 Open Access Article
Open Access ArticleNanomaterial disposal by incineration
Amara L.
Holder
a,
Eric P.
Vejerano
b,
Xinzhe
Zhou
b and
Linsey C.
Marr
*b
aUnited States Environmental Protection Agency, National Risk Management Research Laboratory, 109 T. W. Alexander Dr, Research Triangle Park, NC 27711, USA
bCivil and Environmental Engineering, Virginia Tech, 411 Durham Hall, Blacksburg, VA 24061, USA. E-mail: lmarr@vt.edu; Fax: +1 540 231 7916; Tel: +1 540 231 6071
First published on 11th July 2013
Abstract
As nanotechnology-based products enter into widespread use, nanomaterials will end up in disposal waste streams that are ultimately discharged to the environment. One possible end-of-life scenario is incineration. This review attempts to ascertain the potential pathways by which nanomaterials may enter incinerator waste streams and the fate of these nanomaterials during the incineration process. Although the literature on incineration of nanomaterials is scarce, results from studies of their behavior at high temperature or in combustion environments for other applications can help predict their fate within an incinerator. Preliminary evidence suggests nanomaterials may catalyze the formation or destruction of combustion by-products. Depending on their composition, nanomaterials may undergo physical and chemical transformations within the incinerator, impacting their partitioning within the incineration system (e.g., bottom ash, fly ash) and the effectiveness of control technology for removing them. These transformations may also drastically affect nanomaterial transport and impacts in the environment. Current regulations on incinerator emissions do not specifically address nanomaterials, but limits on particle and metal emissions may prove somewhat effective at reducing the release of nanomaterials in incinerator effluent. Control technology used to meet these regulations, such as fabric filters, electrostatic precipitators, and wet electrostatic scrubbers, are expected to be at least partially effective at removing nanomaterials from incinerator flue gas.
Environmental impactManufactured nanomaterials are entering the waste stream, and some of these will be subject to incineration as part of their end-of-life treatment. The behavior and fate of nanomaterials during the incineration process and the environmental impacts of this disposal option are largely unknown. In this literature review, we draw upon current knowledge of nanomaterial combustion, waste incineration, and air pollution control technology to identify critical knowledge gaps. |
Introduction
The global market for nanotechnology-based products was about $254 billion in 2009 and has been increasing at a rate of 25% per year.1 As nanotechnology-based products enter into widespread use, many will end up in disposal waste streams.2 Disposal is the phase in the product life cycle at which most nanomaterials are predicted to enter the environment.3,4 To date, most research has ignored this phase,5 likely due to a lack of information available on nanomaterial disposal.6 However, this topic has gained recent attention with several life cycle analyses and reviews specifically addressing nanomaterial release through different disposal pathways.7–9 We are aware of two nanomaterial life cycle analyses that have included end-of-life processes.7,9 The analyses considered disposal of nanomaterials into a waste incinerator, followed by energy release and emissions to air and water. However, results were based on extrapolation of data for conventional materials of bulk size due to lack of information on energy recovery and emissions for nanoscale materials.Incineration is a key waste treatment technique with great potential for modifying nanomaterials and either controlling them effectively or releasing them to the environment. Nanomaterials may enter waste streams that will be incinerated through several different pathways: disposal of consumer products as municipal solid waste (MSW), wastes generated from nanotechnology research and development, hazardous wastes, medical and infectious wastes, and sewage sludge from wastewater treatment plants (WWTP) handling nanomaterial-laden water. At least 13% (greater than 32 million tons) of MSW is incinerated in the US, much of it for energy recovery, and this fraction is expected to rise in the future.10 As a whole, EU member states incinerate approximately 20% of MSW, and some countries (Denmark, Switzerland, and Norway) incinerate over half of their MSW.11 Countries with limited space for landfilling tend to incinerate larger fractions; for example, Japan incinerates 79% of its MSW.11 Incineration is used to treat over 3 million tons of the 44 million tons of hazardous waste, including research waste, generated each year in the US.12,13 Incineration is also used to treat almost 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of infectious waste each year in the US, some of which will contain nanomaterials used for medical applications.14 Additionally, a considerable fraction of WWTP sludge is incinerated: 19% in the US, 25% in Europe, and 100% in Switzerland.15
000 tons of infectious waste each year in the US, some of which will contain nanomaterials used for medical applications.14 Additionally, a considerable fraction of WWTP sludge is incinerated: 19% in the US, 25% in Europe, and 100% in Switzerland.15
Understanding the fate of nanomaterials during the incineration process is imperative because physical and chemical transformations of nanomaterials could drastically affect their transport and toxicity in the environment. Not only may the nanomaterials themselves be modified, but they may catalyze the formation and destruction of other pollutants (e.g., dioxins). However, a lack of knowledge concerning the behavior of nanomaterials under high-temperature, highly oxidative conditions hinders accurate prediction of their effect on pollutant formation.
The objective of this review is to critically assess the state of knowledge of the incineration of nanomaterial wastes. The review considers potential types of nanomaterial wastes, the possible routes through which nanomaterials may enter into incineration waste streams, and regulation of those routes. A brief description of the physical processes occurring during incineration will be presented. Additionally, the regulations on incinerator effluents and air pollution controls that may minimize nanoparticle emissions to the environment will be evaluated. Insight into the behavior and fate of nanomaterial wastes in incinerators will be drawn from descriptions of the incineration process, experimental studies of nanomaterials in various combustion systems, and experimental evidence from a limited number of studies investigating nanomaterial incineration. Finally, critical knowledge gaps and recommendations for future investigations will be discussed.
Potential for nanowaste incineration
In the US in 2010, it was estimated that of the 250 million tons of waste generated, 13% were plastics, 30% were paper and packaging materials, and 9% were metals.17 Although plastics account for a small amount of the waste, they have the potential to be a large source of nanomaterials. Nanomaterials are frequently incorporated into polymer matrices to form nanocomposite plastics, and the volume of nanomaterials is expected to increase as novel applications emerge.Almost all types of nanomaterials can be incorporated into polymers. Clay, SiO2, and TiO2nanoparticles as well as carbon nanotubes (CNT) are used as fillers to improve the mechanical, electrical, magnetic, and thermal properties of polymers.18 Nanocomposites containing TiO2 and nanoclay incorporated in polyethylene terephthalate (PET) bottles are used for high-barrier packaging to minimize oxygen penetration.19–21 Nanoscale metal and metal oxides have been incorporated into plastics and packaging materials as pigments or to prevent color degradation.22,23 Nanocomposite foams containing metal oxides such as Al2O3, SiO2, clay, and polystyrene latex are being developed for use in a variety of applications including insulators, batteries, scaffolds, catalyst supports, and sensors.24,25 Nanosilica is used in building materials and is incorporated into plastics as part of pigment formulation.
Another potentially large source of nanomaterials in the waste incineration stream is scrap tires. Tires may incorporate nanoscale amorphous silica, carbon black, clay, and CNT26 and are often processed into rubber crumb and used as fuel in cement kilns, utility boilers, pulp and paper mills, industrial boilers, and dedicated scrap tire-to-energy facilities.27–29 In 2003, it was estimated that more than 100 million tires (almost half of all those produced) were burned to supplement coal and wood energy use in the US.27
Personal care products are another class of consumer products that may comprise a substantial portion of waste incinerated. Nanomaterials incorporated into fabrics, usually nanosilver, can be washed off into wastewater during laundering.30 About 95% of Ag and TiO2 nanomaterials used in personal care products is estimated to end up in wastewater.16 Considering that anywhere from 19% to 100% of WWTP sludge is incinerated15 and assuming that almost all nanomaterials partition to the sludge rather than the liquid effluent,31–35 a considerable portion of nanomaterials entering the wastewater stream may eventually be transferred to incinerators.
The US and the EU are the major manufacturers of nanomaterials, accounting for 49% and 30% of annual global production, respectively.36 Actual production quantities of nanomaterials remain elusive, and estimates of the amounts have large uncertainties. Piccinno et al.37 estimated global production levels ranging from 0.6 to 5500 tons per year, depending upon the material. SiO2 and TiO2 had the largest estimates at several thousand tons per year, followed by ZnO and CNT with several hundred tons per year. The technology for large-scale production of nanosized metals, clay, ceramics, and SiO2 is already well-developed, and these nanomaterials may be used in numerous commercial applications. Thus, these classes of nanomaterials are expected to make up a considerable fraction of nanowastes. Despite growing production capacities,38 estimates of fullerene production remain low, in the range of 0.6–80 tons per year.37,39 Much uncertainty still exists, as US production estimates39 are similar to or larger than global estimates for some nanomaterials.37
While no industry-wide information on nanowaste flows is available, researchers have begun to estimate releases of nanomaterials to the environment. Nowack and colleagues15,16,40 estimated the mass flow of nanomaterials in waste treatment compartments, including WWTP, incinerators, and landfills. Cosmetics (nanoscale TiO2, ZnO, Ag), coatings and cleaning agents (nanoscale TiO2, ZnO, Ag), and dietary supplements (nanoscale TiO2, ZnO, Ag) were assumed to be released into the wastewater stream, while composites (CNT and fullerenes), plastics (nanoscale TiO2, ZnO, Ag), light bulbs (nanoscale TiO2), and glass and ceramics (nanoscale TiO2, Ag) were assumed to be nearly totally released to incinerators and landfills.15Table 1 shows estimated mass flows of nanomaterials to incinerators from production, manufacturing, and consumption. The flows are separated into two streams, one that goes directly to incineration and one that passes through WWTPs (i.e., disposed into wastewater first, partitioned to the sludge during treatment, and then incinerated as part of the sludge). The data are based on estimates of mass flows of Ag, TiO2, and CNT to incinerators in Switzerland under a high-exposure scenario16 and on estimates of mass transfer of TiO2, ZnO, Ag, and CNT to the environment for the US using a probabilistic method of environmental exposure analysis.15 Among the nanomaterials considered, the quantity of TiO2 is two orders of magnitude higher than that of Ag, CNT, and ZnO. For both Ag and TiO2, over half of the input to incinerators arrives via WWTPs, while for CNT, almost all input to incinerators is direct. This distribution of nanomaterials in solid and liquid waste concurs with the global production of different nanomaterials, as TiO2 is the most prevalent nanomaterial currently.15,16
Regulation of nanowaste disposal
In the US, there are no guidelines or regulations that specifically address the disposal of nanomaterials. Rather, they fall under existing legislation, primarily the Resource Conservation and Recovery Act (RCRA), which gives the Environmental Protection Agency (EPA) the authority to regulate the generation, transportation, treatment, storage, and disposal of hazardous waste.41 It is widely accepted that RCRA offers broad statutory authority to EPA to regulate wastes containing nanomaterials.42 However, the current characteristics used to define hazardous wastes may not encompass nanomaterials adequately.42 The agency could conceivably add a new category to handle “nanowaste” if sufficient evidence for such action became available.41RCRA gives EPA broad authority to regulate waste treatment plants, including incinerators. Under the Clean Water Act (CWA) and the Clean Air Act (CAA), EPA is able to regulate potential releases of nanomaterials into water and air. However, as Powell et al.43 pointed out, the lack of adequate tools and methods to measure emissions of nanoparticles accurately and significant gaps in data, monitoring, and technology hinder the use of those statutes to regulate nanoparticle emissions. This is particularly true for emissions from combustion sources, as they produce incidental nanoparticles that may be difficult to distinguish from nanoparticles derived from nanotechnology-based products.
In Europe, Directive 2008/98/EC of the European Parliament and of the Council and Council Directive 1991/689 govern waste management. However, neither of them mentions nanoparticles,44,45 as the paucity of toxicological data makes it difficult to assess whether nanomaterials meet the criteria of hazardous waste. Austria's Waste Management Act also lacks provisions specific to nanomaterials.46 A review of Australian regulation on nanomaterials47 shows that the Hazardous Waste Act (HWA) and the Environment Protection Act fail to regulate waste containing nanomaterials; however, some waste streams associated with nanotechnology-based products might fall within the broadly defined hazardous and prescribed wastes that are regulated by the acts. In Japan, the Ministry of the Environment (MOE) released a guideline recognizing that companies are expected to control and prevent the environmental release of nanomaterials.48 It appears that no country's environmental agency has yet developed effective strategies for management of nanowaste. This situation is not surprising given that researchers are still trying to understand the toxicity of nanomaterials.
Nanomaterials pose a challenge to EPA's current framework to define and regulate hazardous waste. The primary challenge is knowing whether nanomaterials in waste pose a novel environmental risk. The major barrier for EPA to consider nanomaterials as hazardous waste is the difficulty in determining their toxicity via the test of leaching capacity, Toxicity Characteristic Leaching Procedure (TCLP), since nanomaterials often do not behave as traditional bulk materials do. The TCLP is designed to simulate leachate from an unlined landfill. As nanomaterials may adhere to soils, undergo transport in groundwater, or infiltrate into drinking water supplies in a different manner from bulk materials, results of the TCLP might not sufficiently represent the toxicity of nanomaterials. Another problem is that some nanowastes will be disposed of as household waste, which is exempt from hazardous waste regulations.41 For manufacturers of nanomaterials, a major exemption is the current 100 kg annual production threshold. Below this threshold, manufacturers are not required to notify EPA of their activities or establish contingency plans, and they can store wastes for more than 90 days.42 Because nanomaterials may not need to be produced in the same volume as bulk materials to achieve commercially viable applications, it is possible that a larger fraction of nanomaterial manufacturers than expected will be exempt from hazardous waste regulations. For the reason of commercial confidentiality, the volume of nanowastes and anticipated methods of disposal are not publicly available, making it difficult for EPA to track nanowaste and evaluate its risk.
In the absence of guidelines from EPA addressing disposal of nanomaterial-containing waste, many universities have established their own policies, including identifying and evaluating or collecting nanomaterials for special waste disposal.49 Since some studies50–54 suggest possible toxic effects, MIT's Environment, Health, and Safety (EHS) office treats nanomaterials as particularly hazardous substances to be collected for special disposal. The health and safety offices of some universities, including Virginia Tech, the University of California at Berkeley, the University of Pennsylvania, and the California Institute of Technology, state that all solutions and solid materials contaminated with nanoparticles must be disposed of as hazardous waste. The California Nanosafety Consortium of Higher Education has developed the Nanotoolkit,55 a document covering best practices and guidelines for working with nanomaterials in academic research settings. According to the Nanotoolkit, solid waste contaminated with nanoparticles is required to be collected in a rigid container with a tight fitting lid, while researchers handling liquid solutions containing nanomaterials should use leak-proof containers that are compatible with all contents. Additionally, waste containers must be labeled to specify nanomaterial types and their hazardous characteristics. However, the effectiveness of these methods in protecting workers and environment has not been proven.57
Less is known about nanomaterial disposal in industrial settings, since companies are not required to provide information on their nanomaterial handling and disposal practices. A survey of industrial practices found that companies have yet to collect extensive data on the fate of nanomaterials in the use and disposal stages.58 As nanotechnology expands beyond the research and development phase, nanowaste will grow to include much larger quantities of consumer products and industrial waste.
Waste incineration physical conditions
In contrast to landfilling, incineration can be used to remove highly toxic organic wastes, reduce the volume of wastes, and potentially recover some of the energy stored in wastes. Incineration facilities burn hazardous wastes at high temperatures (850–1200 °C) in oxidative environments to ensure complete combustion of the waste before release to the atmosphere. There are various types of incinerators, including water-wall, modular, multiple hearth, catalytic combustion, waste-gas flare, direct-flame, liquid injection, fluidized bed, rotary kiln, and grate incinerators (moving and fixed). The choice of incinerator type depends on the type, volume, and hazard of the waste to be destroyed.In the US, EPA estimates that as of 2010, there were 176 industrial incinerators, 167 MSW incinerators, and 107 hazardous and medical waste incinerators (22 of them are hazardous chemical incinerators).59 Moving grate incinerators are the most common type in the US and account for 90% of the MSW incinerators in Europe.9 Moving grate incinerators can handle large volumes of waste with heterogeneous composition and calorific value. Rotary kiln incinerators are commonly used because they can combust various types of wastes, including solid, liquids, and sludge, with minimal processing. Fluidized bed incinerators are also common due to their high combustion efficiency and low emissions compared to other incinerator types.
Since the majority of incinerators used for MSW are grate types, the details of the processes in them that may alter, destroy, and form new nanomaterials are presented in greater detail. The schematic of a grate incinerator is illustrated in Fig. 1.56 A typical incinerator operation includes four general processes: pretreatment, combustion, energy recovery, and flue gas cleaning. If needed, pretreatment includes shredding and sorting. The waste is then introduced to the combustion chamber by moving grates. First, wastes are dried by heating at 100 °C, and air is injected to remove as much moisture as possible. As the drying stage wanes, the wastes are heated at 250 °C under pyrolytic conditions, which causes some components to outgas. Oxygen is supplied to the incinerator and the gases ignite. During this process, the wastes are burned at lower temperature (450 °C), and easily oxidizable solid wastes volatilize and decompose. More oxygen (primary and secondary air) is introduced to the combustion chamber, and the environment becomes highly oxidizing, with temperatures reaching around 1100–1200 °C. In most cases, the large amount of heat produced during combustion is recovered for energy use. After much of the combustible materials and gases are oxidized, the incineration process enters the last phase—burn-out—and the non-combustible materials subsequently cool. The bottom ash from the primary combustion chamber, which typically accounts for 15–25% of the waste by weight, is sent to a landfill.60 Flue gases containing combustion by-products and PM are routed to air pollution control devices where fly ash (i.e., PM) and other combustion by-products are treated or removed, and the cleaned gas is released into the atmosphere.
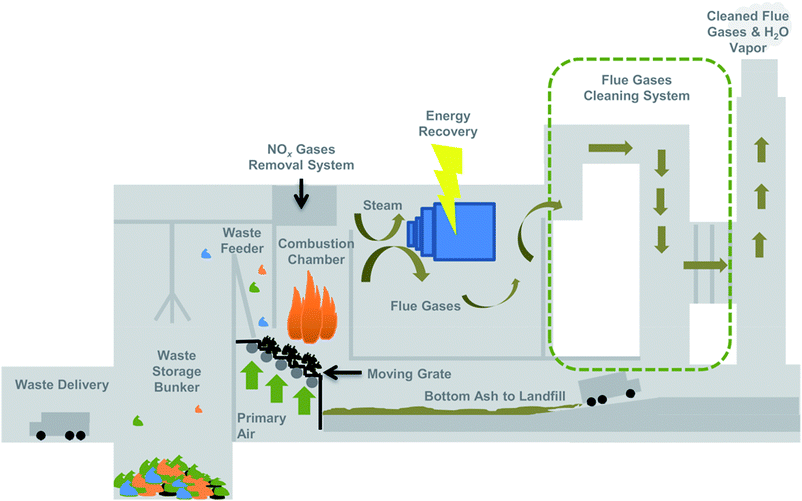 | ||
| Fig. 1 Schematic of grate incinerator operation.56 | ||
During the combustion of waste, myriads of hazardous pollutants and products of incomplete combustion are formed at different stages. The most common pollutants released from waste incineration include acid gases such as hydrogen chloride (HCl) and sulfur dioxide (SO2); nitrogen oxides (NOx); heavy metals such as mercury, lead, and cadmium; PM; polycyclic aromatic hydrocarbons (PAH) and other semi-volatiles; and polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/F). While incinerators typically operate at higher temperature to increase pollutant destruction efficiencies, some zones in the combustion region, as well as in the post-combustion region, facilitate pollutant formation. A portion of NOx (thermal NOx) is formed at higher temperatures (>1450 °C) in the combustion zone. At lower temperatures (150 °C to 400 °C), PCDD/F are formed, primarily from wastes containing chlorine (e.g. PVC).61,62 Metals present in wastes, depending upon volatility, can partition to the bottom ash or to PM63,64 where they can catalyze the formation of other pollutants such as PAH and PCDD/F.67,70–72 Emissions of most of these pollutants are regulated to minimize their adverse environmental and health effects. Most modern incinerators are equipped with advanced air pollution control devices: fabric filters (baghouses), scrubbers, electrostatic precipitators, etc., which are described in a subsequent section.
Regulation of incinerator emissions
In the US, incinerators must operate under an air permit, and thus their emissions are regulated. Emission limits are set by EPA, but individual states may set more stringent requirements. However, some facilities may obtain an exemption from meeting these regulations depending upon the type of waste they incinerate and other related process variables (e.g., power generation or material recovery). Emission limits vary depending upon the type of incinerator and waste incinerated, as do the amount and type of emissions vary with the ‘fuel’ being incinerated.Hazardous waste incineration is a special sub-category that falls under RCRA and therefore has reporting requirements and emissions guidelines that are separate from those for incinerators burning non-hazardous waste. Regulated emissions from hazardous waste incinerators include metals and PM.65 Regulated metal emissions are lumped into a semi-volatile category (Pb and Cd) and a low-volatility category (As, Be, and Cr). Some nanomaterials contain toxic metals, such as Cd or Pb in quantum dots, which may route these nanoparticles into the hazardous waste stream. The emissions of these types of nanomaterials are therefore regulated based on their composition. Additionally, hazardous waste incinerators must meet destruction efficiencies for principal organic hazardous constituents, 99.99% for most organics or 99.9999% for particularly hazardous compounds such as chlorinated hydrocarbon wastes.
In the US, non-hazardous incinerators are regulated on the basis of facility size and waste source. Incinerator categories are small MSW (35–250 tons per day);73,74 large MSW (>250 tons per day);79,80 sewage sludge;69,70 medical, infectious, and hospital wastes;77,78 commercial and industrial wastes;75,76 and other solid wastes.71,72 The ‘other solid waste’ category is a catchall, which includes very small MSW incinerators and institutional waste incinerators. The same pollutants are regulated for all incinerators: PM, CO, NOx, SO2, HCl, Pb, Cd, Hg, PCDD/F, and opacity. The limits for each of these pollutants vary by category, incinerator type, size, and in some instances location (i.e., distance to urban center). The range of emission limits encompassing all incinerator categories is presented in Table 2. Regulations on incinerator effluents in other countries are similar to those in the US and, for the most part, standards fall within the range specified for the different incinerator categories. It is possible that the presence of nanomaterials in the waste stream could impact emissions of the pollutants listed in Table 2.
| Region | PM (mg m−3) | Cd (mg m−3) | Hg (mg m−3) | PCDD/Fa (ng m−3) | Opacity (%) |
|---|---|---|---|---|---|
| a Provided as toxic equivalency (TEQ). b Values encompass all nonhazardous incinerator types designated by EPA regulations.69–80 c Combined with thallium containing compounds. | |||||
| USb | 0.18–115 | 0.001–18 | 0.001–0.55 | 0.0022–5 | 10–20 |
| EU66 | 10 | 0.05c | 0.05 | 0.1 | — |
| Japan67 | 150 | — | — | 0.1–5 | 10–20 |
| Taiwan68 | 80–180 | 0.02–0.04 | 0.05–0.1 | — | — |
Stringent regulations, promulgated in the US in the last two decades, on these pollutants as well as the many options for inexpensive landfilling have led to a large reduction in the amount of wastes being incinerated since the late 1990s. Currently, less than 20% of solid wastes are combusted as opposed to landfilled; nonetheless, this amounts to nearly 40 million tons of material incinerated in the US every year.
Particle emission control technology
Although nanoparticles are not overtly addressed in regulations pertaining to incinerator emissions, controls implemented to meet emission limits on PM, opacity, and metals may be at least partially effective at reducing nanoparticle concentrations in the flue gas. There are numerous control technology options for particle removal from waste incinerators, and many incinerators have several equipped in series. Particle control technology categories include older technologies such as cyclones and wet scrubbers, which also treat acid gases, and more modern technologies such as electrostatic precipitators, wet electrostatic precipitators, and fabric filters. However, there are many different designs and operation parameters, all of which can affect particle collection efficiency. The effectiveness of these control technologies for removing nanoparticles is not well known, as most assessments have focused on particle mass reduction, since this is the regulated quantity. Nanoparticles themselves contribute very little to the total mass of PM. More recent measurements have focused on the removal of nanoparticles in control technologies and their emissions from incinerators.81–87Simultaneous removal of gases and particles can be achieved with a wet scrubber. Collection efficiencies for nanoparticles can be low; diffusion is the primary mechanism by which droplets and nanoparticles collide, and this mechanism is only efficient for particles smaller than 5 nm.88 Minimum collection efficiency, 5% or less of the particles, is predicted at a particle size of approximately 100 nm.88,89 Design modifications to increase relative velocities between particles and droplets, like venturi scrubbers, have little effect on nanoparticle removal.90 However, charging of the droplet, particle, or both with opposite charges, as is done in wet electrostatic scrubbers or ionizing wet scrubbers, can dramatically increase collection efficiency for nanoparticles.89,90 Removal efficiencies of up to 70% were calculated for 80 nm particles when only the droplets were charged and close to 100% when droplets and particles were oppositely charged.89
Electrostatic precipitators have a minimum collection efficiency at 300–600 nm91 that is also dependent upon the mechanical rapping scheme used to remove particles collected on the precipitator walls. How much and what size particles are re-entrained is dependent upon the frequency of rapping, with some schemes preferentially releasing smaller size particles.92 Measurements involving an electrostatic precipitator treating exhaust from a grate boiler burning moist forest residue found removal efficiencies of ∼96% for 30–100 nm particles and ∼50% for 300–600 nm particles.93 The rapping process is avoided in a wet-electrostatic precipitator, which uses a liquid spray to remove particles from the precipitator plates. Measurements in a MSW incinerator exhibited an order-of-magnitude reduction of ultrafine particles through the wet-electrostatic precipitator, although some ultrafine particles could be attributed to condensation downstream of the control device as the flue gases cooled.84 However, electrostatic precipitator collection efficiency cannot be generalized because collection efficiency is dependent upon particle composition, which can vary by fuel and incinerator type.94
Fabric filters, or baghouses, force the flue gas through filter media to remove particles. Buonanno et al.82,86 have investigated the efficiency of fabric filters for nanoscale particle removal from several different waste-to-energy plants (grate type incinerators) and found that particle emissions were highest during removal of the particle cake that builds up on the filter media over time. However, even during cake removal, the collection efficiency of nanoscale particles was 99.9%, only slightly lower than that for larger particles.
Of the air pollution control technologies discussed above, fabric filters present the best option for removal of nanoparticles from flue gases. Electrostatic precipitators and ionizing wet scrubbers are also expected to have relatively high nanoparticle collection efficiencies. Cyclones are expected to be largely ineffective at nanoparticle removal. The prevalence of these control technologies at different facilities is variable and dependent upon incinerator category. For example, fabric filters are operated at about 30% of medical waste incinerators and only about 1% of sewage sludge incinerators in the US.14,95 At facilities without effective controls, it is likely that nanomaterials aerosolized or newly formed during incineration may be released to the environment.
Nanomaterial combustion
Currently there is a dearth of information on the incineration of nanomaterials. However, there are several lines of research that can provide some insight into the behavior of nanomaterials at high-temperature, oxidative, or combustion conditions. Many of these research areas such as aluminum combustion96 or metal oxide sorbents generated in situ97 are an extension of previous research with larger micron sized particles.Nanoparticle thermal behavior
Early investigations of nanomaterials focused on fundamental thermal properties such as melting or evaporation.98–100 Studies of nanocrystals like silver or quantum dots showed that evaporation and melting are size-dependent phenomena.98,99 Because of their high surface energy, nanoparticles begin to evaporate at much lower temperatures in a manner that could be described by the Kelvin effect.99 However, particles supported on a substrate or embedded in a material have lower surface energy and behave more like the bulk material.99 Carbonaceous nanomaterials, like fullerenes and CNT, also evaporate at much lower temperatures than graphite (a frequent reference for bulk carbonaceous material).100–102 In inert atmospheres, fullerenes begin to evaporate at 600 °C102 and CNT at 1000 °C.103 In air, oxidation of the materials begins at much lower temperatures: fullerenes at 444 °C, soot at 565 °C, graphite at 644 °C, diamond at 629 °C,100 and CNT at 695 °C.103Nanoparticle behavior during combustion
As nanomaterial applications have been developed, research on nanoparticlecombustion has become more focused. Due to the potential of higher fuel energy densities and enhanced combustion, nanomaterials, particularly aluminum, have been investigated for propulsion systems.96 The ignition temperature of aerosolized nanoparticle dusts was found to be dependent on particle size. For particles larger than 100 μm, the ignition temperature was roughly constant at approximately the melting point of the aluminum oxide passivating coating. Below 100 μm, the ignition temperature decreased as the particle size decreased.104 However, at smaller sizes, the volume of metal oxide layer dominated the particle, reducing the amount of fuel to combust. Similar results were observed for aerosolized carbonaceous nanoparticle dusts. Bouillard et al.105 observed a reduction of ignition temperature with decreasing particle diameter. However, below 30 nm, the minimum ignition temperature increased, which the authors attributed to the enhanced aggregation of the smaller particles.CNT and nanoscale metals such as boron, iron, and aluminum have been added to liquid fuels to create nanofluids with improved burning characteristics.106,107 High concentrations of aluminum nanoparticles led to the formation of one large aggregate that ignited if the flame temperature of the suspending liquid was high enough to melt the particle, whereas in dilute suspensions the liquid and the particles burned simultaneously.106 Similar to the nanoparticle dusts described above, particle size was a critical factor in determining combustion characteristics. The 80 nm aluminum nanoparticles in the nanofluid aggregated into a larger particle which combusted as an aluminum droplet flame after the liquid fuel was consumed. The 5 μm particles formed an aggregate that was oxidized in a microexplosion, and the 25 μm particles did not burn at all.108 The composition of the suspending liquid was also important. Nanoparticles tended to be ejected from the droplet and combusted outside of the droplet when a surfactant was present. The authors hypothesized the difference in boiling points between the liquid fuel and surfactant created bubbles that forcefully ejected the particles from the liquid droplet.106
At the other end of the combustion spectrum, nanoparticles have attracted interest as flame retardant additives to polymers. Nanoscale clays are most prevalent in nanocomposites, but CNT and, metal oxidenanoparticles have also been used.109–111 Nanomaterials must be sufficiently dispersed within the polymer and present at relatively high concentrations (0.5–2% by wt) to provide flame retardant properties.109,112 For example, the addition of CNT to polymers increased the temperature at the onset of combustion by ∼10%113 and reduced the peak heat release rate in half.112 During polymerdecomposition, it is believed that nanomaterials accumulated at the polymer surface, forming a dense network that acted as a barrier to mass transfer of vapor-phase reactive species to the combustion zone and prevented heat transfer into the polymer. CNT with their high aspect ratio formed dense networks, which then accumulated into a protective char on the polymer surface.112,114 Additionally, the CNT altered the rheology of the polymer, preventing dripping that can lead to a pool fire.114
Nanoparticle impact on pollutant emissions
Somewhat less is known about the impacts of nanomaterials on pollutant formation and particle emissions from combustion systems. Much of the existing research has focused on pollutant reduction, as several applications have exploited nanomaterial characteristics such as large surface area and enhanced catalytic activity to reduce emissions. For example, TiO2nanoparticles have been used to catalyze the destruction of gas-phase pollutants and their precursors.115 The addition of TiO2nanoparticles to PVC reduced dioxin formation during PVC decomposition at 350 °C. Increased TiO2 concentration and dispersion within the polymer resulted in greater reductions in dioxins compared to the combustion of pristine PVC.Fuel-borne CeO2nanoparticles are another example of nanomaterials used to reduce pollutants from combustion systems. Jung et al.116 found that CeO2nanoparticles added to diesel fuel acted as a catalyst and reduced the total mass of PM formed.116 The addition of CeO2 led to a dramatic increase in the oxidation rate of diesel soot, reducing the temperature at which particles begin to shrink by ∼250 °C. The CeO2nanoparticles persisted through the combustion zone and remained as individual particles or attached to the soot surface. The authors speculated that the nanoparticles were able to remain unchanged in the engine because of the high melting temperature of CeO2 (∼2300 °C).
Other fuel-borne catalysts like ferrocene are not initially nanoparticles but oxidize in the flame and form low-volatility metal oxides that nucleate and form nanoparticles.117 This behavior is exploited in combustion synthesis of nanoparticles118 and for sorbents generated in situ for pollutant removal in coal and incinerator effluents.97,119 The large surface area of nanoscale sorbents allows them to adsorb greater amounts of condensable pollutants compared to larger micron sized sorbents.119 For example, nanoscale SiO2 formed in the effluent from munitions incineration has been used as a sorbent for Cd and Pb species.119 The available surface area of the sorbent particles was an important parameter in suppressing CdO and PbO nanoparticle nucleation. In the presence of a sorbent, CdO and PbO condensed onto the sorbent surface, making these toxic metals easier to capture in particle control devices.
Only scant research exists indicating that nanomaterials may enhance pollutant formation or particle emissions, as this is still an emerging research topic. Bouillard et al.113 discovered a substantial number of CNT in the aerosol emissions produced by combustion of a CNT polymer composite. Motzkus et al.120 investigated particle emissions from the combustion of polymers with the addition of several different nanoparticles used to confer flame retardant properties. Although the addition of nanoparticles (SiO2, Al2O3, and multiwalled CNT) reduced the emitted particle number concentrations compared to pristine polymer, the size distribution shifted to larger diameters.120 Intact CNT of aerodynamic diameter < 30 nm were found in the combustion exhaust. However, analysis by microscopy was limited to only the smallest particles collected and only to emissions from the combustion of samples containing CNT, so it is unknown if CNT were present in the larger size fractions or emitted from the other nanocomposites. Despite being a combustible material, CNT are still observed in emissions from the combustion of CNT-containing nanocomposites. Several other examples of nanomaterials increasing pollutant formation in incinerator systems will be discussed in the next section.
State of knowledge of nanomaterial waste incineration
Nanowaste incineration has been addressed in reviews or models of nanomaterial waste handling.7–9 Petersen et al.8 highlighted incineration as a potential pathway in which CNT in polymer composite materials may be released to the environment. The authors drew upon the literature assessing nanocomposite flammability and concluded that CNT would not likely persist through the combustion zone but may persist in incinerator ash. This hypothesis was based on studies identifying nanofibrous material in the ash residues. Roes et al.9 also focused on the fate of polymers, including a broader range of nanomaterials (metallic, oxide, etc.), within incineration systems. They used a thermodynamic analysis to predict the final composition of the original nanomaterial components under incineration conditions and assumed that all solid-phase species would exist in the nanoscale range. They concluded that there was potential for release of both the original nanomaterials and of those formed in the incineration system. However, these analyses ignored any kinetic considerations and did not attempt to resolve the partitioning of nanomaterials (i.e. to bottom ash or fly ash). A model of nanomaterial waste flows in Switzerland by Mueller et al.7 predicted that the majority of TiO2, ZnO, and Ag nanomaterials partitioned to the bottom ash and that almost all CNT were consumed by combustion. The behavior of the nanomaterials in the incinerator system was based on the properties of the bulk material or on the behavior of other carbonaceous materials, as in the case of the CNT incineration. Additionally, the assumed nanomaterial distribution between fly ash, bottom ash, and quench water and removal efficiency of pollution control technology were derived from a full-scale study of CeO2 incineration (discussed below) and applied to all nanomaterials, regardless of particle characteristics. The validity of the assumptions used in these analyses is uncertain, but such assumptions are necessary because of the lack of experimental studies on fate and behavior of nanomaterials within incinerator systems.To our knowledge, only three experimental studies have been published specifically addressing the incineration of nanowastes. Of these, two have been carried out in bench-scale systems and have examined the impact of the addition of nanomaterials on the formation of other pollutants,121,122 while a third used both a full-scale MSW incinerator and a laboratory-scale furnace.123 A bench-scale experiment by Font et al.121 investigated the impact of zero-valent iron nanoparticles on emissions of hydrocarbons, chlorinated hydrocarbons, and PCDD/F from PVC combustion. They carried out a two-stage reaction scenario in a furnace and found that at low temperature (375 °C), the addition of the iron nanoparticles (∼40% by mass) resulted in a substantial increase in hydrocarbon, chlorinated hydrocarbon, and PCDD/F emissions compared to the case when only PVC was present. However, in the second combustion phase at a higher temperature (850 °C), emissions of hydrocarbons and chlorinated hydrocarbons were low, and PCDD/F formation was lower than in the case without the nanoparticles present. The authors hypothesized that the iron was oxidized during the first phase to Fe2O3, which then acted as a catalyst to increase the oxidation of PCDD/F precursors, thus reducing their formation in the second stage. Another bench-scale experiment by Vejerano et al.122 investigated PAH and PCDD/F emissions from the combustion of surrogate wastes spiked with a variety of different nanomaterials. The presence of nanoparticles (Ag, NiO, TiO2, CeO2, fullerene) enhanced PAH emissions, particularly of those with medium vapor pressure. Moreover, the increase was larger when the material was in the nanoscale phase compared to the same mass added in bulk form for all particle types except fullerene, which did not have a bulk equivalent. No PCDD emissions were detected under any conditions, but PCDF emissions increased with Ag and TiO2nanoparticles and decreased with CdSe/ZnS quantum dots, CeO2, and Fe2O3.
The only full-scale investigation, by Walser et al.,123 focused on the fate of nanoparticles in a modern MSW incinerator including a heat recovery unit, electrostatic precipitator, and wet scrubber. In one scenario a suspension of CeO2nanoparticles (10 kg total) was sprayed on the waste before it was fed into the system, representing a single large input into the waste stream. The CeO2nanoparticles preferentially partitioned to the slag and fly ash and to a lesser extent the quench water of the wet scrubber. There were no significant changes in the morphology or composition of the nanoparticles, although particle size was not quantified. Removal rates for the CeO2nanoparticles were greater than 99.6% in the electrostatic precipitator and greater than 99.9% in the wet scrubber. No cerium could be detected in the cleaned flue gas. In an alternative scenario, nanoparticles were sprayed into the flue gas above the combustion zone to represent a case in which raw material escaped unburned. In this scenario, the majority of the cerium was detected in the quench water as well as in the fly ash and the slag from the heat recovery unit directly downstream of where the nanoparticles were injected. In this worst case scenario, some cerium was detected in the cleaned flue gas, albeit at a low concentration.
Fate of nanomaterials within an incinerator
These initial studies on the incineration of nanoparticle-spiked wastes have shown that (1) some nanoparticles can penetrate through the combustion zone largely unchanged, (2) modern air pollution control equipment may be effective at removing some nanoparticles, and (3) nanoparticles can potentially impact the production or destruction of hazardous pollutants. These studies evaluated at most just a few particle types; therefore, these conclusions are likely specific to the particular nanomaterial investigated and cannot readily be extended to other nanomaterials.Our current understanding from the patchwork of studies on the behavior of nanomaterials in combustion systems or at high temperatures provides a glimpse of the possible fate of nanomaterials in waste incinerators. Fig. 2 describes the possible pathways that a nanoparticle, or nanotube or other shape, can follow inside the incinerator system. Nanoparticles may exist in the waste as free particles (i.e., a powder), dispersed in a liquid, or embedded in a solid material. This initial state is likely to be an important determinant of whether the particle will become aerosolized, which largely dictates its fate in the combustion zone. Based on the behavior of nanoparticle fire retardants114 and nanofluids106,108 we hypothesize that nanoparticles contained within a solid or liquid system are more likely to aggregate. These larger aggregates may or may not burn depending upon the local conditions in the combustion chamber.
 | ||
| Fig. 2 Possible pathways describing the fate of nanomaterials within waste incinerators. | ||
Chemical composition is also likely to play an important role in determining the fate of nanomaterials. Particles that are already oxidized, especially those with high melting points, like the CeO2 used in the full-scale incinerator study123 and as a fuel-borne catalyst in diesel engines,116 may exit the combustion zone essentially unchanged. Alternatively, reduced nanoparticles, such as aluminum, will combust given high enough temperatures, as was seen with the nanofluid fuels106,108 and energetic nanoparticles.96 However, complete combustion may depend on the particle size and aggregation state. For example, the supermicron aluminum particles suspended in hydrocarbonfuels108 and the CNT added to polymers114 did not fully combust.
In the post-combustion region, aerosolized nanoparticles that persisted through the flame zone are mixed with other particles produced inside the combustion zone. Particle aggregation may occur, shifting the original size distribution toward larger diameters. Additionally, other species may condense on the nanoparticle, changing its composition, which may increase the health hazard of these particles, as was the case with nanoscale sorbents.119 All of these changes may impact the effectiveness of particle control technology at removing these nanoparticles. Fabric filters are expected to be most effective among existing control technologies at removing nanoparticles and larger aggregates, regardless of particle composition. Aggregation state and particle size will be key factors in determining nanoparticle capture efficiency in other air pollution control devices.
At any point in the process, nanoparticles may impact the formation of other pollutants, particularly PAH and PCCD/F. Again, this interaction is likely to be dependent on the particle composition and available surface area. The limited experimental evidence shows that formation of pollutants can increase in some cases and decrease in others.115,121,122
Table 3 summarizes combustion behavior for several common nanomaterials. To provide some context on how important each nanomaterial may be within incineration systems, the nanomaterials are categorized by their global production levels. For most nanomaterials the amount incinerated is likely to be a small fraction of that produced, and the amount for CNT is slightly higher due to their prevalence in polymer materials.15 The two nanomaterials with highest production levels (TiO2 and SiO2) are not combustible, likely persist through the combustion zone, and may impact pollutant formation. This suggests that nanomaterials produced in the largest quantities may impact the incinerator system and require greater scrutiny to limit releases to the environment.
| Nanomaterial | Production levela,37 | Combustible | Fire retardant111 | Persist through combustion zone | Increased pollutant emissions122 |
|---|---|---|---|---|---|
| a High > 1000 t/year, medium < 1000 t/year and >100 t/year, low < 100 t/year. | |||||
| SiO2 | High | − | + | +119 | ? |
| TiO2 | High | − | + | +97 | + |
| CNT | Medium | + | + | +113,120 | ? |
| CeO2 | Low | − | + | +116 | + |
| Ag | Low | ? | ? | ? | + |
| Fullerene | Low | + | ? | ? | + |
Conclusions and recommendations
The preliminary experimental evidence suggests that a portion of nanomaterials, including CNT and fullerenes, may persist through the combustion zone and may impact the formation of other pollutants. The use of adequate control technology (i.e. fabric filters, ionizing wet scrubbers, or wet electrostatic precipitators) is expected to remove the majority of nanoparticles from the incinerator effluent. However, nanomaterials may become enriched in the bottom ash and fly ash, both of which may require special handling and disposal to prevent release into the environment.Many questions remain, and data on the behavior and fate of nanomaterials within incinerators are needed for life-cycle models. We recommend several areas that should be investigated further to fill these data gaps. First, basic research into the behavior of nanomaterials in the combustion zone is needed. Combustion of different nanomaterial classes (e.g., metals, metal oxides, carbonaceous) in different matrices (aerosolized, nanofluids, nanocomposites) that are most likely to be incinerated in the largest quantities should be investigated. Second, the impact of nanomaterials on pollutant formation and the dependence on temperature or redox conditions needs to be determined. With this information, one may be able to define optimal conditions for combustion and treatment of the incinerator effluent to minimize or prevent pollutant formation. Finally, the effectiveness of control technology at removing nanoparticles needs to be thoroughly assessed. This is important not only for preventing the release of engineered nanoparticles but also for controlling those generated during incineration. With further research, incineration may prove to be a safe and effective end-of-life treatment to limit the impact of nanoparticles on the environment and human health.
Acknowledgements
This work was partially supported by the US EPA National Center for Environmental Research STAR program (grant number 83485601) and the Virginia Tech Institute for Critical Technology and Applied Science. The views expressed in this paper are those of the authors and do not necessarily reflect the views or policies of the US EPA.References
- M. C. Roco, C. A. Mirkin and M. C. Hersam, J. Nanopart. Res., 2011, 13, 897–919 CrossRef.
- G. Bystrzejewska-Piotrowska, J. Golimowski and P. L. Urban, Waste Manage., 2009, 29, 2587–2595 CrossRef CAS.
- D. E. Meyer, M. A. Curran and M. A. Gonzalez, Environ. Sci. Technol., 2009, 43, 1256–1263 CrossRef CAS.
- U.S. Environmental Protection Agency, Nanotechnology White Paper, EPA 100/B-07/001, Office of the Science Advisor, Washington D. C., 2007 Search PubMed.
- R. Hischier and T. Walser, Sci. Total Environ., 2012, 425, 271–282 CrossRef CAS.
- V. K. K. Upadhyayula, D. E. Meyer, M. A. Curran and M. A. Gonzalez, J. Cleaner Prod., 2012, 26, 37–47 CrossRef CAS.
- N. C. Mueller, J. Buha, J. Wang, A. Ulrich and B. Nowack, Environ. Sci.: Processes Impacts, 2013, 15, 251–259 CAS.
- E. J. Petersen, L. W. Zhang, N. T. Mattison, D. M. O'Carroll, A. J. Whelton, N. Uddin, T. Nguyen, Q. G. Huang, T. B. Henry, R. D. Holbrook and K. L. Chen, Environ. Sci. Technol., 2011, 45, 9837–9856 CrossRef CAS.
- L. Roes, M. K. Patel, E. Worrell and C. Ludwig, Sci. Total Environ., 2012, 417–418, 76–86 CrossRef CAS.
- U.S. Environmental Protection Agency, Office of Solid Waste, Municipal Solid Waste in the United States: 2007 Facts and Figures, EPA530-R-08-010, Washington, DC, 2008 Search PubMed.
- M. J. Quina, J. C. M. Bordado and R. M. Quinta-Ferreira, in The Impact of Air Pollution on Health, Economy, Environment and Agricultural Sources, ed. M. K. Khallaf, InTech, Rijeka, Croatia, 2011 Search PubMed.
- U.S. Environmental Protection Agency, 2007 National RCRA Hazardous Waste Biennial Report, EPA530-R-08-012, Washington, DC, 2008 Search PubMed.
- U.S. Environmental Protection Agency, National Biennial RCRA Hazardous Waste Report, http://www.epa.gov/waste/inforesources/data/br05/national05.pdf, 2006 Search PubMed.
- U.S. Environmental Protection Agency, Proposed Amended Federal Plan Hospital/Medical/Infectious Waste Incinerators (HMIWI) Inventory, EPA-HQ-OAR-2011-0405-0002, in rulemaking docket: Federal Plan Requirements for Hospital/Medical/Infectious Waste Incinerators Constructed on or Before December 1, 2008, http://www.regulations.gov, 2012 Search PubMed.
- F. Gottschalk, T. Sonderer, R. W. Scholz and B. Nowack, Environ. Sci. Technol., 2009, 43, 9216–9222 CrossRef CAS.
- N. C. Mueller and B. Nowack, Environ. Sci. Technol., 2008, 42, 4447–4453 CrossRef CAS.
- U.S. Environmental Protection Agency, Wastes - Resource Conservation – Common Wastes & Materials, http://www.epa.gov/osw/conserve/materials/plastics.htm, accessed 21 August, 2012.
- K. Arivalagan, S. Ravichandran, K. Rangasamy and E. Karthikeyan, Int. J. ChemTech Res., 2011, 3, 534–538 Search PubMed.
- M. D. Sanchez-Garcia, E. Gimenez and J. M. Lagaron, J. Plast. Film Sheeting, 2007, 23, 133–148 CrossRef CAS.
- D. Cava, E. Giminez, R. Gavara and J. M. Lagaron, J. Plast. Film Sheeting, 2006, 22, 265–274 CrossRef CAS.
- M. A. Priolo, D. Gamboa and J. C. Grunlan, ACS Appl. Mater. Interfaces, 2009, 2, 312–320 Search PubMed.
- Ecosustainable Polymer Nanomaterials for Food Packaging: Innovative Solutions, Characterization Needs, Safety and Environmental Issues, ed. C. Silvestre and S. Cimmino, CRC Press, Boca Raton, 2013 Search PubMed.
- U.S. Environmental Protection Agency, Nanomaterial Risk Assessment Worksheet DuPont™ Light Stabilizer, http://www.epa.gov/opptintr/nano/dupont1.pdf.
- L. J. Gibson and M. F. Ashby, Cellular Solids: Structure and Properties, Cambridge University Press, Cambridge, UK, 1988 Search PubMed.
- C. A. L. Colard, R. A. Cave, N. Grossiord, J. A. Covington and S. A. F. Bon, Adv. Mater., 2009, 21, 2894–2898 CrossRef CAS.
- F. Schmitz, G. M. V. Thielen,N. Costantini, G. Agostini,I. L. L. Lambert, R. M. D’Sidocky,L. A. Gordon, X. Yang, B. R. Hahn, R. L. Dando, J. E. Varner and L. A. Haller, Tire with component containing carbon nanotubes, US Pat. App. 12/643242, 2011.
- U.S. Environmental Protection Agency, Wastes – Resource Conservation – Common Wastes & Materials-Scrap Tires, http://www.epa.gov/osw/conserve/materials/tires/tdf.htm, accessed 20 August, 2012.
- X. Zhou, Y. Zhu, J. Liang and S. Yu, J. Mater. Sci. Technol., 2010, 26, 1127–1132 CAS.
- Reinforcing fillers in the rubber industry: Assessment as potential nanomaterials with a focus on tires, ChemRisk LLC, 2011 Search PubMed.
- L. Geranio, M. Heuberger and B. Nowack, Environ. Sci. Technol., 2009, 43, 8113–8118 CrossRef CAS.
- M. Farré, S. Pérez, K. Gajda-Schrantz, V. Osorio, L. Kantiani, A. Ginebreda and D. Barceló, J. Hydrol., 2010, 383, 44–51 CrossRef.
- H. P. Jarvie, H. Al-Obaidi, S. M. King, M. J. Bowes, M. J. Lawrence, A. F. Drake, M. A. Green and P. J. Dobson, Environ. Sci. Technol., 2009, 43, 8622–8628 CrossRef CAS.
- B. Kim, C. S. Park, M. Murayama and M. F. Hochella, Environ. Sci. Technol., 2010, 44, 7509–7514 CrossRef CAS.
- M. A. Kiser, P. Westerhoff, T. Benn, Y. Wang, J. Perez-Rivera and K. Hristovski, Environ. Sci. Technol., 2009, 43, 6757–6763 CrossRef CAS.
- L. K. Limbach, R. Bereiter, E. Mueller, R. Krebs, R. Gaelli and W. J. Stark, Environ. Sci. Technol., 2008, 42, 5828–5833 CrossRef CAS.
- R. J. Aitken, M. Q. Chaudhry, A. B. A. Boxall and M. Hull, Occup. Med., 2006, 56, 300–306 CrossRef CAS.
- F. Piccinno, F. Gottschalk, S. Seeger and B. Nowack, J. Nanopart. Res., 2012, 14, 1109 CrossRef.
- Mitsubishi's fullerene frontier, Photovoltaics Bulletin, 2002.
- C. O. Hendren, X. Mesnard, J. Droge and M. R. Wiesner, Environ. Sci. Technol., 2012, 45, 2562–2569 CrossRef.
- F. Gottschalk, T. Sonderer, R. W. Scholz and B. Nowack, Environ. Toxicol. Chem., 2010, 29, 1036–1048 CAS.
- L. K. P. Breggin, Jobn, Where Does the Nano Go?, New Report on End-of-Life Regulation of Nanotechnologies, Woodrow Wilson International Center for Scholars, 2007 Search PubMed.
- T. Hester, RCRA Regulation of Wastes from the Production, Use, and Disposal of Nanomaterials, American Bar Association, Section of Environment, Energy, and Resources, 2006 Search PubMed.
- M. C. Powell, M. P. A. Griffin and S. Tai, Environ. Manage., 2008, 42, 426–443 CrossRef.
- A. Franco, S. F. Hansen, S. I. Olsen and L. Butti, Regul. Toxicol. Pharmacol., 2007, 48, 171–183 CrossRef CAS.
- S. F. Hansen and A. Baun, Dose-Response, 2012, 10, 364–383 CrossRef CAS.
- I. Eisenberger, M. Nentwich, U. Fiedeler, A. Gazsó and M. Simkó, Nano Regulation in Austria (II): Workplace Safety, Industrial Law and Environmental Law, Austrian Academy of Sciences, 2011 Search PubMed.
- K. Ludlow, D. Bowman and G. Hodge, A Review of Possible Impacts of Nanotechnology on Australia's Regulatory Framework, Monash University, 2007 Search PubMed.
- Japan Ministry of the Environment, Guideline for Preventive Environmental Impact from Industrial Nanomaterials, Tokyo, 2009 Search PubMed.
- M. F. Hallock, P. Greenley, L. DiBerardinis and D. Kallin, J. Chem. Health Saf., 2009, 16, 16–23 CrossRef CAS.
- J. P. Elijah and B. H. Theodore, in Biotechnology and Nanotechnology Risk Assessment: Minding and Managing the Potential Threats around Us, American Chemical Society, 2011 Search PubMed.
- Y. Ge, J. P. Schimel and P. A. Holden, Environ. Sci. Technol., 2011, 45, 1659–1664 CrossRef CAS.
- B. Halford, Chemical Engineering News Archive, 2007, 85, 11a Search PubMed.
- H. L. Karlsson, P. Cronholm, J. Gustafsson and L. Möller, Chem. Res. Toxicol., 2008, 21, 1726–1732 CrossRef CAS.
- M. Li, L. Zhu and D. Lin, Environ. Sci. Technol., 2011, 45, 1977–1983 CrossRef CAS.
- Nanotoolkit: Working Safely with Engineered Nanomaterials in Academic Research Settings, ed. J. de la Rosa Ducut, California Nanosafety Consortium of Higher Education, 2012 Search PubMed.
- Ecomaine, Waste-to-Energy Electricity Generation, http://www.ecomaine.org, accessed 21 August, 2012.
- N. Musee, Environ. Int., 2011, 37, 112–128 CrossRef CAS.
- A. Helland, M. Scheringer, M. Siegrist, H. G. Kastenholz, A. Wiek and R. W. Scholz, Environ. Sci. Technol., 2007, 42, 640–646 CrossRef.
- Proposed Rule Standards of Performance for New Stationary Sources and Emission Guidelines for Existing Sources: Commercial and Industrial Solid Waste Incineration Units, Fed. Regist., 2010, 75, 31938.
- U.S. Environmental Protection Agency, Wastes – Non-Hazardous Waste – Municipal Solid Waste, http://www.epa.gov/osw/nonhaz/municipal/wte/basic.htm, accessed 2 August, 2012.
- S. P. Ryan and E. R. Altwicker, Environ. Sci. Technol., 2004, 38, 1708–1717 CrossRef CAS.
- L. Stieglitz, H. Vogg, G. Zwick, J. Beck and H. Bautz, Chemosphere, 1991, 23, 1255–1264 CrossRef CAS.
- S. Abanades, G. Flamant, B. Gagnepain and D. Gauthier, Waste Manage. Res., 2002, 20, 55–68 CrossRef CAS.
- W. P. Linak, C. A. Miller and J. O. L. Wendt, J. Air Waste Manage. Assoc., 2000, 50, 1532–1544 CAS.
- National Emissions Standards for Hazardous Air Pollutants from Hazardous Waste Combustors, Code of Federal Regulations Title 40, Part 63 Subpart EEE, 1999.
- The European parliament and the council of the European Union, EU Standards for the Incinerator Exhaust, Directive 2000/76/EC, 2000.
- K. Inoue, K. Yasuda and K. Kawamoto, Waste Manage. Res., 2009, 27, 617–622 CrossRef CAS.
- R. O. C. (Taiwan) Environmental Law Library, Waste incinerator air pollutant emissions standards, http://law.epa.gov.tw/en/laws/622766802.html, accessed 25 March, 2013.
- Emission Guidelines and Compliance Times for Existing Sewage Sludge Incineration Units, Code of Federal Regulations Title 40, Part 60 Subpart MMMM, 2011.
- Standards of Performance for New Sewage Sludge Incineration Units, Code of Federal Regulations Title 40, Part 60 Subpart LLLL, 2011.
- Standards of Performance for Other Solid Waste Incineration Units for Which Construction is Commenced After December 9, 2004, or for Which Modification or Reconstruction is Commenced on or After June 16, 2006, Code of Federal Regulations Title 40, Part 60 Subpart EEEE, 2005.
- Emission Guidelines and Compliance Times for Other Solid Waste Incineration Units That Commenced Construction On or Before December 9, 2004, Code of Federal Regulations Title 40, Part 60 Subpart FFFF, 2005.
- Standards of Performance for Small Municipal Waste Combustion Units for Which Construction is Commenced After August 30, 1999 or for Which Modification or Reconstruction is Commenced After June 6, 2001, Code of Federal Regulations Title 40, Part 60 Subpart AAAA, 2000.
- Emission Guidelines and Compliance Times for Small Municipal Waste Combustion Units Constructed on or Before August 30, 1999, Code of Federal Regulations Title 40, Part 60 Subpart BBBB, 2000.
- Emissions Guidelines and Compliance Times for Commercial and Industrial Solid Waste Incineration Units that Commenced Construction On or Before November 30, 19999, Code of Federal Regulations Title 40, Part 60 Subpart DDDD, 2000.
- Standards of Performance for Commercial and Industrial Solid Waste Incineration Units for Which Construction Is Commence After November 30, 1999 or for Which Modification or Reconstruction Is Commenced on or After June 1, 2001, Code of Federal Regulations Title 40, Part 60 Subpart CCCC, 2000.
- Standards of Performance for Hospital/Medical/Infectious Waste Incinerators for Which Construction is Commenced After June 20, 1996, Code of Federal Regulations Title 40, Part 60 Subpart Ec, 1997.
- Emission Guidelines and Compliance Times for Hospital/Medical/Infectious Waste Incinerators, Code of Federal Regulations Title 40, Part 60 Subpart Ce, 1997.
- Emissions Guidelines and Compliance Times for Large Municipal Waste Combustors That are Constructed on or Before September 20, 1994, Code of Federal Regulations Title 40, Part 60 Subpart Cb, 1995.
- Standards of Performance for Large Municipal Waste Combustors for Which Construction is Commenced After September 20, 1994 or for Which Modification or Reconstruction is Commenced After June 19, 1996, Code of Federal Regulations Title 40, Part 60 Subpart Eb, 1995.
- S. Cernuschi, M. Giugliano, S. Ozgen and S. Consonni, Sci. Total Environ., 2012, 420, 319–326 CrossRef CAS.
- G. Buonanno, M. Scungio, L. Stabile and W. Tirler, J. Air Waste Manage. Assoc., 2012, 62, 103–111 CAS.
- S. Ozgen, G. Ripamonti, S. Cernuschi and M. Giugliano, Rev. Environ. Sci. Biotechnol., 2012, 11, 407–415 CAS.
- J. Maguhn, E. Karg, A. Kettrup and R. Zimmermann, Environ. Sci. Technol., 2003, 37, 4761–4770 CrossRef CAS.
- J. H. Zeuthen, A. J. Pedersen, J. Hansen, F. J. Frandsen, H. Livbjerg, C. Riber and T. Astrup, Combust. Sci. Technol., 2007, 179, 2171–2198 CrossRef CAS.
- G. Buonanno, L. Stabile, P. Avino and E. Belluso, Waste Manage., 2011, 31, 2253–2262 CrossRef CAS.
- S. H. Huang and C. C. Chen, Environ. Sci. Technol., 2002, 36, 4625–4632 CrossRef CAS.
- H. T. Kim, C. H. Jung, S. N. Oh and K. W. Lee, Environ. Eng. Sci., 2001, 18, 125–136 CrossRef CAS.
- H. B. Zhao and C. G. Zheng, Chem. Eng. Technol., 2008, 31, 1824–1837 CrossRef CAS.
- C. Carotenuto, F. Di Natale and A. Lancia, Chem. Eng. J., 2010, 165, 35–45 CrossRef CAS.
- S. H. Kim, H. S. Park and K. W. Lee, J. Electrost., 2001, 50, 177–190 CrossRef.
- T. Ferge, J. Maguhn, H. Felber and R. Zimmermann, Environ. Sci. Technol., 2004, 38, 1545–1553 CrossRef CAS.
- M. Strand, J. Pagels, A. Szpila, A. Gudmundsson, E. Swietlicki, M. Bohgard and M. Sanati, Energy Fuels, 2002, 16, 1499–1506 CrossRef CAS.
- E. Cereda, G. M. B. Marcazzan, M. Pedretti, G. W. Grime and A. Baldacci, J. Aerosol Sci., 1996, 27, 607–619 CrossRef CAS.
- U.S. Environmental Protection Agency, Sewage Sludge Incinerators Final Inventory and Emissions Database, EPA-HQ-OAR-2009-0559-0149, in rulemaking docket: NSPS/Emission Guidelines (EG) for Sewage Sludge Incinerators, http://www.regulations.gov, 2011 Search PubMed.
- R. A. Yetter, G. A. Risha and S. F. Son, Proc. Combust. Inst., 2009, 32, 1819–1838 CrossRef CAS.
- A. Suriyawong, M. Smallwood, Y. Li, Y. Zhuang and P. Biswas, Aerosol Air Qual. Res., 2009, 9, 394–403 CAS.
- A. N. Goldstein, C. M. Echer and A. P. Alivisatos, Science, 1992, 256, 1425–1427 CAS.
- K. K. Nanda, A. Maisels, F. E. Kruis, H. Fissan and S. Stappert, Phys. Rev. Lett., 2003, 91, 106102 CrossRef CAS.
- J. D. Saxby, S. P. Chatfield, A. J. Palmisano, A. M. Vassallo, M. A. Wilson and L. S. K. Pang, J. Phys. Chem., 1992, 96, 17–18 CrossRef CAS.
- F. Cataldo, Fullerenes, Nanotubes, Carbon Nanostruct., 2002, 10, 293–311 CrossRef CAS.
- A. M. Vassallo, L. S. K. Pang, P. A. Coleclarke and M. A. Wilson, J. Am. Chem. Soc., 1991, 113, 7820–7821 CrossRef CAS.
- L. S. K. Pang, J. D. Saxby and S. P. Chatfield, J. Phys. Chem., 1993, 97, 6941–6942 CrossRef CAS.
- Y. Huang, G. A. Risha, V. Yang and R. A. Yetter, Combust. Flame, 2009, 156, 5–13 CrossRef CAS.
- J. Bouillard, A. Vignes, O. Dufaud, L. Perrin and D. Thomas, J. Hazard. Mater., 2010, 181, 873–880 CrossRef CAS.
- Y. A. Gan, Y. S. Lim and L. Qiao, Combust. Flame, 2012, 159, 1732–1740 CrossRef CAS.
- Y. A. Gan and L. Qiao, Energy Fuels, 2012, 26, 4224–4230 CrossRef CAS.
- Y. A. Gan and L. Qiao, Combust. Flame, 2011, 158, 354–368 CrossRef CAS.
- G. P. Cai, H. D. Lu, Y. Zhou, J. W. Hao and C. A. Wilkie, Thermochim. Acta, 2012, 549, 124–131 CrossRef CAS.
- F. Laoutid, L. Bonnaud, M. Alexandre, J. M. Lopez-Cuesta and P. Dubois, Mater. Sci. Eng., R, 2009, 63, 100–125 CrossRef.
- L. J. Wang, X. J. He and C. A. Wilkie, Materials, 2010, 3, 4580–4606 CrossRef CAS.
- T. Kashiwagi, F. M. Du, K. I. Winey, K. A. Groth, J. R. Shields, S. P. Bellayer, H. Kim and J. F. Douglas, Polymer, 2005, 46, 471–481 CrossRef CAS.
- J. Bouillard, B. R'Mili, D. Moranviller, A. Vignes, O. Le Bihan, A. Ustache, J. S. Bomfim, E. Frejafon and D. Fleury, J. Nanopart. Res., 2013, 15, 1–11 CrossRef.
- T. Kashiwagi, F. M. Du, J. F. Douglas, K. I. Winey, R. H. Harris and J. R. Shields, Nat. Mater., 2005, 4, 928–933 CrossRef CAS.
- S. H. Kim, S. Y. Ahn and S. Y. Kwak, Appl. Catal., B, 2008, 79, 296–305 CrossRef CAS.
- H. J. Jung, D. B. Kittelson and M. R. Zachariah, Combust. Flame, 2005, 142, 276–288 CrossRef CAS.
- A. Miller, G. Ahlstrand, D. Kittelson and M. Zachariah, Combust. Flame, 2007, 149, 129–143 CrossRef CAS.
- S. T. Aruna and A. S. Mukasyan, Curr. Opin. Solid State Mater. Sci., 2008, 12, 44–50 CrossRef CAS.
- A. Suriyawong, X. Chen and P. Biswas, Aerosol Sci. Technol., 2010, 44, 676–691 CrossRef CAS.
- C. Motzkus, C. Chivas-Joly, E. Guillaume, S. Ducourtieux, L. Saragoza, D. Lesenechal, T. Mace, J. M. Lopez-Cuesta and C. Longuet, J. Nanopart. Res., 2012, 14, 687 CrossRef.
- R. Font, A. Galvez, J. Molto, A. Fullana and I. Aracil, Chemosphere, 2010, 78, 152–159 CrossRef CAS.
- E. Vejerano, A. L. Holder and L. C. Marr, Environ. Sci. Technol., 2013, 47, 4866–4874 CrossRef CAS.
- T. Walser, L. K. Limbach, R. Brogioli, E. Erismann, L. Flamigni, B. Hattendorf, M. Juchli, F. Krumeich, C. Ludwig, K. Prikopsky, M. Rossier, D. Saner, A. Sigg, S. Hellweg, D. Gunther and W. J. Stark, Nat. Nanotechnol., 2012, 7, 520–524 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
