); 3, G. sulphuraria extract (Viscozyme digestion followed by viscozyme, trypsin and pepsin); 4, G. sulphuraria extract (trypsin and pepsin digestion). | ||
To develop an effective treatment to disrupt the polysaccharide cell wall, making the protein and other constituents accessible for digestive enzymes, a screening of commercial enzymatic preparations was carried out. Viscozyme L, which is a mixture of polysaccaridases from Aspergillus sp., proved to be very effective in disrupting the G. sulphuraria cell wall. In Fig. 1 panel B, the SDS profile obtained after hydrolysis of algal dry matter with Viscozyme L is shown. The electrophoretic pattern of G. sulphuraria, after Viscozyme digestion (lane 2), revealed several main bands with apparent molecular weight between 97.0 and 45.0 kDa and other less abundant bands with molecular masses ranging from approximately 45.0 to 12.0 kDa.
A successive treatment of the Viscozyme-extracted proteins with a mixture of human digestive enzymes (pepsine and trypsine) at physiological conditions resulted in a marked decrease of the relative intensity of proteins at high molecular weight with the appearance of new bands at low molecular weight, and particularly of an intense band at 28.0 kDa (lane 3). The overall intensity of the bands is decreased, indicating that most of the proteins are degraded into peptides lower that 10 kDa. This evidence indicates that after Viscozyme extraction G. sulphuraria proteins became a good substrate for human digestive enzymes. Interestingly, when G. sulphuraria biomass was incubated directly with digestive enzymes, skipping the treatment with Viscozyme (lane 4), a limited amount of proteins bands was visible, but an intense band with molecular mass at about 25.0 kDa could be seen. This suggested that digestive enzymes were able to cleave and solubilise some parts of the proteins which are not completely wrapped in the cell wall polysaccharides structure.
Despite their high content of protein rich essential amino acids, dried microalgae have not gained significant importance as food or food ingredients thus far. This is due to many technological and sensorial obstacles: the powder-like consistency of the dried biomass; its dark green color, easily turning to brown; and its slightly fishy odor, which becomes a rancid smell during the product's shelf life. Moreover, the digestibility of microalgae proteins is limited as well as the amount of high quality (i.e. not bacterial contaminated) microalgae biomass on the market.
Data of this paper suggested that G. sulphuraria biomass obtained under heterotrophic conditions can potentially overcome most of these hurdles. As shown for other microalgae species,47,48 the growth in heterotrophy allows reaching high cell densities also using cheap byproducts such as glycerol. G. sulphuraria biomass was colorless, thus avoiding the appearance of an unattractive green-brownish color after incorporation into foods. It had a low lipid content mainly of monounsaturated acid, therefore the oxidation during shelf life is negligible. Moreover, the developed enzymatic extraction protocol allowed the extraction of proteins which proved to be good substrates of human digestive enzymes.
G. sulphuraria can be used to develop new food ingredients, and thanks to its favorable macro and micronutrient profiles, it can be used to design food preparations rich in bioavailable proteins and dietary fiber.
Acknowledgements
This work was carried out in the framework of the project “Phytocosmetics: cosmetic formulations based on water soluble extracts from microalgae with replenishing action on skin tissue” funded by Institute of Foreign Commerce (ICE) and Conference of Italian University Rectors (CRUI).References
- T. D. Brock, Life at High Temperatures, The Yellowstone Association for Natural Science, History & Education, Inc, Yellowstone National Park, Wyoming 82190, USA, 1994 Search PubMed.
- H. W. Yoon, K. M. Müller, R. G. Sheath, F. D. Ott and D. Bhattacharya, Defining the major lineages of red algae (Rhodophyta), J. Phycol., 2006, 42, 482–492 CrossRef CAS.
- P. Albertano, C. Ciniglia, G. Pinto and A. Pollio, The taxonomic position of Cyanidium, Cyanidioschyzon and Galdieria: an update, Hydrobiologia, 2000, 433, 137–1443 CrossRef.
- W. Gross and C. Schnarrenberger, Heterotrophic growth of two strains of the acido thermophilic alga Galdieria sulphuraria, Plant Cell Physiol., 1995, 36, 633–638 CAS.
- W. Gross, J. Küwer, G. Tischendorf, N. Bouchaala and W. Büsch, Cryptoendolithic growth of the red alga Galdieria sulphuraria in volcanic areas, Eur. J. Phycol., 1998, 33, 25–31 CrossRef.
- Y. Chisti, Biodiesel from microalgae, Biotechnol. Adv., 2007, 25, 294–306 CrossRef CAS.
- N. T. Eriksen, The technology of microalgal culturing, Biotechnol. Lett., 2008, 30, 1525–1536 CrossRef CAS.
- P. M. Cook, Chemical engineering problems in large scale culture of algae, Ind. Eng. Chem., 1951, 43, 2385–2389 CrossRef CAS.
- L. C. Powell, E. M. Nevels and M. E. Mc Dowell, Algae feeding in humans, J. Nutr., 1961, 75, 7–12 Search PubMed.
- S. Varfolomeev and L. Wasserman, Microalgae as source of biofuel, food, fodder, and medicines, Appl. Biochem. Microbiol., 2011, 47, 789–807 CrossRef CAS.
- O. Perez-Garcia, M. E. Escalante, L. de-Bashan and Y. Bashan, Heterotrophic cultures of microalgae: metabolism and potential products, Water Res., 2011, 45, 11–36 CrossRef CAS.
- O. V. Graverholt and N. T. Eriksen, Heterotrophic high-cell density fed-batch and continuous-flow cultures of Galdieria sulphuraria and production of phycocyanin, Appl. Microbiol. Biotechnol., 2007, 77, 69–75 CrossRef CAS.
- R. W. Bailey and L. A. Staehelin, The chemical composition of isolated cell wall of Cyanidium caldarium, J. Gen. Microbiol., 1968, 54, 269–276 CrossRef CAS.
- E. W. Becker, Micro-algae as a source of protein, Biotechnol. Adv., 2007, 25, 207–210 CrossRef CAS.
- M. M. Allen, Simple conditions for growth of unicellular blue-green algae on plates, J. Phycol., 1968, 4, 1–4 CrossRef.
- G. Olivieri, A. Marzocchella, R. Andreozzi, G. Pinto and A. Pollio, Biodiesel production from Stichococcus strains on laboratory scale, J. Chem. Technol. Biotechnol., 2011, 86, 776–783 CrossRef CAS.
- J. P. Yuan, F. Chen, X. Liu and X. Z. Li, Carotenoid composition in the green microalga Chlorococcum, Food Chem., 2002, 76, 319–325 CrossRef CAS.
- J. P. Yuan and F. Chen, Purification of trans-astaxanthin from a high-yielding astaxanthin ester-producing strain of the microalga haematococcus pluvialis, Food Chem., 2000, 68, 443–448 CrossRef CAS.
- V. Fogliano, C. Andreoli, A. Martello, M. Chiazzo, O. Lo bosco, F. Formisano, P. A. Carlino, G. Meca, G. Graziani, V. Di Martino Rigano, V. Vona, S. Carfagna and C. Rigano, Functional ingredients produced by culture of koliella antarctica, Aquaculture, 2010, 299, 115–120 CrossRef CAS.
- AOAC Official Method 985.29, Total Dietary in Foods—EnzymaticGravimetric Method, Official Methods of Analysis, AOAC International, Gaithersburg, MD, 16th ed, 1995 Search PubMed.
- O. Heudi, T. Kilinç and P. Fontannaz, Separation of water-soluble vitamins by reversed-phase high performance liquid chromatography with ultra-violet detection: application to polyvitaminated premixes, J. Chromatogr., A, 2005, 1070, 49–56 CrossRef CAS.
- S. Siebenhandl, H. Grausgruber, N. Pellegrini, D. Del Rio, V. Fogliano, R. Pernice and E. Berghofer, Phytochemical profile and total antioxidant capacity of purple and blue wheat, and black barley, J. Agric. Food Chem., 2007, 55, 8541–8547 CrossRef CAS.
- A. Reis, A. Mendes, H. Lobo-Fernandes, J. A. Empis and J. Maggiolly Novais, Production, extraction and purification of phycobiliproteins from Nostoc sp., Bioresour. Technol., 1998, 66, 181–187 CrossRef CAS.
- T. A. Kursar and R. S. Alberte, Photosynthetic unit organization in a red alga, Plant Physiol., 1983, 72, 409–414 CrossRef CAS.
- V. Gökmen, A. Serpen and V. Fogliano, Direct measurement of the total antioxidant capacity of foods: the ‘QUENCHER’ approach, Trends Food Sci. Technol., 2009, 20, 278–288 CrossRef.
- P. Spolaore, C. Joannis-Cassan, E. Duran and A. Isambert, Commercial applications of microalgae, J. Biosci. Bioeng., 2006, 101, 87–96 CrossRef CAS.
- M. M. Rebolloso Fuentes, G. G. Acién Fernández, J. A. Sánchez Pérez and G. L. Guil Guerrer, Biomass nutrient profiles of the microalga Porphyridium cruentum, Food Chem., 2000, 70, 345–353 CrossRef CAS.
- J. J. Ortega-Calvo, C. Mazuelos, B. Hermosin and C. Saiz-Jimenez, Chemical composition of Spirulina and eukaryotic algae food products marketed in Spain, J. Appl. Phycol., 1993, 5, 425–435 CrossRef CAS.
- R. Órpez, M. E. Martínez, G. Hodaifa, E. L. Yousfi, N. Jbari and S. Sánchez, Growth of the microalga Botryococcus braunii in secondarily treated sewage, Desalination, 2009, 246, 625–630 CrossRef.
- J. Liu, J. Huang, Z. Sun, Y. Zhong, Y. Jiang and F. Chen, Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zoofingiensis: assessment of algal oil for biodiesel production, Bioresour. Technol., 2011, 102, 106–110 CrossRef CAS.
- E. Ryckebosch, K. Muylaert, M. Eeckhout, T. Ruyssen and I. Foubert, Influence of drying and storage on Lipid and carotenoid stability of the microalga Phaeodactylum tricornutum, J. Agric. Food Chem., 2011, 59, 11063–11069 CrossRef CAS.
- A. Chiovitti, A. Bacic, D. J. Craik, S. L. A. Munro, G. T. Kraft and M. Liao, Cell-wall polysaccharides from Australian red algae of the family Solieriaceae (Gigartinales, Rhodophyta): novel, highly pyruvated carrageenans from the genus Callophycus, Carbohydr. Res., 1997, 299, 229–243 CrossRef CAS.
- A. I. Usov and M. I. Bilan, Fucoidans-sulfated polysaccharides of brown algae, Russ. Chem. Rev., 2009, 78, 785–789 CrossRef CAS.
- T. Shimonaga, S. Fujiwara, M. Kaneko, A. Izumo, N. Satoko, B. F. Perigio, A. Satoh, N. Fujita, Y. Nakamura and M. Tsuzuki, Variation in storage α-polyglucans of red algae: amylose and semi-amylopectin types in Porphyridium and glycogen type in Cyanidium, Mar. Biotechnol., 2007, 9, 192–202 CrossRef CAS.
- I. N. Stadniciuck, L. R. Semenova, G. P. Smirnova and A. I. Usov, A highly branched storage polyglucan in the thermoacidophilic red microalga Galdieria maxima cells, Appl. Biochem. Microbiol., 2007, 43, 78–83 CrossRef.
- A. T. Southgate, Dietary Fiber and Health, in, Dietary Fiber: Chemical and Biochemical Aspects, ed. A. T. Southgate, Royal Society of Chemistry, Cambridge, UK, 1990, pp. 10–25 Search PubMed.
- M. Plaza, A. Cifuentes and E. Ibáñez, In the search of new functional food ingredients from algae, Trends Food Sci. Technol., 2008, 19, 31–39 CrossRef CAS.
- M. R. Brown, M. Mular, I. Miller, C. Farmer and C. Trenerry, The vitamin content of microalgae used in aquaculture, J. Appl. Phycol., 1999, 11, 247–255 CrossRef CAS.
- M. R. Brown and C. L. Farmer, Riboflavin content of six species of microalgae used in mariculture, J. Appl. Phycol., 1994, 6(1), 61–65 CrossRef CAS.
- Y. De Roeck-Holtzhauer, I. Quere and C. Claire, Vitamin analysis of five planktonic microalgae and one macroalga, J. Appl. Phycol., 1991, 3, 259–264 CrossRef CAS.
- N. T. Eriksen, Production of phycocyanin-a pigment with applications in biology, biotechnology, foods and medicine, Appl. Microbiol. Biotechnol., 2008, 80, 1–14 CrossRef CAS.
- J. Moreno, H. Rodríguez, M. A. Vargas, J. Rivas and M. G. Guerrero, Nitrogen-fixing cyanobacteria as source of phycobiliprotein pigments. Composition and growth performance of ten filamentous heterocystous strains, J. Appl. Phycol., 1995, 7(1), 17–23 CrossRef CAS.
- G. Kaur, J. I. S. Khattar, D. P. Singh, Y. Singh and J. NaddaMicroalgae: A Source of Natural Colours. Algal Biology and Biotechnology, 2009, 10, pp. 129–144 Search PubMed.
- O. Pulz and W. Gross, Valuable products from biotechnology of microalgae, Appl. Microbiol. Biotechnol., 2004, 65, 635–648 CrossRef CAS.
- N. Pellegrini, M. Serafini, B. Colombi, D. Del Rio, S. Salvatore, M. Bianchi and F. Brighenti, Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays, J. Nutr., 2003, 133, 2812–2819 CAS.
- A. J. Darragh and S. M. Hodgkinson, Quantifying the digestibility of dietary protein, J. Nutr., 2000, 130, 1850–1856 Search PubMed.
- O. S. Graverholt and N. T. Eriksen, Heterotrophic high-cell-density fed-batch and continuous-flow cultures of Galdieria sulphuraria and production of phycocyanin, Appl. Microbiol. Biotechnol., 2007, 77, 69–75 CrossRef CAS.
- Z. Wu and X. Shi, Optimization for high-density cultivation of heterotrophic Chlorella based on a hybrid neural network model, Lett. Appl. Microbiol., 2007, 44, 13–18 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
