Effects of prebiotic inulin-type fructans on structure, quality, sensory acceptance and glycemic response of gluten-free breads
Vanessa D.
Capriles
a and
José A. G.
Arêas
*b
aDepartamento de Biociências, campus Baixada Santista, Universidade Federal de São Paulo, Av. Ana Costa, 95, CEP 11060-001, Santos, SP, Brazil. E-mail: vanessa.capriles@unifesp.br
bDepartamento de Nutrição, Faculdade de Saúde Pública, Universidade de São Paulo, Av. Dr. Arnaldo, 715, CEP 01246-904, São Paulo, SP, Brazil. E-mail: jagareas@usp.br; Fax: +55-11-30617705; Tel: +55-11-30617701
First published on 3rd October 2012
Abstract
The effect of adding increasing levels of prebiotic inulin-type fructans (ITFs) (0, 4, 8, 10 and 12%) on the sensory and nutritional quality of gluten-free bread (GFB) was assessed. ITFs can provide structure and gas retention during baking, thus improving GFB quality by yielding better specific volume, softer crumb, improved crust and crumb browning with enhanced sensory acceptance. During baking, approximately one-third of the ITFs was lost. The addition of 12% ITFs to the basic formulation is required in order to obtain GFB enriched with 8% ITFs (4 g of fructans per 50 g bread serving size), levels that can provide health benefits. 12% ITFs-addition level decreased GFB glycemic index (from 71 to 48) and glycemic load (from 12 to 8). Prebiotic ITFs are a promising improver for GFB that can provide nutritional (11% dietary fiber content, low glycemic response) and functional benefits to patients with celiac disease, since ITFs are prebiotic ingredients that can also increase calcium absorption.
Introduction
Celiac disease (CD) is a genetically based autoimmune disease characterized by life-long intolerance to the ingestion of gluten. It is a term used to encompass specific storage proteins from wheat (gliadin), rye (secalin), and barley (hordein). Currently, the only effective and safe treatment for CD sufferers is a strict life-long adherence to a gluten-free diet (GFD).1A markedly reduced bone mineral density (BMD) has been consistently found in patients with CD. Therefore, interventions with supplemental calcium and vitamin D have been tested, albeit with limited success. This is, possibly due to the presence of negligible amounts of calcium-binding protein (calbindin-D9k) in celiac patients' small intestine.1 Thus, new therapeutic approaches must be tested, such as the consumption of inulin-type fructans (ITFs), with potential to increase calcium absorption and BMD.2,3
ITFs (inulin and oligofructose) are soluble dietary fiber, and the most studied prebiotic food ingredients. Prebiotics are selectively fermented ingredients that allow specific changes in both the composition and/or activity of the gastrointestinal microbiota which confer benefits to host well-being and health.4 Colonic absorption represents approximately 70% of the increased calcium absorption induced by consumption of ITFs.3 This benefit becomes especially important when absorption in the small intestine is impaired for anatomic or physiological reasons3, like in CD sufferers.1
Compliance with GFD is often difficult due to the lack of good quality gluten-free food products and the negative impact of this food restriction on social life.5 Thus, there is a demand for gluten-free products, especially bread.
A range of GFB recipes have been developed, and usually contain mainly refined flours and starches from gluten-free raw materials. Consequently, this kind of bread has inherently low levels of dietary fiber.6 Therefore, the enrichment of GFB with dietary fibers has proven necessary, since it has been reported that celiac patients have a low intake of fibers attributable to their GFD.7 Fiber enrichment can decrease GFB glycemic response, which is highly desirable due to the association of CD and insulin-dependent diabetes.8
Although ITFs are attractive ingredients to be incorporated into GFB recipes, information regarding their effects on GFB structure is scant9,10, and no investigations have evaluated the impact of ITFs on GFB post-prandial glycemic response.
The aim of the present study was to investigate the impact of ITFs addition levels (0, 4, 8, 10, and 12%) on the sensory and nutritional quality of GFB. Physical properties and sensory analyses were used as tools to evaluate the resultant bread structure and quality. The nutritional value and fructans content were also evaluated. The glycemic response was also investigated in two steps. Firstly, an in vitro study was performed in order to evaluate the importance of ITFs level effects on starch digestibility. Subsequently, an in vivo study was performed in a group of healthy volunteers.
Material and methods
The experimental protocol was approved by the Human Research Ethics Committee of the Faculdade de Saúde Pública da Universidade de São Paulo (FSP/USP), under the research protocol number 1404. The participants were recruited from staff, students and lecturers of FSP/USP to participate voluntarily in sensory evaluation and glycemic response analysis. All subjects gave written informed consent.Bread formulations
To prepare the GFB all ingredients were acquired from local markets, except for ITFs mixture (Raftilose®Synergy1) supplied by Orafti NV (Tienen, Belgium). According to the manufacturer's specifications, this commercial ITFs mixture powder contained 89% fructans, 8% sugar (glucose, fructose, and sucrose) and 3% moisture.The control GFB formulation was defined after preliminary tests and consisted, for the flour/starch base, of: 50% rice flour and 50% potato starch, 25% egg, 10.5% whole milk powder, 6% sugar, 6% soy oil, 2% salt, 0.8% instant dry yeast, 0.3% xanthan gum, 0.3% carboxymethylcellulose and 85% water. Subsequently, 8.6%, 17.9%, 22.7% and 28% (flour/starch base) of fructans were added to the control formulation in order to obtain 4, 8, 10 and 12% ITFs-enriched baked loaves, respectively. These quantities were defined taking into account each formulation's total weight and 12% bake loss (observed in baking preliminary tests).
All ingredients were placed in the bowl of a stand mixer (Arno S.A. - Indústria e Comércio, Brazil), and mixing was performed for 4 min at a medium speed (level 3 of 5). The resultant batters were scaled (220 g) into previously greased and floured baking pans (16 × 7 × 5 cm) and proofed at 40 °C for 45 min. Baking was done at 180 °C for 50 min. After baking, the loaves were depanned and cooled for 1.5 h on cooling racks at room temperature. The loaves were then stored in polyethylene bags, to prevent moisture loss, at room temperature (approximately 25 °C) until they were analysed.
Eighteen loaves for each of the five bread types were prepared from two independent batches (nine loaves per batch). Three random loaves were used for specific volume and color analyses. Six random loaves were used for sensory evaluation. Nine loaves were used for moisture and texture evaluation (3 random units were analyzed after 2, 24 and 48 h post-baking). Three of these loaves were dried at 60 °C for 18 h, and ground into powder (<0.250 mm) for proximate composition and in vitro starch digestibility analyses. An extra five loaves (one batch) of selected treatments were produced for in vivo glycemic response evaluation.
Bread quality evaluation
After cooling, loaves were weighed and loaf volume measured by millet-seed displacement. Loaf specific volume (volume [cm3]/weight [g]) was calculated. Bake loss was also calculated ([weight of the loaf before baking − weight of the loaf after baking and cooling]/[weight of the loaf before baking] × 100). Analyses were performed in triplicate.Color of crust and crumb were measured using a colorimeter (Color Quest XE, Hunter Lab, US) and expressed as Hunter L* a* b* values (L* value defines the lightness, a* value the red-greenness and b* value the blue-yellowness, respectively). Crust color (six values) was measured at two opposite points on top of the loaf in three different loaves. The bread was then sliced transversely to obtain uniform 25 mm-thick slices. Crumb color (twelve values) was measured in the center on both sides of each slice (two slices taken from the centre of three different loaves).
Aging effects on crumb moisture and firmness were assessed 2, 24 and 48 h after baking. Crumb moisture was evaluated, in triplicate, according to the AACC method 44-15A.11 Crumb firmness was determined according to the AACC method 74-09 (ref.11) using a texture analyzer (TA-XT2i, Stable Micro Systems, Surrey, UK). Texture measurements (six values) were made on two bread slices taken from the centre of three different loaves.
Sensory evaluation for acceptance
Sensory acceptability of breads was evaluated one day after their production. Sixty untrained panelists (57 females and 3 males, aged 19–54 years) evaluated the appearance, color, texture, taste and overall acceptability of the GFB on a 9-point hedonic scale (1 = dislike extremely, 5 = neither like nor dislike, 9 = like extremely). Each panelist evaluated three random bread types. Bread slices (25 mm thick) were offered in polyethylene bags codified with 3-digit numbers. Panelists evaluated the breads in a testing area and were instructed to rinse their mouths with water between samples to minimize any residual effect.Proximate composition
Moisture, ash, fat and protein contents were determined according to AOAC methods 950.46, 923.03, 920.39 C and 960.52, respectively.12 The dietary fiber (total, soluble and insoluble) was quantified by the enzymatic-gravimetric AOAC method 999.03 modified by Mc Cleary and Rossiter13. This consisted of adding a mixture of fructanases (E-FRMXLQ; Megazyme International Ireland Limited, Bray, Ireland) to ensure complete removal of fructans, so they are not quantified in the dietary fiber measurement. Total fructans' content was analyzed separately, according to the enzymatic-UV method13 using a commercial assay kit (K-FRUCHK; Megazyme International Ireland Limited, Bray, Ireland). Total starch content was assessed following the enzymatic-UV method.14 Each value was the average of three determinations.In vitro predicted glycemic response
The resistant starch content was determined using the enzymatic-UV method.15 The available starch level was ascertained by calculating the difference between the content of total starch and resistant starch.The in vitro kinetics of starch digestion was evaluated, in triplicate, according to the method proposed by Goñi, Garcia Alonso and Saura Calixto14. The rate of starch digestion was expressed as the percentage of total starch hydrolyzed at 30, 60, 90 and 120 min of incubation. The hydrolysis index (HI) was derived from the ratio between the area under the hydrolysis curve of the GFB and the standard food (wheat white bread). This HI was found to be a good predictor of the glycemic response to food ingestion and highly correlated with the glycemic index (GI) in vivo. The predicted GI was estimated using the equation pGI = 39.71 + 0.549(HI), which yielded a correlation coefficient of r = 0.89, P < 0.05.14
In vivo glycemic response
Healthy adults were recruited. Exclusion criteria included: cigarette smoking; pregnancy; lactating; allergy to any food ingredients used in the test breads; diagnosed diabetes or gastrointestinal disease; family history of diabetes; use of medications known to affect glycemic response. Ten healthy volunteers (nine females, one male) aged 26.1 ± 3.1 years, and with body mass indexes (BMI) of 22.3 ± 1.8 kg m−2 (mean ± standard deviation) were included in the study.The GI produced by the control, and by the 12% ITFs-added GFB was determined. Subjects were studied once a week in the morning after a 10 to 12 h overnight fast. Wheat white bread (standard food) was tested twice during the first two weeks. On subsequent weeks, the volunteers ingested a portion of control or 12% ITFs-added loaf. Subjects were blinded to which GFB they were receiving. Each portion of food contained 25 g of available carbohydrates.17
The volunteers had 10 min to ingest each portion with up to 500 mL of water. Finger-prick capillary blood samples were taken using a lancing device (Accu-Check® Softclix, Roche Diagnostics, Mannheim, Germany) before the meal and at 15, 30, 45, 60, 90, and 120 min after starting to eat.16,17 Timing for blood samples started with the first bite of the test meal. Blood glucose concentration was measured using a blood-glucose meter (Accu-Check® Active, Roche Diagnostics, Mannheim, Germany).
The incremental areas under the blood-glucose curve (IAUC), excluding area below fasting, were calculated using the trapezoidal method.16 To calculate GI, the IAUC for each gluten-free bread was expressed as a percentage of the mean IAUC for white-bread taken by the same subject. The resulting mean values for all subjects represented the GI of the food16. As wheat white bread was the standard food used (GI of white bread = 100), the GI values were multiplied by 0.7 to obtain the GI value with glucose as the standard food (GI of glucose = 100)17. The glycemic load (GL) of each food was calculated according to the following equation: GL = [(GI(glucose=100) × available carbohydrate (g) per portion)/100]18.
Taking glucose as a standard food, GI of food can be classified into low (≤55), medium (56–69) and high (≥70), and GL into low (≤10), medium (11–19) and high (≥20)18.
Statistical analysis
Differences in treatment means were identified by one-way analysis of variance (ANOVA), and Tukey's test. Simple linear correlation (Pearson correlation) was also evaluated. Data were processed using Minitab 15.0 statistical software (Minitab Inc., Pennsylvania, USA).Results and discussion
Bread quality
Table 1 shows the effect of different ITFs levels on several quality parameters of fresh GFB. The ITFs treatments produced no significant changes in bake loss or loaf weight. All ITFs addition levels increased the specific volume of GFB, which showed a significant improvement compared with the control bread (0% ITFs).| Gluten-free breadsa | |||||||
|---|---|---|---|---|---|---|---|
| 0% ITFsb | 4% ITFsb | 8% ITFsb | 10% ITFsb | 12% ITFsb | |||
| a Gluten-free bread prepared in order to present 0, 4, 8, 10 and 12% of inulin-type fructans (ITFs) in the baked loaf. b Values are means ± standard deviations. Values followed by a different superscript in each row are significantly different (P < 0.05). | |||||||
| Bake loss (%) | 10.75a ± 0.69 | 11.67a ± 0.08 | 11.32a ± 0.56 | 11.94a ± 0.69 | 11.39a ± 0.46 | ||
| Loaf weight (g) | 200.09a ± 8.88 | 201.63a ± 1.49 | 200.92a ± 2.19 | 196.91a ± 2.58 | 199.94a ± 1.96 | ||
| Loaf volume (cm3) | 360.00b ± 22.91 | 393.33b ± 16.07 | 466.67a ± 2.89 | 488.33a ± 2.89 | 463.33a ± 5.77 | ||
| Loaf specific volume (cm3 g−1) | 1.79d ± 0.04 | 1.95c ± 0.06 | 2.32b ± 0.01 | 2.48a ± 0.03 | 2.36b ± 0.02 | ||
| Crust color | L* | 79.45a ± 1.33 | 67.76b ± 1.64 | 70.08b ± 2.24 | 64.54c ± 1.19 | 67.47b ± 1.23 | |
| a* | 6.66d ± 0.95 | 13.66a ± 0.57 | 10.27c ± 0.98 | 13.72a ± 0.61 | 12.15b ± 1.06 | ||
| b* | 31.40a ± 1.96 | 33.89a ± 3.05 | 34.09a ± 1.47 | 35.92a ± 2.37 | 34.53a ± 2.15 | ||
| Crumb color | L* | 81.45a ± 0.64 | 80.92a,b ± 0.42 | 80.37b ± 0.59 | 78.76c ± 0.58 | 79.08c ± 0.59 | |
| a* | 0.22a ± 0.02 | 0.10b ± 0.01 | 0.26a ± 0.03 | 0.16c ± 0.03 | 0.22a ± 0.03 | ||
| b* | 22.65a ± 0.45 | 22.32a ± 0.50 | 20.32c ± 0.18 | 19.77d ± 0.15 | 20.84b ± 0.33 | ||
| Crumb moisture (%) | 49.62a ± 0.25 | 47.47b ± 0.01 | 45.78c ± 0.02 | 45.29c ± 0.25 | 43.86d ± 0.19 | ||
| Crumb firmness (N) | 12.00a ± 1.34 | 9.62b ± 0.92 | 9.02b ± 0.58 | 8.86b ± 0.47 | 7.24c ± 0.17 | ||
Thus, the data suggests that ITFs can provide structure and CO2 retention capability during proofing and baking, in the same way as some hydrocolloids which have been used as structuring agents in GFB formulations.19 Hydrocolloids interact with water and produce a gel network structure that serves to increase batter viscosity and to strengthen the boundaries of the expanding cells, increasing gas retention through baking, enhancing the volume, and the structural characteristics and texture of GFB.6
The results of this study agreed with those reported by Korus et al.9 describing partial replacement of starch in GFB recipes for 3, 5 and 8% inulin. They observed a gradual increase in volume up to 9% according to inulin levels in the formulation, effects which were similar to those observed in this study. Addition of 4 to 12% ITFs in the formulation, equivalent to the addition of 8.6, 17.9, 22.7 and 28% fructans in GFB recipe (% flour/starch base), increased the specific volume of bread by between 8.2 and 38% (Table 1). The specific volume of bread formulated with 8.6% fructans addition, 4% ITFs-enriched GFB (1.95 ± 0.06 cm3 g−1, Table 1), is equal to the value found by Hager et al.10 for a 9% inulin added GFB (1.99 ± 0.18 cm3 g−1).
No significant effect of ITFs levels on crust color of GFB was detected (Table 1). Generally, the crust color of all ITFs-enriched breads was darker (lower L* and higher a* values) than that of control breads. It was noted that the higher the fructans level, the lower the L* values of bread crumb (r = −0.926, P = 0.024). This result is consistent with the literature,10,20 which reported that the presence of fructans in bread formulating favored the Maillard reaction. The darkening of the crust and crumb color was desirable as GFB tends to have a lighter color than wheat breads.21
The ITFs mixture contains reduced sugars that can participate in the Maillard reaction. In addition, hydrolysis of low molecular weight fructans may have occurred during the baking process, thereby increasing the quantity of free sugar (especially fructose), again favoring the Maillard reaction. The yeast (Saccharomyces cerevisiae) produces invertase that can hydrolyse low molecular weight fructans, and are also susceptible to hydrolysis during baking.22
Higher ITFs levels proportionally decrease the batter moisture (50.0%, 48.5%, 46.9%, 46.1% and 45.3% for batter with 0 to 12% fructans, respectively), which, consequently, decreases crumb moisture, giving values ranging from 50 to 44% for 0 to 12% ITFs-enriched GFB according to Table 1.
After production, no differences were observed in crumb firmness of 4 to 10% ITFs-containing breads, whereas the 12% fructans-enriched loaves resulted in a much softer crumb than control GFB (Table 1). These results suggest that ITFs may be used in the production of GFB with a softer crumb structure. This is a desirable characteristic as GFB are often characterized by a harder texture.21
Moisture loss was observed in all GFB breads during 48 h storage. The higher the ITFs levels, the lower the crumb moisture observed throughout the storage period. The rate of moisture loss for all breads was similar, at approximately 1% per 48 h (Fig. 1). Crumb hardening was observed during the storage of the breads, as evidenced by higher firmness values with increasing storage time (Fig. 1). Crumb hardening during storage is expected as a result of moisture loss as well as being due to starch retrogradation. GFBs age faster because, in the absence of gluten, migration of water from crumb to crust is facilitated, leading to more rapid moisture loss.6
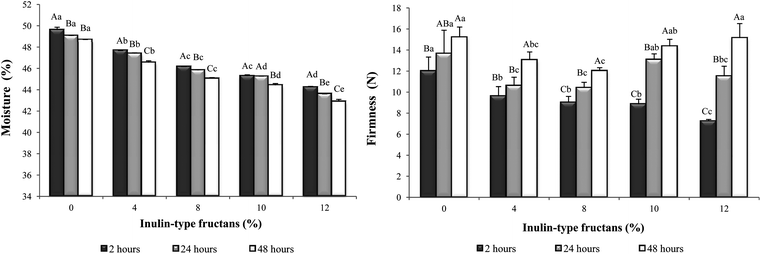 | ||
| Fig. 1 Crumb moisture and firmness of gluten-free breads enriched with different inulin-type fructans levels at 2, 24 and 48 h post-baking. Gluten-free bread prepared in order to present 0, 4, 8, 10 and 12% of inulin-type fructans (ITFs) in the baked loaf. Bars represent the mean values; error bars represent standard deviation. Bars with different lowercase letters within the same bread type are significantly different (P < 0.05). Bars for a given storage time with different capital letters are significantly different (P < 0.05). | ||
The higher the ITFs levels, the higher the firming rate observed during 48 h of storage. Firming rates were 27%, 36%, 33%, 62% and 109% for 0, 4, 8, 10 and 12% ITFs-containing breads, respectively. As previously reported, the addition of inulin to GFB resulted in an increased rate of hardening.9,10
Despite presenting a lower rate of hardening, control bread exhibited the firmest crumb 2, 24 and 48 h post baking. Thus, these data suggest that ITFs-containing breads present softer breadcrumbs, possibly due to this fiber water-retention capacity.
Sensory acceptance
ITFs-addition significantly increased the appearance, color, texture and overall acceptability of GFB (Table 2). All ITFs-enriched GFB formulations were acceptable, with scores ranging from 6.2 to 7.4. No significant differences in scores for sensory attributes were observed in ITFs-enriched breads.| Gluten-free breadsa | |||||
|---|---|---|---|---|---|
| 0% ITFsb | 4% ITFsb | 8% ITFsb | 10% ITFsb | 12% ITFsb | |
| a Gluten-free bread prepared in order to present 0, 4, 8, 10 and 12% of inulin-type fructans (ITFs) in the baked loaf. b Values are means ± standard deviations. Values followed by a different superscript in each row are significantly different (P < 0.05). | |||||
| Appearance | 5.11b ± 1.60 | 7.08a ± 1.60 | 7.28a ± 1.26 | 6.97a ± 1.34 | 7.03a ± 1.48 |
| Color | 5.94b ± 1.91 | 7.36a ± 1.62 | 7.14a ± 1.31 | 7.17a ± 1.36 | 7.14a ± 1.38 |
| Texture | 4.97b ± 1.95 | 6.67a ± 1.84 | 6.19a ± 1.75 | 6.42a ± 1.63 | 6.61a ± 1.61 |
| Taste | 6.06a ± 1.88 | 6.92a ± 1.64 | 6.72a ± 1.58 | 7.03a ± 1.18 | 6.97a ± 1.56 |
| Overall | 5.44b ± 1.80 | 7.00a ± 1.51 | 6.75a ± 1.44 | 6.86a ± 1.24 | 7.03a ± 1.28 |
Panelists' scores agree with the instrumental analysis that showed ITFs-addition improved bread quality by yielding better specific volume, softer crumb, and darker crust and crumb, all of which enhance the sensory characteristics of GFB. These results show that prebiotic ITFs could be successfully used to improve GFB quality and acceptability.
Proximate composition
ITFs increased dietary fiber content and proportionally decreased ash, lipids, protein and starch quantities (Table 3).| Gluten-free breadsa | |||||
|---|---|---|---|---|---|
| 0% ITFsb | 4% ITFsb | 8% ITFsb | 10% ITFsb | 12% ITFsb | |
| a Gluten-free bread prepared in order to present 0, 4, 8, 10 and 12% of inulin-type fructans (ITFs) in the baked loaf. b Values are means ± standard deviations. Values followed by a different superscript in each row are significantly different (P < 0.05). c HI = hydrolysis index, pGI = predicted glycemic index, pGL = predicted glycemic load. d Reference sample (white bread) data: HI = 100 ± 0.00, pGI(bread=100) = 94.61 ± 0.00, pGI(glucose=100) = 66.23 ± 0.00, pGL = 19.85 ± 0.00. | |||||
| Proximate composition | |||||
| Ash | 2.41a ± 0.02 | 2.30b ± 0.01 | 1.97c ± 0.06 | 1.97c ± 0.03 | 1.86d ± 0.15 |
| Lipids | 7.93a ± 0.03 | 7.56b ± 0.01 | 7.23c ± 0.02 | 7.07d ± 0.03 | 6.50e ± 0.01 |
| Protein | 7.84a ± 0.19 | 7.87a ± 0.07 | 6.68b ± 0.25 | 6.50b ± 0.05 | 6.33b ± 0.16 |
| Total starch | 73.57a ± 1.83 | 68.15b ± 1.23 | 66.82b,c ±1.25 | 64.80b,c±2.29 | 62.96c ± 1.75 |
| Resistant starch | 3.03a ± 0.14 | 2.81b ± 0.03 | 2.68b ± 0.04 | 2.65b ± 0.02 | 2.46c ± 0.02 |
| Digestible starch | 70.54 | 65.34 | 64.15 | 62.14 | 60.49 |
| Total dietary fiber | 7.03 | 11.35 | 16.22 | 18.37 | 20.14 |
| Insoluble fiber | 4.54a ± 0.09 | 4.39a,b ± 0.20 | 4.21a,b ± 0.07 | 4.04b ± 0.13 | 4.11b ± 0.16 |
| Soluble fiber | 2.49 | 6.96 | 12.01 | 14.33 | 16.03 |
| Total fructans | 0.74e ± 0.01 | 5.18d ± 0.05 | 10.24c ± 0.07 | 12.50b ± 0.04 | 14.12a ± 0.16 |
| Other soluble fibers | 1.75a ± 0.08 | 1.78a ± 0.13 | 1.77a ± 0.08 | 1.83a ± 0.05 | 1.91a ± 0.10 |
| Predicted glycemic response , | |||||
| HI | 97.30a ± 0.79 | 89.98a,b ± 1.77 | 84.88b,c ± 2.13 | 82.43b,c ± 4.53 | 80.86c ± 0.25 |
| pGI (bread=100) | 93.13a ± 0.44 | 89.11a,b± 0.98 | 86.31b,c ± 1.17 | 84.97b,c ± 2.49 | 84.10ca ± 0.61 |
| pGI (glucose=100) | 65.19a ± 0.31 | 62.38a,b ± 0.68 | 60.42b,c ± 0.82 | 59.48b,c ± 1.74 | 58.87c ± 0.43 |
| pGL – 50 g portion | 11.58a ± 0.05 | 10.66b ± 0.12 | 10.43b,c ± 0.14 | 10.30b,c ± 0.30 | 9.93c ± 0.07 |
Conversion of ITFs levels of breads to a wet basis revealed that 4%, 8%, 10% and 12% addition levels resulted in 2.7%; 5.5%; 6.8% and 7.9% fructans in the final baked product, respectively. These results show that approximately one third of the ITFs added were hydrolyzed during proofing and baking. ITFs loss was calculated based on the difference between the expected value (the content added in the recipes) and quantified values in the GFB. The total fructans levels in the loaves produced were higher than in the GFB obtained by Korus et al.9, ranging from 0.7 to 3.3%.
There is scant information in the literature on ITFs stability during thermal processing. Bohm et al.23 observed 40% inulin loss after heating at 180 °C for 60 min, values consistent with those observed in the present study. Praznik et al.22 noted 35% inulin loss and 47% oligofructose loss in the wheat-rye breads supplemented with these fructans. Korus et al.9 found 59 to 78% inulin loss in GFB enriched with inulin. ITFs loss is likely to be due to hydrolysis of low molecular weight fructans during proofing (by the yeast Saccharomyces cerevisiae) and baking.22
The addition of 12% ITFs to the basic formulation is required in order to obtain GFB enriched with 8% ITFs (4 g of fructans per 50 g bread serving size), levels that can provide health benefits given that a prebiotic effect has been observed with an intake of 4–5 g of inulin and oligofructose per day24, while calcium absorption enhancement has been obtained with 8 g ITFs mixture per day2,3. This is particularly important because celiac patients are more susceptible to presenting lower calcium absorption, lower BMD, and suffering from osteoporosis.1
Generally, GFB is characterized by low fiber content because of the formulation normally employed.6 It has been reported25,26 values of dietary fiber raging from 1.2 to 7.2 g per 100 g in commercial GFB, whereas in enriched bread those values varied from 6.1 to 9.6 g per 100 g.25 Compared with those commercial products, the formulated 4–12% ITFs-containing GFB presented higher fiber content, ranging from 11 to 20% on a dry matter basis, and from 6 to 11% on a wet matter basis.
In vitro predicted glycemic response
Firstly, an in vitro study was performed in order to evaluate the importance of ITFs level effects on starch digestibility. The hydrolysis index (HI) was calculated based on areas under the starch hydrolysis curve. To predict in vivo effects, a hydrolysis index based predicted glycemic index (pGI) and predicted glycemic load (pGL) were also calculated (Table 3).Data demonstrate a high predicted glycemic response following ingestion of control GFB, similar to that of wheat white bread. ITFs-enrichment significantly reduced pGI (r = −0.994, P = 0.001) and pGL (r = −0.956, P = 0.011). Data suggests that 12% ITFs-addition reduced pGI by 10% and pGL by 14% (Table 3).
In vivo glycemic response
Based on in vitro results, a control and 12% ITFs-enriched GFBs were selected for in vivo analysis. Participants consumed food portions that contained 25 g of available carbohydrates, corresponding to 41.7 g of wheat white bread (standard food), 70.3 g of control GFB, and 74.1 g of 12% ITFs-enriched GFB.The mean blood-glucose responses to the tested meals are shown in Fig. 2. Fasting blood-glucose levels did not differ before the treatments. Data clearly demonstrated that 12% ITFs-addition decreased glucose response to GFB. The mechanism involved remains to be fully characterized.
 | ||
| Fig. 2 Blood glucose response of control and fructans enriched gluten-free bread. All tests were 25 g portions of available carbohydrates. Values are means and standard error of ten health individuals. | ||
The shapes of the glucose response curve (Fig. 2) are consistent with the results of Brand-Miller et al.27 who assessed the glycemic response of over one thousand foods, and reported that high-GI and low-GI foods both present similar curve shapes, and differ only in blood-glucose levels at certain points of the curve (30, 60 and 90 min).
Similarly to Packer, Dornhorst and Frost28, we also noted that GFB bread produced a high glycemic response, and found no difference in GI between white wheat bread and conventional GFB. According to Segura and Rossel26 commercial GFB presents a high pGI(bread=100 ), values between 83.3 and 96.1.
Control GFB (0% ITFs) presented high GI and moderate GL. The addition of 12% ITFs caused a reduction of 32% GI and 36% GL, effects greater than those expected based on in vitro analysis (Tables 3 and 4). This was because ITFs decreased the glycemic response to GFB, mainly through mechanisms related to human digestion, effects that cannot be predicted by in vitro kinetics of starch.
| White bread | Gluten-free breadsa | ||
|---|---|---|---|
| 0% ITFsb | 12% ITFsb | ||
| a Gluten-free bread prepared in order to present 0, 4, 8, 10 and 12% of inulin-type fructans (ITFs) in the baked loaf. b Values are means ± standard error. Values followed by a different superscript in each row are significantly different (P < 0.05). c IAUC = incremental area under the blood glucose response curve, GI = glycemic index, GL = glycemic load. | |||
| IAUCc (mmol min L−1) | 88.81a ± 5.97 | 88.32a ± 4.77 | 60.26b ± 4.06 |
| GI(bread = 100) | 100a ± 0.00 | 100.89a ± 4.06 | 68.46b ± 3.58 |
| GI(glucose = 100) | 70a ± 0.00 | 70.62a ± 2.84 | 47.92b ± 2.51 |
| GL – 50 g portion | 20.99a ± 0.00 | 12.55b ± 0.50 | 8.08c ± 0.42 |
The 12% ITFs-enriched GFB is a low-GI and low-GL food that combines a physiologically significant supply of prebiotic soluble dietary fiber, high-quality and acceptability.
Conclusions
Prebiotic ITFs is a promising improver for GFB, yielding better specific volume, softer crumb, improved crust and crumb browning, all of which enhance the sensory acceptability of the product.Due to about one third fructans loss during the baking process, a 12% ITFs addition to the formulation is needed in order to obtain GFB enriched with 4 g of fructans/portion (50 g), levels consistent with their prebiotic properties. This ITFs addition level decreased glycemic response of GFB by 30% resulting in a low glycemic index (GI = 48) and low glycemic load (GL = 8) product that can benefit patients with both celiac disease and diabetes.
Prebiotic ITFs are feasible ingredients in the manufacture of high-quality health GFBs, improving bread quality while providing nutritional and functional benefits to patients with celiac disease. Therefore, ITFs-enriched GFBs can contribute to a nutritionally diversified gluten-free diet and increase calcium absorption in CD sufferers.
Acknowledgements
The authors gratefully acknowledge the volunteers who kindly participated in this study. Appreciation is extended to Rosana Ap. Manólio Soares for her assistance with some analyses performed. This study was financially supported by the State of São Paulo Research Foundation (FAPESP) under scholarship agreement no. 04/14127-3.References
- V. Capriles, L. Martini and J. Arêas, Metabolic osteopathy in celiac disease: importance of a gluten-free diet, Nutr. Rev., 2009, 67, 599–606 CrossRef.
- S. Abrams, I. Griffin, K. Hawthorne, L. Liang, S. Gunn, G. Darlington and K. Ellis, A combination of prebiotic short- and long-chain inulin-type fructans enhances calcium absorption and bone mineralization in young adolescents, Am. J. Clin. Nutr., 2005, 82, 471–476 CAS.
- S. Abrams, K. Hawthorne, O. Aliu, P. Hicks, Z. Chen and I. Griffin, An inulin-type fructan enhances calcium absorption primarily via an effect on colonic absorption in humans, J. Nutr., 2007, 137, 2208–2212 CAS.
- G. Gibson, H. Probert, J. Loo, R. Rastall and M. Roberfroid, Dietary modulation of the human colonic microbiota: updating the concept of prebiotics, Nutr. Res. Rev., 2004, 17, 259–275 CrossRef CAS.
- A. Lee and J. Newman, Celiac diet: its impact on quality of life, J. Am. Diet. Assoc., 2003, 103, 1533–1535 CrossRef.
- E. Gallagher, T. Gormley and E. Arendt, Recent advances in the formulation of gluten-free cereal-based products, Trends Food Sci. Technol., 2004, 15, 143–152 CrossRef CAS.
- T. Thompson, M. Dennis, L. Higgins, A. Lee and M. Sharrett, Gluten-free diet survey: are Americans with coeliac disease consuming recommended amounts of fibre, iron, calcium and grain foods?, J. Hum. Nutr. Diet., 2005, 18, 163–169 CrossRef CAS.
- J. Murray, Celiac disease in patients with an affected member, type 1 diabetes, iron-deficiency, or osteoporosis?, Gastroenterology, 2005, 128, S52–56 CrossRef.
- J. Korus, K. Grzelak, K. Achremowicz and R. Sabat, Influence of prebiotic additions on the quality of gluten-free bread and on the content of inulin and fructooligosaccharides, Food Sci. Technol. Int., 2006, 12, 489–495 CrossRef CAS.
- A. Hager, L. Ryan, C. Schwab, M. Ganzle, J. O'Doherty and E. Arendt, Influence of the soluble fibres inulin and oat b-glucan on quality of dough and bread, Eur. Food Res. Technol., 2011, 232, 405–413 CrossRef CAS.
- AACC International, Approved methods of the American Association of Cereal Chemists, AACC, St. Paul, MN, 10th edn, 2000 Search PubMed.
- AOAC, Official Methods of Analysis, AOAC, Gaithersburg, Md, 17th edn, 2003 Search PubMed.
- B. McCleary and P. Rossiter, Measurement of novel dietary fibers, J. AOAC Int., 2004, 87, 707–717 CAS.
- I. Goni, A. Garcia Alonso and F. Saura Calixto, A starch hydrolysis procedure to estimate glycemic index, Nutr. Res., 1997, 17, 427–437 CrossRef CAS.
- I. Goni, L. GarciaDiz, E. Manas and F. SauraCalixto, Analysis of resistant starch: a method for foods and food products, Food Chem., 1996, 56, 445–449 CrossRef CAS.
- FAO/WHO – Food and Agriculture Organization/World Health Organization, Carbohydrates in human nutrition, Report of a joint FAO/WHO Expert Consulation, Rome, Apr. 14–18, 1997. Food and Nutrition Paper, 66, 1998 Search PubMed.
- T. Wolever, J. Brand-Miller, J. Abernethy, A. Astrup, F. Atkinson, M. Axelsen, I. Bjorck, F. Brighenti, R. Brown, A. Brynes, M. Casiraghi, M. Cazaubiel, L. Dahlqvist, E. Delport, G. Denyer, D. Erba, G. Frost, Y. Granfeldt, S. Hampton, V. Hart, K. Hatonen, C. Henry, S. Hertzler, S. Hull, J. Jerling, K. Johnston, H. Lightowler, N. Mann, L. Morgan, L. Panlasigui, C. Pelkman, T. Perry, A. Pfeiffer, M. Pieters, D. Ramdath, R. Ramsingh, S. Robert, C. Robinson, E. Sarkkinen, F. Scazzina, D. Sison, B. Sloth, J. Staniforth, N. Tapola, L. Valsta, I. Verkooijen, M. Weickert, A. Weseler, P. Wilkie and J. Zhang, Measuring the glycemic index of foods: interlaboratory study, Am. J. Clin. Nutr., 2008, 87, 247S–257S CAS.
- F. Atkinson, K. Foster-Powell and J. Brand-Miller, International tables of glycemic index and glycemic load values: 2008, Diabetes Care, 2008, 31, 2281–2283 CrossRef.
- A. Anton and S. Artfield, Hydrocolloids in gluten-free breads: a review, Int. J. Food Sci. Nutr., 2008, 59, 11–23 CrossRef CAS.
- P. Poinot, G. Arvisenet, J. Grua-Priol, C. Fillonneau, A. Le-Bail and C. Prost, Influence of inulin on bread: kinetics and physico-chemical indicators of the formation of volatile compounds during baking, Food Chem., 2010, 232, 1474–1484 CrossRef.
- E. Gallagher, T. Gormley and E. Arendt, Crust and crumb characteristics of gluten free breads, J. Food Eng., 2003, 56, 153–161 CrossRef.
- W. Praznik, E. Cieslik and A. Filipiak-Florkiewicz, Soluble dietary fibres in Jerusalem artichoke powders: composition and application in bread, Nahrung, 2002, 46, 151–157 CrossRef CAS.
- A. Bohm, I. Kaiser, A. Trebstein and T. Henle, Heat-induced degradation of inulin, Eur. Food Res. Technol., 2005, 220, 466–471 CrossRef.
- V. Rao, The prebiotic properties of oligofructose at low intake levels, Nutr. Res., 2001, 21, 843–848 CrossRef CAS.
- T. Thompson, Folate, iron, and dietary fiber contents of the gluten-free diet, J. Am. Diet. Assoc., 2000, 100, 1389–1396 CrossRef CAS.
- M. Segura and C. Rosell, Chemical composition and starch digestibility of different gluten-free breads, Plant Foods Hum. Nutr., 2011, 66, 224–230 CrossRef.
- J. Brand-Miller, K. Stockmann, F. Atkinson, P. Petocz and G. Denyer, Glycemic index, postprandial glycemia, and the shape of the curve in healthy subjects: analysis of a database of more than 1000 foods, Am. J. Clin. Nutr., 2009, 89, 97–105 CrossRef CAS.
- S. Packer, A. Dornhorst and G. Frost, The glycaemic index of a range of gluten-free foods, Diabetic Med., 2000, 17, 657–660 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
