Exceptional gravimetric and volumetric hydrogen storage for densified zeolite templated carbons with high mechanical stability†
Eric
Masika
and
Robert
Mokaya
*
University of Nottingham, University Park, Nottingham NG7 2RD, UK. E-mail: r.mokaya@nottingham.ac.uk
First published on 29th October 2013
Abstract
Zeolite templating successfully generates carbons with high surface area and pore volume of ca. 3300 m2 g−1 and 1.6 cm3 g−1, respectively. The templated carbons have an exceptional gravimetric hydrogen uptake of 7.3 wt% at 20 bar and −196 °C, and a projected maximum of ca. 9.2 wt%. These hydrogen uptake values are the highest ever recorded for carbon materials. The zeolite templated carbons have excellent mechanical stability and when compacted at a load of 10 tons (740 MPa) undergo densification to a packing density of ca. 0.72 g cm−3 but with hardly any loss in porosity (surface area and pore volume are little changed at ca. 3000 m2 g−1 and 1.4 cm3 g−1) or gravimetric hydrogen uptake capacity, which remains high at 7.0 wt% at 20 bar and a projected maximum of ca. 8.8 wt%. The effects of densification (i.e., increased packing density) coupled with hardly any loss in porosity or hydrogen uptake means that the densified zeolite templated carbons achieve an exceptional and unprecedented volumetric hydrogen uptake of 50 g l−1 at −196 °C and 20 bar, and an estimated maximum of up to 63 g l−1 at higher pressure.
Broader contextAs part of the anticipated Hydrogen Economy, there are on-going intensive research efforts aimed at finding materials suitable for on-board hydrogen storage. The likely success of storage materials is measured against gravimetric and volumetric uptake targets set at levels that may allow commercial and practical viability. However, the volumetric hydrogen uptake of porous materials is related to packing density, yet high surface area materials that are likely to have large gravimetric hydrogen uptake tend to have low density and therefore low volumetric uptake. To address this conundrum, we present findings on high surface area zeolite templated carbons (ZTCs) that have excellent mechanical stability and which, when compacted undergo densification to a packing density of ca. 0.72 g cm−3 but with hardly any loss in porosity (surface area and pore volume are maintained at ca. 3000 m2 g−1 and 1.4 cm3 g−1) or gravimetric hydrogen uptake, which remains high at 7.0 wt% at 20 bar and −196 °C. The densified ZTCs possess a combination of packing density, textural properties and hydrogen uptake hitherto not achieved in any porous material. The densified ZTCs achieve an exceptional and unprecedented volumetric hydrogen uptake of 50 g l−1 at 20 bar, and an estimated maximum of up to 63 g l−1 at higher pressure. |
Introduction
There are currently considerable research efforts aimed at developing materials that would allow the use of energy generation processes that do not emit greenhouse gases. A key target is the development of materials for efficient and effective energy supply and storage. In this respect, hydrogen has long been considered as a suitable fuel for both the transportation and power industry within the context of the Hydrogen Economy. However, technological barriers related to hydrogen production and in particular storage stand in the way of the use of hydrogen as an energy source.1 Technological barriers with respect to storage are due to a lack of suitable materials that can efficiently capture, store and release hydrogen under appropriate conditions.1 A variety of porous solid state materials are currently under investigation for hydrogen storage including zeolites,2 metal–organic frameworks (MOFs),3,4 and porous carbons (carbon nanotubes, carbon fibers, nanoporous carbons).5–7 In particular, high surface area porous carbons are increasingly being seen as attractive hydrogen stores.8–12 Recently, there have been encouraging reports of porous carbons that exhibit high gravimetric hydrogen uptake at 20 bar and −196 °C, including (i) zeolite templated carbons nanocast using zeolite beta as template that can store up to 6.9 wt%,9 (ii) doubly activated carbon with uptake of 7.08 wt%,10 and (iii) ultrahigh surface area polypyrrole-based activated carbons with uptake capacity of 7.03 wt%.11 Thus, to date, activation (chemical or physical) of carbon precursors, zeolite templating and chlorination of metal carbides to generate carbide-derived carbons have proved to be the most effective methods for preparing carbons with attractive properties for hydrogen storage,5–18 with zeolite templating proving to be very successful in controlling the porosity of carbons towards the preferred pore size range known to be best suited for hydrogen adsorption.12–18Research in materials for hydrogen storage for on-board hydrogen storage systems is geared towards achieving various targets set at levels that would allow commercial and practical viability. Targets that are often quoted are those set by the United States Department of Energy (DOE); the most recent targets for 2017 are a system gravimetric uptake capacity of 5.5 wt% and volumetric uptake capacity of 40 g l−1.19 The DOE also gives ultimate targets as a system gravimetric uptake capacity of 7.5 wt% and volumetric uptake capacity of 70 g l−1.19 Thus although most reports to date emphasise the gravimetric hydrogen uptake of materials, it is clear that volumetric capacity is equally if not even more important. The volumetric hydrogen uptake of a porous solid state material is related to its packing density. Most porous materials by their nature tend to have low density and therefore, despite attractive gravimetric hydrogen uptake, have low volumetric uptake.3–6 The volumetric hydrogen uptake of porous materials can potentially be improved by densification or compaction. However, such densification generally comes at the expense of surface area of the material, which rather negates any gains since the hydrogen uptake is dependent on surface area.7–18 Recently, there have been studies that have considered the compaction behaviour of activated carbons and metal–organic frameworks and its effect on hydrogen uptake.20 In general, whilst activated carbons exhibit good mechanical stability and are amenable to compaction, MOFs, on the other hand, tend to collapse and lose their porosity even at low compaction pressures.20,21
There is renewed interest in the potential of densification as a means of improving the hydrogen volumetric uptake capacity of low density porous materials such as carbons. High surface area activated carbons compacted at 40 MPa have been shown to have a packing density of 0.4–0.5 g l−1 and a volumetric hydrogen uptake of ca. 20 g l−1 at −196 °C and 40 bar.20 Linares-Solano and co-workers recently reported that activated carbon monoliths with a high packing density of 0.7 g cm−3 and good micropore volume can achieve a total volumetric hydrogen uptake capacity of ca. 39 g l−1 at −196 °C and 44 bar, which is amongst the highest values reported so far based on measured packing density of materials, while Zhou and co-workers observed a similar volumetric hydrogen uptake capacity of 41 g l−1 at −196 °C and 60 bar for compacted activated carbon with a packing density of 0.72 g cm−3.22 Under similar conditions, the metal–organic framework, MOF-5, achieves an uptake of ca. 16 g l−1 due to a low packing density. A similar lowly volumetric hydrogen uptake of 15 g l−1 at −196 °C and 40 bar has been reported by Mueller and co-workers for MOF-5.23 The possibility of densification illustrates the potential advantages of porous materials as stores with high volumetric hydrogen uptake. The volumetric hydrogen uptake of MOFs may be improved by densification, which however occurs at the expense of gravimetric uptake due to the loss of pore volume and surface area.24–26 Chahine and co-workers achieved an uptake of 38 g l−1 at 50 bar (48 g l−1 at 130 bar) and −196 °C for densified MOF-177,24 while Muller and co-workers attained a similar uptake of 38 g l−1 at 50 bar (43 g l−1 at 80 bar) and −196 °C for densified MOF-5.25 In a related study Siegel and co-workers obtained an uptake of 38 g l−1 at 50 bar (43 g l−1 at 100 bar) and −196 °C for densified MOF-5.26 Elsewhere, monolithic forms of carbide-derived carbons with a volumetric hydrogen uptake of 35 g l−1 at −196 °C and 60 bar have been prepared via chlorination of fully-dense ceramic titanium carbide plates,27 and binders in combination with powdered carbide-derived carbons may also be used to generate carbon peels with a total volumetric hydrogen uptake of 29 g l−1 at −196 °C and 40 bar.28
On the other hand, Kyotani and co-workers performed densification of zeolite Y-templated microporous carbons via hot-pressing at 300 °C and 147 MPa to achieve a packing density of 0.7–0.9 g cm−3. They observed that commercially available KOH-activated carbons did not undergo such densification when hot-pressed under similar conditions, and attributed the densification behaviour of the templated carbons to their unique molecular structure.29 It is now recognised that the molecular structure of zeolite templated carbons is quite different from that of activated carbons and is considered to be made up of buckybowl-like nanographenes assembled into a three-dimensionally regular network.30 Given the unique molecular structure of zeolite-templated carbons, theoretical simulations have suggested that they may achieve a volumetric hydrogen uptake of 50 g l−1 at −196 °C and 50 bar.31 It is therefore highly desirable to explore the possible densification of zeolite templated carbons at high compaction pressures and to investigate any effects such compaction may have on hydrogen uptake. This is particularly important given that, as described above, previous experimental attempts at densification have so far only achieved a volumetric hydrogen uptake of no more than 40 g l−1 at 50 bar and −196 °C.20–29 Indeed, although it is known that other high surface area materials such as MOFs (i.e., MOF-5) are significantly altered irreversibly after compaction at 180 MPa, while ZIFs are stable only up to 340 MPa, and activated carbons with moderate surface area can withstand compaction pressure of up to 420 MPa,21 there are no data on the mechanical stability of zeolite-templated carbons. Recently, theoretical simulations have predicted that zeolite-templated carbons, despite their high porosity, have a bulk modulus (ca. 100 GPa) that is much higher than that of nonporous minerals such as quartz (ca. 60 GPa).32 We report here on the mechanical properties of high surface area zeolite templated carbons and the consequences of compaction on porosity, densification and both gravimetric and volumetric hydrogen uptake.
Experimental section
Material synthesis
The zeolite-templated carbons were prepared as follows; 0.6 g zeolite 13X was dried in the furnace at 300 °C for 12 h before being impregnated, via the incipient wetness method, with furfural alcohol (FA). The resulting FA/zeolite composite in an alumina boat was placed in a flow through tube furnace and heated under argon flow at 80 °C for 24 h followed by further heating at 150 °C for 8 h. In order to allow the carbonisation of polymerised furfural alcohol (polyfurfuryl alcohol), the temperature was raised at a ramp rate of 5 °C min−1 to 700 °C and held for 3 h under Ar flow. The resulting zeolite/carbon composite was then exposed to ethylene gas (10% in argon by volume) at 700 °C for 3 h. The gas flow was then switched to Ar flow only and the temperature raised to 900 °C and held for 3 h followed by cooling under Ar to room temperature. The resulting zeolite/carbon composite was washed in 10% HF for 24 h and then refluxed in concentrated HCl for 6 h. The final carbon material, designated as ZTC-5 was washed with deionised water and dried at 120 °C for 12 h. A second sample, designated as ZTC-15 was prepared as described above except that a heating ramp rate of 15 °C min−1 was used.To effect densification, ca. 35 mg of zeolite-templated carbon was compacted for 10 min at loads of 5 or 10 tons on a 1.3 cm diameter die, equivalent to compaction pressure of 370 MPa and 740 MPa, respectively. The densified samples were denoted as Cx-ZTC-y, where x is the compaction load (5 or 10 tons) and y is the ramp rate (5 or 15).
Materials characterisation
Powder XRD analysis was performed on a Bruker D8 Advance powder diffractometer using CuKα radiation (λ = 1.5406 Å) and operating at 40 kV and 40 mA, with 0.02° step size and 2 s step time. For porosity analysis, each sample was pre-dried in an oven and then degassed overnight at 200 °C under high vacuum. The textural properties were determined by nitrogen sorption at −196 °C using a Micromeritics ASAP 2020 volumetric sorptometer. The surface area was calculated by using the BET method applied to adsorption data in the relative pressure (P/Po) range of 0.06–0.22. The total pore volume was determined from the amount of nitrogen adsorbed at P/Po = 0.99. The pore size distribution was determined by a non-local density functional theory (NLDFT) method using nitrogen adsorption data.Hydrogen uptake measurements
Hydrogen uptake capacity of the carbons was measured by gravimetric analysis with an Intelligent Gravimetric Analyser, IGA, (Hiden) using 99.9999% purity hydrogen additionally purified by a molecular sieve filter. Prior to analysis, the carbon samples were dried in an oven for 24 h at 80 °C overnight and then placed in the analysis chamber and degassed at 200 °C and 10−10 bar for 4–6 h. The hydrogen uptake measurements were typically performed at −196 °C (in a liquid nitrogen bath) over the pressure range 0 to 20 bar. The uptake data were corrected for the buoyancy of the system and samples. The hydrogen uptake was calculated on the basis of a skeletal density of 1.5 g cm−3 for the carbons, and 0.04 g cm−3 for the adsorbed hydrogen. The skeletal density of the carbons was determined from helium sorption data obtained using the IGA at a pressure of up to 20 bar at 0 °C. We compared our hydrogen uptake data (from the IGA) to measurements obtained using a Sievert's apparatus (PCT-Pro 2000) with high purity hydrogen (performed at General Motors, Warren, Michigan). This allowed verification of the IGA hydrogen uptake measurements.Results and discussion
Material properties
The powder XRD patterns of the templated carbons indicate a reasonably high level of zeolite-like ordering (Fig. S1, ESI†) as evidenced by a sharp peak, similar to that present in zeolite 13X, at 2θ = 6.3° corresponding to d-spacing of ca. 1.4 nm, which is comparable to that of zeolite 13X (d-spacing = 1.4 nm).9,12,14,34,35 The XRD patterns of the zeolite/carbon composite forerunner of sample ZTC-5 (Fig. S1, ESI†) exhibit some sharp peaks ascribable to the zeolite 13X template, which means that the zeolite framework is not destroyed during the carbonisation process and thus can facilitate replication of the zeolite structure to the carbons. The heating ramp rate had little effect on the zeolite-like ordering of the carbons (Fig. S1, ESI†) but exerts some influence on the nature of the carbon; a broad peak at 2θ = 43°, which is ascribable to graphitic domains,36,37 is slightly more prominent for sample ZTC-15 prepared at higher heating ramp rate, while for ZTC-5 the peak is virtually absent. Nitrogen sorption isotherms and corresponding pore size distribution curves (Fig. S2, ESI†) indicate that the zeolite templated carbons are mainly microporous.34–38 The nitrogen sorption isotherms of the carbons are typically type I, with a high nitrogen uptake in the low relative pressure domain (P/Po < 0.01), and are consistent with data previously reported for zeolite templated carbons that exhibit significant levels of zeolite-like structural ordering.9,14,18 The templated carbons have unimodal pore size distribution centred at 1.2 nm (Fig. S2, ESI†), which agrees with previous studies that have shown that zeolite templated carbons with higher levels of zeolite-like ordering do not possess a significant proportion of pores larger than 1.5 nm.9,14,18 As summarised in Table 1, carbon ZTC-5 and ZTC-15 possess a high surface area of 3332 m2 g−1 and 3169 m2 g−1, respectively, and a large pore volume of 1.66 and 1.55 cm3 g−1, respectively. The high surface area of the templated carbons may be attributed to the unique molecular structure of pore ordered nanographene sheets with defects and associated edge structures as described by recent theoretical studies.30–33 Sample ZTC-5 has slightly higher textural properties, which is consistent with the XRD patterns (Fig. S1, ESI†) that indicate lower levels of turbostratic/graphitic character. Noteworthy for both templated carbons is the high proportion of surface area (88%) and pore volume (ca. 75%) that arises from micropores. The templated carbons are clearly well ordered with high surface area and are therefore good starting points for densification. The carbons were found to have a surface area per unit volume (m2 cm−3) of ca. 1430 m2 cm−3 based on a bulk density of 0.43 g cm−3 and 0.45 g cm−3 for ZTC-5 and ZTC-15, respectively. The bulk density (ρd) was calculated after compacting the templated carbons under a mild load of 1 ton for 10 min in a 1.3 cm die (equivalent to 74 MPa). Alternatively relatively similar values were obtained using the equation ρd = (1/ρs + VT)−1, where ρs is skeletal density and VT is total pore volume.| Sample | Surface areaa (m2 g−1) | Pore volumeb (cm3 g−1) | V/Sc m2 cm−3 | Pore sized (nm) | H2 uptake at 20 bare | Maximum H2 uptakef | ||
|---|---|---|---|---|---|---|---|---|
| (wt%) | g l−1 | (wt%) | g l−1 | |||||
| a Values in the parenthesis are micropore surface area. b Values in the parenthesis are micropore volume. c Surface area per unit volume. d Maxima of pore size distribution obtained using non-local density functional theory (NLDFT) analysis. e Measured total hydrogen uptake capacity at −196 °C and 20 bar. f Estimated (using Langmuir plots) maximum hydrogen uptake. | ||||||||
| ZTC-5 | 3332 (2837) | 1.66 (1.18) | 1434 | 1.2 | 7.3 | 31.4 | 9.2 | 39.6 |
| C5-ZTC-5 | 3292 (2840) | 1.64 (1.16) | 2272 | 0.6/1.2 | 7.2 | 49.7 | 9.0 | 62.1 |
| C10-ZTC-5 | 3041 (2523) | 1.41 (0.97) | 2190 | 0.6/1.2 | 7.0 | 50.4 | 8.8 | 63.4 |
| ZTC-15 | 3169 (2760) | 1.55 (1.15) | 1426 | 1.2 | 6.2 | 28.0 | 7.9 | 35.6 |
| C5-ZTC-15 | 3192 (2769) | 1.60 (1.15) | 2266 | 0.6/1.2 | 6.3 | 44.7 | 8.1 | 57.5 |
| C10-ZTC-15 | 2782 (2430) | 1.27 (0.91) | 2059 | 0.6/1.2 | 5.9 | 43.7 | 7.5 | 55.5 |
To achieve densification, the templated carbons were compacted at a load of 5 tons (370 MPa) or 10 tons (740 MPa), i.e., 5 and 10 ton load on a 1.3 cm diameter die. This compaction pressure was much higher that any previously used to densify carbons or related porous materials.20–29 We therefore first assessed the mechanical stability of the carbons by observing their structural ordering after compaction. Fig. 1 shows representative XRD patterns of sample ZTC-15 before and after compaction. The XRD patterns do not change after compaction; the sharp peak, similar to that present in zeolite 13X, at 2θ = 6.3° with d-spacing of ca. 1.4 nm is retained after compaction. This indicates excellent mechanical stability and shows that the zeolite-like structural ordering of the zeolite templated carbon can withstand mechanical pressure as high as 740 MPa for 10 min.
 | ||
| Fig. 1 Powder XRD patterns of sample ZTC-15 before and after compaction at a load of 5 tons (C5-ZTC-15) and 10 tons (C10-ZTC-15). | ||
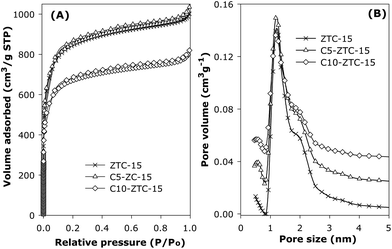
The more important property with respect to retention of gas uptake capability is the porosity. Representative nitrogen sorption isotherms and corresponding pore size distribution curves for sample ZTC-15 before and after compaction are shown in Fig. 2. Compaction at 370 MPa (sample C5-ZTC-15) has virtually no effect on the isotherm, which indicates no change in porosity. After compaction at 740 MPa (sample C10-ZTC-15) there is still no change in the shape of the isotherm but slightly lower overall nitrogen adsorption is observed. The pore size distribution (PSD) curves clearly show that the pore size remains unchanged after compaction at both 370 and 740 MPa. The nitrogen sorption isotherms and PSD curves of compacted sample ZTC-5 exhibited similar trends to those observed in Fig. 2. The textural properties summarised in Table 1 provide further evidence of the mechanical stability of the zeolite templated carbons. Compaction at 370 MPa causes virtually no change in the surface area and pore volume of both templated carbons. The total and micropore component of the surface area and pore volume of both samples remain unchanged attesting to their remarkable mechanical stability. Compaction at 370 MPa, however, caused significant densification; the packing density increased to 0.69 g cm−3 for C5-ZTC-5 and 0.71 g cm−3 for C5-ZTC-15. We note that in previous studies,20–29 densification of porous materials to a packing density of ca. 0.7 g cm−3 is accompanied by reduction of surface area to below 2000 m2 g−1 and typically ca. 1000 m2 g−1 or lower compared to ca. 3200 m2 g−1 for the present zeolite-templated carbons. The surface area per unit volume increased by 54% from ca. 1430 m2 cm−3 to ca. 2200 m2 cm−3 after compaction at 370 MPa (Table 1). It is remarkable that compaction at the much higher pressure of 740 MPa caused only a small reduction in porosity as shown in Fig. 2 and Table 1; the surface area of ZTC-5 and ZTC-15 reduced by only 9% and 12% respectively, while the pore volume was lowered by 15% and 18%. The proportion of micropore surface area (ca. 85%) and pore volume (ca. 70%) remained unchanged after compaction at 740 MPa. The packing density increased to 0.72 g cm−3 for C10-ZTC-5 and 0.74 g cm−3 for C10-ZTC-15 yielding surface area per unit volume of ca. 2100 m2 cm−3.
Hydrogen uptake
The excess and total hydrogen uptake isotherms obtained for the zeolite-templated carbons, ZTC-5 and ZTC-15, via gravimetric analysis at −196 °C and pressures up to 20 bar are shown in Fig. 3A. The total hydrogen uptake at 20 bar and −196 °C is summarised in Table 1. Sample ZTC-5, which has a higher surface area and micropore surface area of 3332 and 2837 m2 g−1 respectively and pore volume of 1.66 cm3 g−1, exhibits a total hydrogen uptake capacity of 7.3 wt% at 20 bar and −196 °C compared to 6.2 wt% for ZTC-15. The excess hydrogen uptake achieved at 20 bar is 6.3 wt% and 5.6 wt% for ZTC-5 and ZTC-15, respectively. The present zeolite-templated carbons, and particularly sample ZTC-5, exhibit hydrogen uptake higher than most carbon materials reported so far. Indeed, an uptake of 7.3 wt%, at 20 bar and −196 °C is, as far as we know, the highest ever recorded for any carbon material.5–11,14–17,39 This value is higher than 6.9 wt% previously reported for a zeolite EMC-2 templated carbon,9 7.03 wt% for polypyrrole-derived activated carbon11 and 7.08 wt% for a doubly activated carbon.10Fig. 3B shows both the excess and total volumetric hydrogen uptake calculated on the basis of a packing/bulk density of 0.43 g cm−3 for ZTC-5 and 0.45 g cm−3 for ZTC-15. As shown in Fig. 3B, the non-compacted ZTCs achieve a high excess volumetric hydrogen uptake (at relatively low pressure of 20 bar and −196 °C) of 27 g l−1 and 25.4 g l−1 for ZTC-5 and ZTC-15, respectively. As shown in Fig. 3B and summarised in Table 1, the corresponding total volumetric hydrogen uptake, at 20 bar, is 31.4 g l−1 and 28 g l−1 for ZTC-5 and ZTC-15, respectively. Such an uptake, for the non-compacted ZTCs, is already much higher than that observed for activated carbons and MOFs of similar packing density.20–29 | ||
| Fig. 3 Gravimetric (A) and volumetric (B) excess and total hydrogen uptake isotherms at −196 °C of zeolite templated carbons ZTC-5 and ZTC-15. | ||
As described above, compaction (i.e., densification) of the templated carbons significantly increased their packing density, which should be beneficial for volumetric hydrogen uptake as long as the densification process does not compromise gravimetric uptake. The total hydrogen uptake of the carbons after densification at a load of 5 tons (370 MPa) is given in Table 1. It is remarkable that, in line with the trend in textural properties, the gravimetric hydrogen uptake capacity after compaction at 370 MPa is virtually unchanged at 7.2 wt% and 6.3 wt% for C5-ZTC-5 and C5-ZTC-15, respectively compared to 7.3 wt% and 6.2 wt% before compaction. This means that the total volumetric hydrogen uptake (at 20 bar) rises to 49.7 g l−1 and 44.7 g l−1 for C5-ZTC-5 and C5-ZTC-15, respectively. Such a volumetric hydrogen uptake, at 20 bar, is by far the highest ever reported for any porous material, including densified carbons and MOFs, which have so far been limited to ca. 40 g l−1 at much higher pressure (≥40 bar).20–29
The excess and total hydrogen uptake isotherms obtained after densification at a load of 10 tons (740 MPa), i.e., carbons C10-ZTC-5 and C10-ZTC-15, are shown in Fig. 4A. The total gravimetric hydrogen uptake at 20 bar and −196 °C is summarised in Table 1. After densification at 740 MPa there was a small decrease in gravimetric hydrogen uptake, which again is consistent with the changes in textural properties (Table 1); at 20 bar, the total gravimetric hydrogen uptake of sample ZTC-5 reduces only slightly from 7.3 wt% to 7.0 wt% for C10-ZTC-5, while C10-ZTC-15 has an uptake of 5.9 wt% compared to 6.2 wt% for the non-compacted ZTC-15 analogue. As shown in Fig. 4A, the corresponding excess gravimetric hydrogen uptake achieved at 20 bar is 6.0 wt% and 5.4 wt% for C10-ZTC-5 and C10-ZTC-15, respectively. Fig. 4B shows both the excess and total volumetric hydrogen uptake calculated on the basis of packing density of 0.72 g cm−3 for C10-ZTC-5 and 0.74 g cm−3 for C10-ZTC-15. The densified zeolite templated carbons achieve a very high excess volumetric hydrogen uptake (at 20 bar and −196 °C) of 43 and 40 g l−1 for C10-ZTC-5 and C10-ZTC-15, respectively. As summarised in Table 1, the total volumetric hydrogen uptake, at 20 bar, is 50.4 and 43.7 g l−1 for C10-ZTC-5 and C10-ZTC-15, respectively. The volumetric uptake after densification at 740 MPa is therefore quite similar to that of samples densified at 370 MPa. We note that a total volumetric uptake of 50.4 g l−1, at 20 bar and −196 °C, is by far the highest ever recorded for any carbon material.20–29 Moreover, the encouraging uptake of 50.4 g l−1 is achieved at a relatively low pressure of 20 bar. The uptake of the present densified zeolite templated carbons thus appears to comfortably exceed the 2017 targets set by the United States Department of Energy (DOE), i.e., a system gravimetric uptake capacity of 5.5 wt% and volumetric uptake capacity of 40 g l−1. We, however, note that the DOE targets are for the whole system rather than on a material-only basis as reported here. Nevertheless, the targets may be achievable given that the densified ZTC carbons can store even greater amounts of hydrogen at pressures above 20 bar, vide infra.
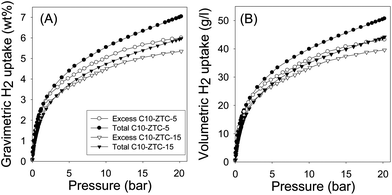 | ||
| Fig. 4 Gravimetric (A) and volumetric (B) excess and total hydrogen uptake isotherms at −196 °C of zeolite templated carbons C10-ZTC-5 and C10-ZTC-15 after densification at 740 MPa. | ||
The hydrogen uptake isotherms in Fig. 3 and 4 do not show any hysteresis, which indicates that the uptake is totally reversible. It is also clear that the total hydrogen uptake isotherms do not reach saturation at 20 bar, which means, as mentioned above, that higher hydrogen storage is possible above 20 bar as demonstrated by the uptake of a representative ZTC at a pressure of up to 50 bar (Fig. S3, ESI†). Motivated by the nonsaturation at 20 bar and even up to 50 bar (Fig. S3, ESI†), and the fact that ZTCs can therefore have higher uptake at pressures above 50 bar, we estimated the maximum total hydrogen uptake via fitting of the data up to 20 bar using the Langmuir model40 (Fig. S4, ESI†). We, inter alia, confirmed that such fitting using the Langmuir model (i.e., Langmuir plots) gives acceptable maximum hydrogen storage data by applying it to published experimental data for a metal–organic framework (NOTT-112).41 The comparison between published experimental data for NOTT-112 and an estimate from Langmuir plots was performed so as to illustrate the veracity of the Langmuir plots in estimating maximum hydrogen uptake. The experimentally determined maximum total hydrogen storage for NOTT-112 is 10 wt% at 77 bar,41 while fitting uptake data up to 20 bar using a Langmuir plot (Fig. S4, ESI†) estimates a maximum total uptake of 9.96 wt%. Thus the experimentally determined value (10 wt%) and estimated value from a Langmuir plot (9.96 wt%) are virtually identical for NOTT-112,41 which confirmed the veracity of our approach to estimating maximum total gravimetric hydrogen uptake for the zeolite templated carbons. The estimated maximum total gravimetric hydrogen uptake for the ZTCs before and after densification is summarised in Table 1. For ZTC-5, the estimated maximum is 9.2 wt%, decreasing slightly to 9.0 wt% (C5-ZTC-5) and 8.8 wt% (C10-ZTC-5) after densification. For ZTC-15, the maximum is 7.9 wt%, and remains largely unchanged at 8.1 wt% for densified C5-ZTC-15, and then decreases to 7.5 wt% after densification at 740 MPa (sample C10-ZTC-15). The very high estimated maximum gravimetric hydrogen uptake means that, as summarised in Table 1, the ZTCs can achieve a total volumetric uptake of up to 40 g l−1 before densification, and exceptionally reach storage of 56–58 g l−1 for densified ZTC-15 and an unprecedented 63 g l−1 for densified ZTC-5. The estimated maximum uptake presented here far exceeds the 2017 DOE systems targets, i.e., 5.5 wt% gravimetric uptake and 40 g l−1 volumetric uptake. However, as the DOE targets are for the whole system rather than on a material-only basis as reported here, more work is needed to clarify how material-only gravimetric hydrogen uptake of 8.8 wt% and volumetric uptake of 63 g l−1 (for densified ZTC-5) translate to system values. Nevertheless, the fact that the material-only values presented here are higher than the DOE systems targets is very encouraging.
More generally, the volumetric uptake observed here is higher than that of any carbon or known MOFs and even compares very favourably with the theoretical maximum for MOFs.42 It is also worth noting, as discussed above, that MOFs are very susceptible to loss of porosity on compaction, which rather limits their densification.24–26,42 The excellent mechanical stability, densification and hydrogen storage exhibited by the present template carbons indicate that zeolite templated carbons are one of the key materials for use in hydrogen storage. The exceptional hydrogen uptake is attributed to a combination of the presence of 1.2 nm pores, which are in the pore size range useful for hydrogen uptake,43,44 high surface area and unique molecular structure that allows densification without loss of textural fidelity. More widely, the data presented here illustrate the potential of porous carbons as hydrogen stores.
Summary
A hard templating technique using zeolite as the template has been employed to generate porous carbons with zeolite-like structural ordering, high surface area and pore volume and excellent mechanical stability. The high mechanical stability of the resulting zeolite templated carbons (ZTCs) means that they readily undergo densification and can be safely compacted at extreme loads of up to 10 tons (740 MPa) while still retaining their zeolite-like structural ordering and high porosity. On densification, the ZTCs reach packing density of 0.72–0.74 g cm−3, and retain surface area of ca. 3000 m2 g−1 and pore volume of ca. 1.4 cm3 g−1, and thus possess a combination of textural properties hitherto not been achieved in any porous material. Unusually, the densified zeolite templated carbons retain high gravimetric hydrogen uptake, at −196 °C, of ca. 7 wt% at 20 bar, rising to 8.8 wt% above 20 bar. The high packing density and high gravimetric hydrogen uptake translate to exceptional and unprecedented volumetric hydrogen storage of 50 g l−1 at 20 bar and up to 63 g l−1 above 20 bar. The porosity in the zeolite templated carbons, before and after densification, arises mainly from micropores of size 1.2 nm. This study and the findings herein described break new ground with respect to levels of volumetric hydrogen uptake achievable for porous carbons and highlight a very promising route for fabrication and post synthesis densification of carbon materials. The possibility of densification accompanied with little loss in porosity or gravimetric hydrogen uptake is, as far as we know, unique to ZTCs and means that appropriately densified zeolite templated carbons are excellent hydrogen stores with high gravimetric and exceptional volumetric capacity. The findings add new insights in the development of carbonaceous materials with enhanced hydrogen storage capacity.Acknowledgements
This research was funded by the University of Nottingham. We are grateful to Dr Anne Dailly (General Motors, Warren, Michigan) for some hydrogen uptake measurements.References
- (a) L. Schlapbach and A. Züttel, Nature, 2001, 414, 353 CrossRef CAS PubMed; (b) J. A. Turner, Science, 1999, 285, 687 CrossRef CAS; (c) G. W. Crabtree, M. S. Dresselhaus and M. V. Buchanan, Phys. Today, 2004, 57, 39 CrossRef CAS; (d) C.-J. Winter, Int. J. Hydrogen Energy, 2009, 34, 1 CrossRef PubMed; (e) S. Dunn, Int. J. Hydrogen Energy, 2002, 27, 235 CrossRef CAS; (f) M. A. Deluchi, Int. J. Hydrogen Energy, 1989, 14, 81 CrossRef CAS; (g) A. W. C. van den Berg and C. O. Arean, Chem. Commun., 2008, 668 RSC.
- (a) M. G. Nijkamp, J. Raaymakers, A. J. van Dillen and K. P. de Jong, Appl. Phys. A, 2001, 72, 619 CrossRef CAS; (b) A. Zecchina, S. Bordiga, J. G. Vitillo, G. Ricchiardi, C. Lamberti, G. Spoto, M. Bjorgen and K. P. Lillerud, J. Am. Chem. Soc., 2005, 127, 6361 CrossRef CAS PubMed; (c) F. Stephanie-Victoire, A. M. Goulay and E. C. de Lara, Langmuir, 1998, 14, 7255 CrossRef CAS.
- (a) H. K. Chae, D. Y. Siberio-Perez, J. Kim, Y. Go, M. Eddaoudi, A. J. Matzger, M. O'Keeffe and O. M. Yaghi, Nature, 2004, 427, 523 CrossRef CAS PubMed; (b) N. L. Rosi, J. Eckert, M. Eddaoudi, D. T. Vodak, J. Kim, M. O'Keeffe and O. M. Yaghi, Science, 2003, 300, 1127 CrossRef CAS PubMed; (c) J. L. C. Rowsell and O. M. Yaghi, Angew. Chem., Int. Ed., 2005, 44, 4670 CrossRef CAS PubMed; (d) J. L. C. Rowsell, A. R. Millward, K. S. Park and O. M. Yaghi, J. Am. Chem. Soc., 2004, 126, 5666 CrossRef CAS PubMed.
- (a) S. Q. Ma and H. C. Zhou, Chem. Commun., 2010, 46, 44 RSC; (b) Y. H. Hu and L. Zhang, Adv. Mater., 2010, 22, 117 CrossRef PubMed; (c) L. J. Murray, M. Dinca and J. R. Long, Chem. Soc. Rev., 2009, 38, 1294 RSC; (d) S. S. Han, J. L. Mendoza-Cortes and W. A. Goddard, Chem. Soc. Rev., 2009, 38, 1460 RSC; (e) K. M. Thomas, Dalton Trans., 2009, 1487 RSC; (f) D. Zhao, D. Q. Yuan and H. C. Zhou, Energy Environ. Sci., 2008, 1, 222 RSC.
- (a) P. Chen, X. Wu, J. Lin and K. L. Tan, Science, 1999, 285, 91 CrossRef CAS; (b) C. Liu, Y. Y. Fan, M. Liu, H. T. Cong, H. M. Cheng and M. S. Dresselhaus, Science, 1999, 286, 1127 CrossRef CAS; (c) A. Chambers, C. Park, R. T. K. Baker and N. M. Rodriguez, J. Phys. Chem. B, 1998, 102, 4253 CrossRef CAS; (d) C. C. Ahn, Y. Ye, B. V. Ratnakumar, C. Witham, R. C. Bowman and B. Fultz, Appl. Phys. Lett., 1998, 73, 3378 CrossRef CAS; (e) G. G. Tibbetts, G. P. Meisner and C. H. Olk, Carbon, 2001, 39, 2291 CrossRef CAS; (f) M. Ritschel, M. Uhlemann, O. Gutfleisch, A. Leonhardt, A. Graff, C. Taschner and J. Fink, Appl. Phys. Lett., 2002, 80, 2985 CrossRef CAS; (g) H. M. Cheng, Q. H. Yang and C. Liu, Carbon, 2001, 39, 1447 CrossRef CAS.
- (a) M. Hirscher and B. Panella, J. Alloys Compd., 2005, 404, 399 CrossRef PubMed; (b) A. C. Dillon and M. J. Heben, Appl. Phys. A: Mater. Sci. Process., 2001, 72, 133 CrossRef CAS; (c) A. Zuttel, P. Sudan, P. Mauron, T. Kiyobayashi, C. Emmenegger and L. Sclapbach, Int. J. Hydrogen Energy, 2002, 27, 203 CrossRef CAS; (d) R. Ströbel, J. Garche, P. T. Moseley, L. Jörissen and G. Wolf, J. Power Sources, 2006, 159, 781 CrossRef PubMed; (e) K. M. Thomas, Catal. Today, 2007, 120, 389 CrossRef CAS PubMed.
- (a) Y. Xia and R. Mokaya, J. Phys. Chem. C, 2007, 111, 10035 CrossRef CAS; (b) X. B. Zhao, B. Xiao, A. J. Fletcher and K. M. Thomas, J. Phys. Chem. B, 2005, 109, 8880 CrossRef CAS PubMed; (c) J. Pang, J. E. Hampsey, Z. Wu, Q. Hu and Y. Lu, Appl. Phys. Lett., 2004, 85, 4887 CrossRef CAS; (d) E. Terres, B. Panella, T. Hayashi, Y. A. Kim, M. Endo, J. M. Dominguez, M. Hirscher, H. Terrones and M. Terrones, Chem. Phys. Lett., 2005, 403, 363 CrossRef CAS PubMed; (e) B. Fang, H. Zhou and I. Honma, J. Phys. Chem. B, 2006, 110, 4875 CrossRef CAS PubMed; (f) N. Texier-Mandoki, J. Dentzer, T. Piquero, S. Saadallah, P. David and C. Vix-Guterl, Carbon, 2004, 42, 2744 CrossRef CAS PubMed.
- (a) M. Sevilla, A. B. Fuertes and R. Mokaya, Energy Environ. Sci., 2011, 4, 1400 RSC; (b) M. Sevilla, R. Foulston and R. Mokaya, Energy Environ. Sci., 2010, 3, 223 RSC; (c) M. Sevilla, A. B. Fuertes and R. Mokaya, Int. J. Hydrogen Energy, 2011, 36, 15658 CrossRef CAS PubMed; (d) A. Almasoudi and R. Mokaya, J. Mater. Chem., 2012, 22, 146 RSC; (e) Y. Xia and R. Mokaya, Chem. Vap. Deposition, 2010, 16, 322 CrossRef CAS; (f) M. Sevilla and R. Mokaya, J. Mater. Chem., 2011, 21, 4727 RSC.
- Z. Yang, Y. Xia and R. Mokaya, J. Am. Chem. Soc., 2007, 129, 1673 CrossRef CAS PubMed.
- H. Wang, Q. Gao and J. Hu, J. Am. Chem. Soc., 2009, 131, 7016 CrossRef CAS PubMed.
- M. Sevilla, R. Mokaya and A. B. Fuertes, Energy Environ. Sci., 2011, 4, 2930 CAS.
- (a) Y. Xia, Z. Yang and R. Mokaya, Nanoscale, 2010, 2, 639 RSC; (b) H. Nishihara and T. Kyotani, Adv. Mater., 2012, 24, 4473 CrossRef CAS PubMed.
- T. Kyotani, Bull. Chem. Soc. Jpn., 2006, 79, 1322 CrossRef CAS.
- (a) N. Alam and R. Mokaya, Energy Environ. Sci., 2010, 3, 1773 RSC; (b) Y. Xia, G. S. Walker, D. M. Grant and R. Mokaya, J. Am. Chem. Soc., 2009, 131, 16493 CrossRef CAS PubMed; (c) E. Masika and R. Mokaya, Prog. Nat. Sci. Mater. Int., 2013, 23, 308 CrossRef PubMed.
- I. Cabria, M. J. López and J. A. Alonso, Carbon, 2007, 45, 2649 CrossRef CAS PubMed.
- (a) G. Yushin, R. Dash, J. Jagiello, J. E. Fischer and Y. Gogotsi, Adv. Funct. Mater., 2006, 16, 2288 CrossRef CAS; (b) Y. Gogotsi, R. K. Dash, G. Yushin, T. Yildirim, G. Laudisio and J. E. Fischer, J. Am. Chem. Soc., 2005, 127, 16006 CrossRef CAS PubMed.
- (a) M. Sevilla, N. Alam and R. Mokaya, J. Phys. Chem. C, 2011, 114, 11314 CrossRef; (b) A. Pacula and R. Mokaya, J. Phys. Chem. C, 2008, 112, 2764 CrossRef CAS.
- (a) T. Kyotani, Z. Ma and A. Tomita, Carbon, 2003, 41, 1451 CrossRef CAS; (b) T. Kyotani, T. Nagai, S. Inoue and A. Tomita, Chem. Mater., 1997, 9, 609 CrossRef CAS; (c) Z. Ma, T. Kyotani and A. Tomita, Carbon, 2002, 40, 2367 CrossRef CAS.
- U.S. Department of Energy Website, https://www1.eere.energy.gov/hydrogenandfuelcells/storage/current_technology.html.
- (a) J. P. Marco-Lozar, J. Juan-Juan, F. Suarez-Garcıa, D. Cazorla-Amoros and A. Linares-Solano, Int. J. Hydrogen Energy, 2012, 37, 2370 CrossRef CAS PubMed; (b) J. Juan-Juan, J. P. Marco-Lozar, F. Suarez-Garcia, D. Cazorla-Amoros and A. Linares-Solano, Carbon, 2010, 48, 2906 CrossRef CAS PubMed.
- (a) K. W. Chapman, G. J. Halder and P. J. Chupas, J. Am. Chem. Soc., 2009, 131, 17546 CrossRef CAS PubMed; (b) J. Alcaniz-Monge, G. Trautwein, M. Perez-Cadenas and M. C. Roman-Martinez, Microporous Mesoporous Mater., 2009, 126, 291 CrossRef CAS PubMed.
- (a) M. Jorda-Beneyto, D. Lozano-Castello, F. Suarez-Garcıa, D. Cazorla-Amoros and A. Linares-Solano, Microporous Mesoporous Mater., 2008, 112, 235 CrossRef CAS PubMed; (b) L. Zhou, Y. Zhou and Y. Sun, Int. J. Hydrogen Energy, 2004, 29, 319 CrossRef CAS.
- U. Mueller, M. Schubert, F. Teich, H. Puetter, K. Schierle-Arndt and J. Pastre, J. Mater. Chem., 2006, 16, 626 RSC.
- R. Zacharia, D. Cossement, L. Lafi and R. Chahine, J. Mater. Chem., 2010, 20, 2145 RSC.
- J. J. Purewal, D. Liu, J. Yang, A. Sudik, D. J. Siegel, S. Maurer and U. Muller, Int. J. Hydrogen Energy, 2012, 37, 2723 CrossRef CAS PubMed.
- J. Purewal, D. Liu, A. Sudik, M. Veenstra, J. Yang, S. Maurer, U. Muller and D. J. Siegel, J. Phys. Chem. C, 2012, 116, 20199 CAS.
- S. H. Yeon, I. Knoke, Y. Gogotsi and J. E. Fischer, Microporous Mesoporous Mater., 2010, 131, 423 CrossRef CAS PubMed.
- J. P. Singer, A. Mayergoyz, C. Portet, E. Schneider, Y. Gogotsi and J. E. Fischer, Microporous Mesoporous Mater., 2008, 116, 469 CrossRef CAS PubMed.
- P. X. Hou, H. Orikasa, H. Itoi, H. Nishihara and T. Kyotani, Carbon, 2007, 45, 2011 CrossRef CAS PubMed.
- H. Nishihara, Q. H. Yang, P. X. Hou, M. Unno, S. Yamauchi, R. Saito, J. I. Paredes, A. Martínez-Alonso, J. M. D. Tascón, Y. Sato, M. Terauchi and T. Kyotani, Carbon, 2009, 47, 1220 CrossRef CAS PubMed.
- T. Roussel, C. Bichara, K. E. Gubbins and R. J. M. Pellenq, J. Chem. Phys., 2009, 130, 174717 CrossRef PubMed.
- T. Roussel, A. Didion, R. J. M. Pellenq, R. Gadiou, C. Bichara and C. Vix-Guterl, J. Phys. Chem. C, 2007, 111, 15863 CAS.
- F. O. M. Gaslain, J. Parmentier, V. P. Valtchev and J. Patarin, Chem. Commun., 2006, 991 RSC.
- Z. Yang, Y. Xia and R. Mokaya, Microporous Mesoporous Mater., 2005, 86, 69 CrossRef CAS PubMed.
- Z. X. Yang, Y. D. Xia, X. Z. Sun and R. Mokaya, J. Phys. Chem. B, 2006, 110, 18424 CrossRef CAS PubMed.
- Z. Ma, T. Kyotani and A. Tomita, Chem. Commun., 2000, 2365 RSC.
- (a) Y. Xia, R. Mokaya, D. M. Grant and G. S. Walker, Carbon, 2011, 49, 844 CrossRef CAS PubMed; (b) N. Alam and R. Mokaya, Microporous Mesoporous Mater., 2011, 142, 716 CrossRef CAS PubMed; (c) N. Alam and R. Mokaya, Microporous Mesoporous Mater., 2011, 144, 140 CrossRef CAS PubMed.
- (a) S. A. Johnson, E. S. Brigham, P. J. Ollivier and T. E. Mallouk, Chem. Mater., 1997, 9, 2448 CrossRef CAS; (b) F. Su, X. S. Zhao, L. Lv and Z. Zhou, Carbon, 2004, 42, 2821 CrossRef CAS PubMed.
- (a) M. Armandi, B. Bonelli, C. O. Areán and E. Garrone, Microporous Mesoporous Mater., 2008, 112, 411 CrossRef CAS PubMed; (b) M. Rzepka, P. Lamp and M. A. de la Casa-Lillo, J. Phys. Chem. B, 1998, 102, 10894 CrossRef CAS; (c) W. C. Xu, K. Takahashi, Y. Matsuo, Y. Hattori, M. Kumagai, S. Ishiyama, K. Kaneko and S. Iijima, Int. J. Hydrogen Energy, 2007, 32, 2504 CrossRef CAS PubMed; (d) C. Robertson and R. Mokaya, Microporous Mesoporous Mater., 2013, 179, 151 CrossRef CAS PubMed.
- (a) H. W. Langmi, D. Book, A. Walton, S. R. Johnson, M. M. Al-Mamouri, J. D. Speight, P. P. Edwards, I. R. Harris and P. A. Anderson, J. Alloys Compd., 2005, 404–406, 637 CrossRef CAS PubMed; (b) G. Srinivas, Y. Zhu, R. Piner, N. Skipper, M. Ellerby and R. Ruoff, Carbon, 2010, 48, 630 CrossRef CAS PubMed.
- Y. Yan, X. Lin, S. Yang, A. J. Blake, A. Dailly, N. R. Champness, P. Hubberstey and M. Schroder, Chem. Commun., 2009, 1025 RSC.
- J. Goldsmith, A. G. Wong-Foy, M. J. Cafarella and D. J. Siegel, Chem. Mater., 2013, 25, 3373 CrossRef CAS.
- E. Masika and R. Mokaya, J. Phys. Chem. C, 2012, 116, 25734 CAS.
- Y. Gogotsi, C. Portet, S. Osswald, J. M. Simmons, T. Yildirim, G. Laudisio and J. E. Fischer, Int. J. Hydrogen Energy, 2009, 34, 6314 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Four additional figures showing powder XRD patterns, nitrogen sorption isotherms (and pore size distribution) of the zeolite templated carbons, total hydrogen uptake isotherms at −196 °C and up to 50 bar for a representative zeolite templated carbon, and Langmuir plots for determining maximum total hydrogen uptake of zeolite-templated carbons and metal–organic framework NOTT-122. See DOI: 10.1039/c3ee42239a |
| This journal is © The Royal Society of Chemistry 2014 |