Biomass to liquid transportation fuels (BTL) systems: process synthesis and global optimization framework†
Richard C. Baliban, Josephine A. Elia and Christodoulos A. Floudas*
Princeton University, Princeton, NJ 08544, USA. E-mail: floudas@titan.princeton.edu; Fax: +1 (609) 258 0211; Tel: +1 (609) 259 4595
First published on 14th November 2012
Abstract
An optimization-based process synthesis framework is proposed for the thermochemical conversion of biomass to liquid fuels (BTL). Gasification of biomass is used to generate synthesis gas which can be converted to raw hydrocarbons either directly using Fischer–Tropsch synthesis or indirectly using catalytic conversion of methanol over ZSM-5. Multiple technologies are considered for generation of the raw hydrocarbons including (i) six types of Fischer–Tropsch units with different temperatures, catalyst types, and hydrocarbon effluent compositions, (ii) methanol conversion using methanol-to-gasoline, and (iii) methanol conversion using methanol-to-olefins. The hydrocarbons are upgraded into the final liquid fuel products (i.e., gasoline, diesel, and kerosene) using one or more technologies including ZSM-5 catalytic conversion, oligomerization, hydrocracking, isomerization, alkylation, and hydrotreating. A simultaneous heat, power, and water integration is included within the process synthesis framework to directly examine the costs associated with utility production and wastewater treatment with a particular topological design. A rigorous global optimization branch-and-bound strategy is implemented to mathematically guarantee the development of a BTL refinery that is economically and environmentally superior to all competing designs. Twenty-four case studies are investigated to determine the effect of refinery capacity, liquid fuel composition, and biomass feedstock on the overall system cost, the BTL refinery topological design, the process material/energy balances, and the lifecycle greenhouse gas emissions.
Broader contextIncreasingly high energy prices, volatility of the global oil market, and consistent pressure to reduce lifecycle greenhouse gas emissions are major challenges within the United States transportation sector. Sustainably harvested biomass is a renewable energy source that can absorb atmospheric CO2 during photosynthesis, so it is capable of addressing both the concerns of enhanced domestic fuels production and lifecycle greenhouse gas emissions reduction. A plethora of process designs have been proposed for thermochemical-based biomass to liquid fuel (BTL) processes, but determination of the “best possible” design for a given set of conditions (e.g., type of biomass, refinery capacity, type of liquid fuels) requires a significant amount of computational time and manpower. This study introduces a process synthesis framework for BTL processes that can directly analyze technoeconomic and environmental trade-offs using a large-scale mixed-integer nonlinear optimization model. A superstructure of alternatives for producing the liquid hydrocarbon fuels from biomass is generated from several existing or novel process topologies. The framework can theoretically guarantee the selection of a topology that provides superior economic and environmental performance to all other possibilities in an automated and computationally efficient fashion. Results suggest that BTL products can be economically competitive with petroleum-based fuels when crude oil is maintained at or above a range of $80 to $100 per barrel. |
1 Introduction
Increasingly high energy prices, volatility of the global oil market, and consistent pressure to reduce lifecycle greenhouse gas emissions are major challenges within the United States transportation sector. Continued unrest within OPEC nations along with concern of “peak-oil” domestic crude oil production1,2 have motivated national efforts toward the development of liquid fuels that can be derived from domestic carbon-based feedstocks to provide a significant means for increasing national security through enhanced energy independence. Thus, throughout the next two decades, the additional fuel demand for the country is expected to be largely satisfied through “non-petroleum” based feedstocks.3 Several technologies have been investigated to process alternative feedstocks, but the Energy Information Administration projects that a majority of the liquid fuels supply will come from biomass sources.3 Biomass is a renewable energy source that can absorb atmospheric CO2 during photosynthesis,4–6 so it is capable of addressing both the concerns of enhanced domestic fuels production and lifecycle greenhouse gas emissions reduction. If harvested sustainably, biomass has the potential to play a substantial role in shaping the United States energy future. However, the use of land to grow biomass for liquid fuels production must be analyzed in context with the other potential land requirements including food, feed, or the preservation of natural habitats. Ultimately, removal of crop or forest residues for biofuel production will have to be investigated using a holistic framework that recognizes the connectivity of multiple processes that occur on both the farm and the ecosystem including soil carbon management, erosion mitigation, nutrient management, water/air quality, and global food/feed/fiber production.6 Nevertheless, it is possible to responsibly develop biomass feedstocks in a sustainable manner to generate a significant national supply without disruption of the human and animal food chain or deforestation.7 In fact, it is estimated that roughly 400–500 million sustainable dry tons of biomass are currently available for biofuel production with an estimated 1.3 billion tons potentially available in the future.6,7Liquid fuels derived from coal or natural gas take advantage of a non-renewable resource that will not achieve the same environmental benefit as biomass. Though the ability to extract CO2 from the atmosphere during biomass growth is a strong advocating point for biomass, interest has continued in coal based plants due to the low delivered cost of coal ($2.0–2.5 per MM Btu)3 relative to natural gas ($4.8–5.8 per MM Btu)8 or biomass ($4.0–9.0 per MM Btu).6,9,10 However, the high carbon content of coal may require that a significant portion of the feedstock carbon to be converted to CO2 where it can either be vented, sequestered, or converted back to CO using a non-carbon based source of hydrogen.11–17 Natural gas provides a high hydrogen to carbon ratio that can help to increase the conversion rates of feedstock carbon to final liquid fuels. Recent prospects for shale gas production have helped reduce the delivered cost of natural gas and made this feedstock a more attractive choice for liquid fuels production.8 Hybrid refineries that can utilize both a fossil-based feedstock and biomass can take advantage of the strengths of each feed and produce refineries that are economically or environmentally superior to single feedstock refineries. The three major hybrid refinery classifications that utilize biomass are coal/biomass to liquids,10,18–24 natural gas/biomass to liquids,25–28 and coal/biomass/natural gas to liquids.14,15,29,30 On a national scale, utilization of the national supplies of coal, biomass, and natural gas in an economic and environmentally suitable fashion is of utmost importance to break the strong dependence that the United States has on petroleum supplies. A recent review has highlighted the key developments made by academic groups worldwide to introduce process design alternatives that can produce gasoline, diesel, and kerosene using any one or a combination of coal, biomass, or natural gas feedstocks.31
Though hybrid-feedstock refineries can take advantage of the benefits of using lower-cost fossil-based feedstocks, it may not always be practical to utilize multiple feedstocks within a single refinery. The distributed network of biomass feedstock locations will contain several points where infrastructure for coal or natural gas delivery is minimal or non-existant. To develop biomass to liquid fuel (BTL) systems, it is critical to harness the potential of this resource in a sustainable and economic fashion. The high cost of both capital investment and biomass feedstock in thermochemical-based BTL systems is a strong impetus for the development of novel processes that can use existing unit operations in a more efficient topological design. Previous studies on BTL processes generally will fix the topology (i.e., the combination of process units and streams) to analyze one particular type of process design. Heat and mass balances for the process are then calculated using standard simulation software and an economic analysis is carried out to determine the financial metrics of the plant (e.g., net present value).9,10,18,23,24,32–92 Given the plethora of process designs that have been developed in the academic literature, determination of the “best possible” design would require a significant amount of computational time and manpower. A fair and thorough comparison of the process designs would require a framework that implements a standard set of financial parameters, environmental targets, feedstock properties, product requirements, and unit operating conditions for all designs. The effort required to conduct such an analysis scales with the number of designs to consider and becomes intractable beyond some upper threshold of possibilities. Moreover, this strategy offers no guarantee that the “best” design is superior to novel process topologies that were not considered in the comparative analysis.
Process synthesis strategies have been recently developed14,15,29,30,93–95 that are capable of analyzing thousands of process designs simultaneously in a fully automated and computationally efficient fashion. This study introduces an optimization-based process synthesis framework for BTL processes that will directly analyze the technoeconomic and environmental trade-offs using a large-scale mixed-integer nonlinear optimization (MINLP) model. A superstructure of alternatives for producing the liquid hydrocarbon fuels from biomass is generated from several existing or novel process topologies. Proper unit operation of each process unit in the superstructure is defined through detailed input–output relationships, and the simultaneous consideration of every process unit is incorporated through the formulation of a large-scale MINLP model. To mathematically guarantee the solution quality of the optimal design, a rigorous global optimization branch-and-bound strategy29 is used to solve the MINLP model to global optimality. Modeling of several components of the process superstructure have been described in detail in previous works,14,15,29,30,93 though all key processes for the BTL refinery will be discussed in the text below. A simultaneous heat and power integration13,14 using an optimization-based heat-integration approach96 and a series of heat engines is included in the framework to ensure that waste heat is effectively converted into electricity and process steam utilities.12–14 A comprehensive wastewater treatment network15 that includes a sour stripper, a biological digestor, and a reverse osmosis unit is incorporated using a superstructure approach97–100 to minimize wastewater contaminants and freshwater intake.
The process synthesis framework will be utilized to examine (i) biomass gasification with/without recycle synthesis gas, (ii) synthesis gas conversion via Fischer–Tropsch (FT) or methanol synthesis, (iii) methanol conversion via methanol-to-gasoline (MTG) or methanol-to-olefins (MTO), and (iv) hydrocarbon upgrading via ZSM-5 zeolite catalysis, olefin oligomerization, or carbon number fractionation and subsequent treatment. The key products from the BTL refinery will be gasoline, diesel, and jet fuel (kerosene) with allowable byproducts of liquefied petroleum gas (LPG) and electricity. The quantitative trade-offs associated with key metrics for the BTL refinery are illustrated using twenty-four case studies which are selected to demonstrate the capability of the process synthesis framework.
2 BTL process superstructure: conceptual design and mathematical modeling
This section will outline the design and modeling of the key sections of the BTL refinery.12,14,15,29,30,93 The complete mathematical model, all relevant nomenclature, and a complete set of process flow diagrams are provided as ESI.†2.1 Biomass handling and gasification
The BTL refinery is assumed to input only one type of biomass feedstock to reduce the complexity of the feedstock processing section and help increase the uniformity of the input to the gasifier. Three distinct categories of feedstocks will be considered (i.e., agricultural residues, perennial grasses, and forest residues), each of which has a representative composition shown in Table 1 that is derived from the ECN Phyllis database.101 The biomass is assumed to be delivered to the BTL refinery as woodchips (forest residues) or as bales (agricultural residues/perennial feeds) which must be further processed prior to entry to the biomass gasifier. The woodchips are screened to remove particles with size greater than 2 inches and sent them to a grinder for further size reduction.102 Any bales of feedstock are sent to the grinder to shred the biomass to a usable size for gasification.| Feed type | Ultimate analysis (db, weight %) | |||||
|---|---|---|---|---|---|---|
| C | H | N | Cl | S | O | |
| Agricultural | 46.8 | 5.74 | 0.66 | 0.266 | 0.11 | 41.4 |
| Perennial | 46.9 | 5.85 | 0.58 | 0.501 | 0.11 | 41.5 |
| Forest | 50.19 | 5.9 | 0.32 | 0 | 0.03 | 41.42 |
The process flow diagram for the generation of synthesis gas is detailed in Fig. 1. The biomass moisture content is reduced to 20 wt% through a preliminary drying step9 before the feedstock can be injected to the gasifier. Any heat necessary for the dryer is provided by flue gas generated through combustion within the refinery. The flue gas leaves the dryer at 110 °C and 1.05 bar, and is passed through an air cyclone and a baghouse filter to remove any particulates that are present.102 The heated biomass leaved the dryer at 105 °C and 1.05 bar, and is transferred to a high-pressure gasifier (30 bar) using compressed CO2 (10 wt%) and a lockhopper. The effluent of the biomass gasifier is a mixture of synthesis gas, C1–C2 hydrocarbons, acid gases (e.g., NH3, H2S), tar, char, and ash.12,14 The solid ash and char are separated from the vapor phase using cyclones, and are recycled back to the gasifier. It is assumed that the recycle of char will effectively provide a 100% conversion of the carbon in the biomass to vapor species while the ash is removed from the bottom of the gasifier.9 The composition of the gasifier effluent is a function of the biomass composition, gasifier temperature, and oxidizer flow rate and a detailed mathematical model has been formulated to determine the composition12,14 (see ESI†).
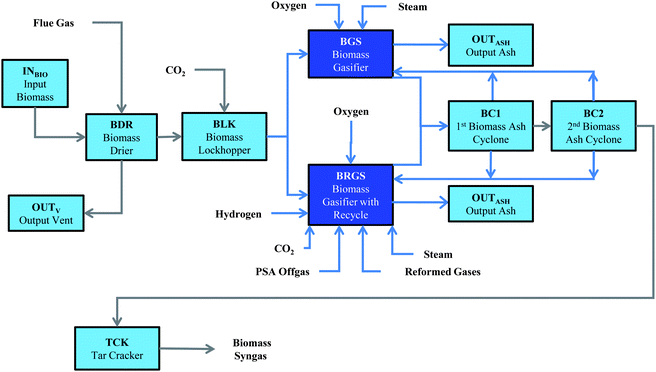 | ||
| Fig. 1 Biomass gasification flowsheet. Biomass is dried to 20 wt% moisture and then transferred to the gasifier system via a lockhopper. The gasifiers will operate with either a solid fuel (biomass) or a combination of solid and recycle gases as fuel. Residual ash and char that are generated within the gasifiers are separated via the cyclones and recycled to the gasifiers. The raw syngas is then transferred to a tar cracker to remove most of the tar species in the vapor phase. | ||
The gasifier is assumed to operate between 800 °C and 1000 °C and will utilize steam for gasification of biomass/char, reforming of C1–C2 hydrocarbons, and reforming of tar species. High-purity oxygen is input to the gasifier to provide the heat needed for the reforming reactions and to help crack the tar species in the gasifier. Operation of the temperature at temperatures of 1000 °C is consistent with the gasifier conditions associated with additional oxygen injection in the freeboard of the gasifier to help promote internal tar cracking.9 The high temperature of the unit will help to facilitate the water–gas-shift (WGS) equilibrium of the synthesis gas effluent, though the concentration of the hydrocarbons in the effluent will be far above the equilibrium values. Using the reverse WGS reaction, CO2 may be consumed within the gasifier unit by reaction with H2 that is present within the gasifier. Therefore, any CO2 that is generated by the process can be recycled to the gasifier along with H2 that is produced from pressure-swing absorption or electrolysis of water.
The effluent of the gasifier is passed through a catalytic tar reformer (925 °C), which will reform (i) tar species to CO and H2, (ii) NH3 to N2 and H2, and (iii) C1–C2 hydrocarbons to CO and H2. Current bench-scale performance of a tar reformer from the National Renewable Energy Laboratory is a conversion of 80% of the CH4, 99.6% of tars, 99% of C2H6, 90% of C2H4, and 90% of NH3.102 Equipment is currently being installed for pilot-scale demonstration of the tar reformer performance over a continuous period of time.103 The steam that is present in the gasifier effluent is sufficiently high enough to reform the syngas without the need of additional input steam.103 Heat for the tar reformer is provided by circulating catalyst between the tar reformer and a catalyst regenerator to remove the coke deposits on the catalyst surface. The level of coke deposited on the catalyst is insufficient to provide the heat needed for the endothermic reforming reactions, so additional combustion gases are passed through the regenerator.103 The syngas exiting the tar reformer is cooled to 60 °C and passed to the cleaning section (see Fig. 2).
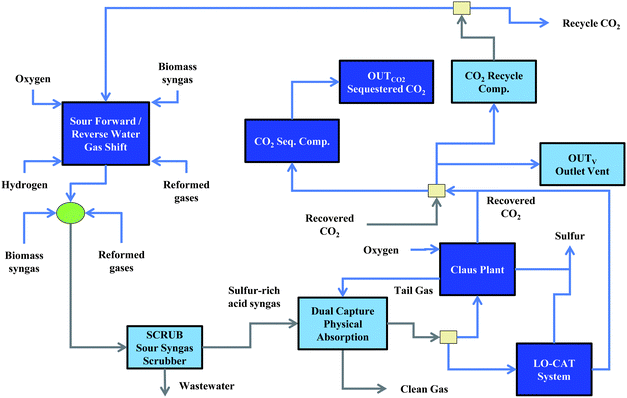 | ||
| Fig. 2 Synthesis gas (syngas) handling flowsheet. Syngas may be passed over a forward/reverse water–gas-shift reactor to alter the H2 to CO/CO2 ratio prior to Fischer–Tropsch or methanol synthesis. The syngas is then cooled, flashed to remove water, and is directed to a dual-capture methanol-based unit for CO2 and H2 removal. The H2S gases are then directed to either a Claus plant or a LO-CAT system for recovery of the sulfur. The captured CO2 may be vented, sequestered, or recycled back to process units. | ||
2.2 Synthesis gas cleaning
The process flow diagram for processing the raw syngas is shown in Fig. 2. The syngas effluent from the gasifier may be directed to a dedicated water–gas-shift reactor that can operate in the presence of sulfur species. The unit will operate at a pressure of 28 bar and a temperature between 400 °C and 600 °C and can be utilized as either a forward or reverse water–gas-shift unit. Hydrogen may be supplied via pressure-swing absorption or electrolysis to facilitate the consumption of recycle CO2via the reverse water–gas-shift reaction. The endothermic heat of reaction is provided by combustion of syngas species via input oxygen. Reformed gases from the auto-thermal reactor may also be directed to the water–gas-shift unit. The syngas from the sour water–gas-shift unit is combined with any bypass syngas, cooled to 185 °C, and sent to a scrubbing system (SCRUB) to remove residual tars, particulates, and NH3 as part of a wastewater stream. The wastewater is sent to a biological digestor (ESI, Fig. S11†) to treat the organic contaminants while the scrubbed syngas is passed over a dual-capture methanol absorption to co-remove the H2S and CO2 from the syngas.10 Note that the acid gas unit must be utilized in the process to remove the sulfur species and prevent poisoning of the downstream hydrocarbon production catalysts. The capture unit will remove 100% of the H2S and 90% of the CO2 from the input gases. Approximately 3 moles CO2/mole H2S are entrained with the H2S in the dual capture system.10 The clean gases are assumed to leave the capture unit at 25 bar and 35 °C while the acid gases will leave at 1 bar and 35 °C. The CO2 may be (a) compressed to 31 bar for recycle to the reformers or the water–gas-shift units or (b) compressed to 150 bar for sequestration. Note that both compression options will utilize multiple compression stages with inter-cooling to control the temperature rise. The CO2 may alternatively be vented to the atmosphere.The sulfur recovery unit in the BTL refinery will either be a Claus recovery system104 or a LO-CAT iron-chelate based process.102 The Claus process will contain an oxygen-blown furnace that can convert up to 95% of the H2 to solid sulfur. The sulfur in the tail gas will be present in various forms (e.g., H2S, SO2, COS, S) which can be hydrogenated to H2S. To recover the sulfur in the tail gas, the tail gas may be recycled back to the dual-capture physical absorption unit. Alternatively, the tail gas may be sent to a dedicated amine recovery unit, as is practiced by Shell in the Shell Claus Off-gas Treating (SCOT) process. If the LO-CAT process is utilized, essentially a 100% recovery of the H2S to sulfur can be realized with a single pass through the system.102
2.3 Hydrocarbon production/upgrading
In addition to the CO2/(CO + CO2) ratio, the amount of inlet hydrogen with respect to both CO and CO2 will play an important role in FT operation. Low-temperature iron-based units have been operated successfully with inlet H2/CO ratios between 0.5 and 1 (ref. 105–107) due to in situ water–gas-shift activity that effectively produces an appropriate outlet H2/CO ratio near 1.7–2.0. Though these FT processes requires substantially less hydrogen than FT processes with a 2/1 inlet ratio, approximately 50% of the CO is converted to CO2. To prevent such a large increase in the outlet CO2 concentration, the inlet ratio of H2/(CO + CO2) must be set to ensure that CO2 can be used as a carbon source due to the reverse water–gas-shift reaction. The Ribblett ratio105,108 is defined such that H2/(2CO + 3CO2) is approximately equal to 1, and is highly useful because the effluent composition of unreacted syngas from an FT unit will maintain roughly the same value as the inlet. Therefore, the internal or external gas loop designs for FT synthesis can be theoretically designed such that very high conversion rates of CO and CO2 are achieved in the BTL refinery.
To examine the effects of the H2/(CO + CO2) ratio, the synthesis gas entering the iron-based FT units will handled in one of two ways. One low-temperature (240 °C) and one high-temperature (320 °C) unit will require an inlet Ribblett ratio that is equal to 1, and should help to facilitate the reverse water–gas-shift reaction as the CO2 inlet concentration increases. The other two units will operate at 267 °C and have an effluent composition that is based two previous DOE reports.30,106,107 These two units will have an H2/CO inlet ratio between 0.5 and 0.7 and will ensure that the H2/CO ratio in the effluent is equal to 1.7 from forward water–gas-shift conversion. Hydrogen may be recycled to any of the FT units to either shift the H2/CO ratio or the H2/CO2 ratio to the appropriate level. Steam may alternatively be used as a feed for the two iron-based fWGS FT units to shift the H2/CO ratio in situ. The two streams exiting the cobalt or iron FT units will be a waxy liquid phase and a vapor phase containing a range of hydrocarbons. The wax will be directed to a hydrocracker (WHC) while the vapor phase is split (SPFTH) for further processing.
The FT hydrocarbons may also be passed over a ZSM-5 catalytic reactor operating at 408 °C and 16 bar106 to be converted into mostly gasoline range hydrocarbons and some distillate.106,107 The ZSM-5 unit will be able to convert the oxygenates to additional hydrocarbons, so no separate processing of the oxygenates will be required for the aqueous effluent. The raw product from FT-ZSM5 is fractionated to separate the water and distillate from the gasoline product. The water is mixed with other wastewater knockout and the distillate is hydrotreated to form a diesel product. The raw ZSM-5 HC product is sent to the LPG–gasoline separation section for further processing (ESI, Fig. S7†).
The water lean FT hydrocarbons are sent to a hydrocarbon recovery column, as shown in ESI, Fig. S5.† The hydrocarbons are split into C3–C5 gases, naphtha, kerosene, distillate, wax, offgas, and wastewater.12,109 The upgrading of each stream will follow a detailed Bechtel design109,110 which includes a wax hydrocracker, a distillate hydrotreater, a kerosene hydrotreater, a naphtha hydrotreater, a naphtha reformer, a C4 isomerizer, a C5/C6 isomerizer, a C3/C4/C5 alkylation unit, and a saturated gas plant.
| CO + 2H2 ↔ CH3OH | (1) |
| CO2 + H2 ↔ CO + H2O | (2) |
The concentration of CO2 input to the methanol synthesis reactor will have significant ramifications on the downstream processing in the BTL refinery. If the inlet CO2 composition is low due to the use of pre-combustion capture, then the extent of the water–gas-shift reaction will be negligible and the concentration of both CO2 and H2O in the effluent stream will be minimal. This ultimately allows for a less energy intensive methanol purification if it is required for downstream conversion. Additionally, the conversion of CO to methanol can approach 45–50% depending on the concentration of inert species that are present in the synthesis reactor. As the inlet concentration of CO2 increases, the reverse water–gas-shift reaction will begin to occur with an increased production of H2O and a decreased per-pass conversion of (CO + CO2). The presence of H2O along with methanol will increase the cost of methanol purification, but if the methanol conversion units are tolerant to high water concentrations, then the purification step will not be needed. In fact, operation of the methanol synthesis reactor with a Ribblett ratio of 1 allows for a very high overall conversion of (CO + CO2) using subsequent recycle of the unreacted syngas. Unlike FT synthesis, no light hydrocarbon gases will be formed during methanol synthesis, so an internal recycle can be employed after appropriate re-compression to the feed inlet pressure. For this study, it is assumed that the methanol conversion units (ZSM-5 based reactors) can tolerate crude methanol containing up to 50 wt% water. Therefore, no additional methanol purification step is required between methanol synthesis and methanol conversion. Note that high levels of water in the crude methanol stream are not anticipated to be a concern because the downstream methanol processing units will yield 50 wt% water from the hydrocarbon synthesis.111
The effluent from the methanol synthesis reactor is cooled to 35 °C and a crude methanol stream is separated using vapor–liquid equilibrium at 48 bar. The amount of methanol that is entrained in the vapor phase is dependent on the input concentration of syngas to the flash unit, but a majority (over 95%) of the methanol can be recovered by enforcing a stoichiometric amount of H2 in the input to the synthesis reactor (i.e., Ribblett ratio = 1). The vapor stream from the flash unit is split so that 5% may be purged to remove inert species and the remaining 95% is compressed to 51 bar and then recycled to the methanol synthesis reactor. The purge stream is recycled back to the process and used as fuel gas. The crude methanol product from the flash unit is heated to 200 °C, expanded to 5 bar to recover electricity, and then cooled to 60 °C prior to entering a degasser distillation column. The degasser will remove all of the entrained gases from the liquid methanol–water while recovering 99.9% of the methanol. The entrained gases are recycled back to the process for use as fuel gas. The bottoms from the degasser will contain methanol and water, with a methanol composition dependent on the level of CO2 input to the synthesis unit.
Any methanol entering the MTO process unit is heated to 400 °C at 1.2 bar. The MTO fluidized bed reactor operates at a temperature of 482 °C and a pressure of 1 bar. The exothermic heat of reaction within the MTO unit is controlled through generation of low-pressure steam. 100% of the input methanol is converted into olefin effluent containing 1.4 wt% CH4, 6.5 wt% C2–C4 paraffins, 56.4 wt% C2–C4 olefins, and 35.7 wt% C5–C11 gasoline.111 The MTO unit is modeled mathematically using an atom balance and a typical composition seen in the literature.111 The MTO product is fractionated (MTO-F) to separate the light gases, olefins, and gasoline fractions. The MTO-F unit is assumed to operate as a separator unit where 100% of the C1–C3 paraffins are recycled back to the refinery, 100% of the C4 paraffins and 100% of the olefins are directed to the MOGD unit, 100% of the gasoline is combined with the remainder of the gasoline generated in the process, and 100% of the water generated in the MTO unit is sent for wastewater treatment.
The separated olefins are sent to the MOGD unit where a fixed bed reactor is used to convert the olefins to gasoline and distillate over a ZSM-5 catalyst. The gasoline/distillate product ratios can range from 0.12 to >100, and the ratio chosen in this study was 0.12 to maximize the production of diesel. The MOGD unit operates at 400 °C and 1 bar and will utilize steam generation to remove the exothermic heat of reaction within the unit. The MOGD unit is modeled with an atom balance and will produce 82% distillate, 15% gasoline, and 3% light gases.111 The product will be fractionated (MTODF) to remove diesel and kerosene cuts from the gasoline and light gases. The MTODF unit will be modeled as a separator unit where 100% of the C11–C13 species are directed to the kerosene cut and 100% of the C14+ species are directed to the diesel cut.
In addition to the distillation columns within this section, there is also an alkylation unit that is used to convert iso-butane and butene to an alkylate blending stock for the gasoline pool. The alkylate was modeled as iso-butane102 and the alkylation unit was modeled using a species balance where the key species, butene, was completely converted to iso-butane. Butene is used as the limiting species in this reaction because it is generally present in a far smaller concentration than iso-butane.
2.4 Light gas handling
Light gases within the refinery consist primarily of C1–C2 hydrocarbons, unreacted syngas (i.e. CO, CO2, and H2), and inert species (e.g., N2, Ar). These gases are generated from (i) unreacted syngas from FT synthesis, (ii) unreacted syngas from methanol synthesis, and (iii) the fuel gases leaving the saturated gas plant. The light gases from FT synthesis and methanol synthesis may contain significant fractions of H2 and CO that could be directly recycled back to the synthesis units to increase the overall conversion to liquid fuels. This configuration is defined as an internal gas loop and is highly beneficial because the yield of liquid product may be improved with minimal additional operational cost to re-compress the gas to the synthesis operating pressure. The key drawback for the internal gas loop is the build-up of inert species in the recycle stream. A fraction of the unreacted syngas must be purged from the internal gas loop to alleviate this problem. This purged syngas is directed to a conversion and/or separation unit before potentially being recycled back to the synthesis units (i.e., an external gas loop design).The unreacted syngas that is purged from the internal gas loop may be directed to one of three processing options (Fig. 3). The auto-thermal reactor will reform the C1–C2 hydrocarbons into a hydrogen-rich syngas that can be recycled to the synthesis units. Alternatively, the syngas may be directed to a fuel combustor to provide high-temperature heat for the process units or to a gas turbine to provide process electricity. The effluent from the latter two units is cooled to 35 °C, passed through a water knock-out unit, and may be directed to a low-pressure amine absorption CO2 recovery unit113 or simply vented to the atmosphere. The light gases exiting from the saturated gas plant can also be directed to one of the three major processing units.
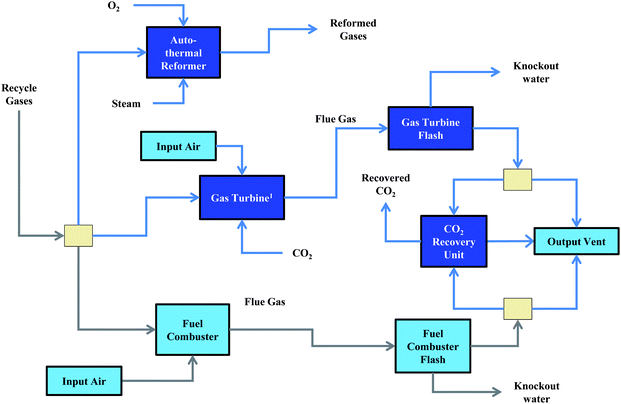 | ||
| Fig. 3 Light gas handling flowsheet. Light gases are generated from (i) external recycle of Fischer–Tropsch synthesis, (ii) external recycle of methanol synthesis, and (iii) upgrading unit offgas. The gases may be split to an auto-thermal reformer to generate additional syngas, a fuel combustor to provide process heating, or a gas turbine to provide process electricity. The flue gas from the gas turbine and the fuel combustor may be directed to a post-combustion capture unit to recover the CO2. | ||
2.5 Hydrogen/oxygen production
Hydrogen is produced via pressure-swing absorption or an electrolyzer unit while oxygen can be provided by the electrolyzer or a separate air separation unit (ESI, Fig. S9†).2.6 Wastewater treatment
A complete wastewater treatment network (ESI, Fig. S10 and S11†) is incorporated that will treat and recycle wastewater from various process units, blowdown from the cooling tower, blowdown from the boilers, and input freshwater.15 Process wastewater is treated using a sour stripper or a biological digestor to remove the sulfur species (e.g., H2S), nitrogen species (e.g., NH3), or hydrocarbon species (e.g., oxygenates) that are expected to be in the wastewater streams. Clean output of the network includes (i) process water to the electrolyzers, (ii) steam to the gasifier, auto-thermal reformer, steam reformer, and water–gas-shift reactor, and (iii) discharged wastewater to the environment.2.7 Unit costs
The total direct costs, TDC, for the BTL refinery hydrocarbon production and upgrading units are calculated using estimates from several literature sources102,104,106,107,112 using the cost parameters in Table 2 and eqn (3)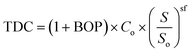 | (3) |
| Description | Co (MM$) | So | Smax | Units | Scale basis | sf | Ref. |
|---|---|---|---|---|---|---|---|
| a Kreutz et al., 2008.b Larson et al., 2009.c Bechtel Corporation, 1998.d National Energy Technology Laboratory, 2010.e National Renewable Energy Laboratory, 2012. | |||||||
| Biomass handling (forest) | 4.65 | 17.9 | 30.6 | kg s−1 | As received biomass | 0.77 | a |
| Biomass handling (non-forest) | 14.42 | 17.9 | 30.6 | kg s−1 | As received biomass | 0.77 | a |
| Biomass gasification, tar cracking, and gas cooling | 55.22 | 17.9 | 33.3 | kg s−1 | Dry biomass | 0.67e | b |
| Auto-thermal reformer | 10.26 | 12.2 | 35.0 | kg s−1 | Natural gas feed | 0.67e | c |
| Water gas shift | 3.75 | 107.9 | 150.0e | kg s−1 | Feed gas | 0.67e | d |
| Dual-capture methanol absorption | 58.3 | 54.9 | 192.0 | kg s−1 | Feed gas | 0.63 | a |
| Claus plant | 27.6 | 1.59 | 10.0 | kg s−1 | Recovered sulfur | 0.67e | d |
| LO-CAT system | 4.2 | 52.2 | 68.0 | kg h−1 | Recovered sulfur | 0.65 | e |
The total plant cost, TPC, for each unit is calculated as the sum of the total direct capital, TDC, plus the indirect costs, IC. The IC include engineering, startup, spares, royalties, and contingencies and is estimated to 32% of the TDC. The TPC for each unit must be converted to a levelized cost to compare with the variable feedstock and operational costs for the process. Using the methodology of Kreutz et al.,10 the capital charges (CC) for the refinery are calculated by multiplying the levelized capital charge rate (LCCR) and the interest during construction factor (IDCF) by the total overnight capital (eqn (4)).
| CC = LCCR × IDCF × TPC | (4) |
Kreutz et al.10 calculates an LCCR value of 14.38% per year and IDCF of 7.6%. Thus, a multiplier of 15.41% per year is used to convert the TPC into a capital charge rate. Assuming an operating capacity (CAP) of 330 days per year and operation/maintenance (OM) costs equal to 5% of the TPC, the total levelized cost (CostU) associated with a unit is given by eqn (5).
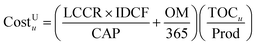 | (5) |
2.8 Objective function
The objective function for the model is given by eqn (6). The summation represents the total cost of liquid fuels production and includes contributions from the feedstocks cost (CostF), the electricity cost (CostEl), the CO2 sequestration cost (CostSeq), and the levelized unit investment cost (CostU). Each of the terms in eqn (6) is normalized to the total volume of products produced (Prod). Note that other normalization factors (e.g., total volume of gasoline equivalent, total energy of products) and other objective functions (e.g., maximizing the net present value) can be easily incorporated into the model framework.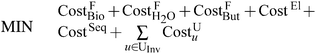 | (6) |
The process synthesis model with simultaneous heat, power, and water integration represents a large-scale non-convex mixed-integer non-linear optimization (MINLP) model that was solved to global optimality using a branch-and-bound global optimization framework.29 At each node in the branch-and-bound tree, a mixed-integer linear relaxation of the mathematical model is solved using CPLEX115 and then the node is branched to create two children nodes. The solution pool feature of CPLEX is utilized during the solution of the relaxed model to generate a set of distinct points (150 for the root node and 10 for all other nodes), each of which is used as a candidate starting point to solve the original model. For each starting point, the current binary variable values are fixed and the resulting NLP is minimized using CONOPT.116 If the solution to the NLP is less than the current upper bound, then the upper bound is replaced with the NLP solution value. At each step, all nodes that have a lower bound that is within an ε tolerance of the current upper bound (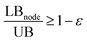 ) are eliminated from the tree. For a more complete coverage of branch-and-bound algorithms, the reader is directed to the textbooks of Floudas117,118 and reviews of global optimization methods.119–121
) are eliminated from the tree. For a more complete coverage of branch-and-bound algorithms, the reader is directed to the textbooks of Floudas117,118 and reviews of global optimization methods.119–121
3 Computational studies
Twenty-four case studies were performed to demonstrate the capability of the process synthesis model using an average representation of either agricultural residues, forest residues, or perennial crops (Table 1). The global optimization framework was terminated if all nodes in the branch-and-bound tree were processed or if 100 CPU hours had passed.29 The effect of scale on the BTL refinery was examined through four representative capacities of 1 thousand barrels per day (kBD), 5 kBD, 10 kBD, and 50 kBD. The gasoline, diesel, and kerosene compositions output from the refinery were selected to either (a) represent the 2010 United States demand (i.e., 67 vol% gasoline, 22 vol% diesel, 11 vol% kerosene)8 or (b) freely output any unrestricted composition of the products. The case studies are labeled as N − C, where N represents the type of product composition (i.e., R: 2010 U.S. ratios, U: unrestricted composition) and C represents the capacity in kBD. For example, the U-1 label represents the 1 kBD capacity refinery with an unrestricted product composition. The life-cycle GHG emissions from the refinery will be measured for each case study and then compared to the emissions of current fossil-fuel based processes. The GHG emissions avoided from petroleum-based liquid fuels (91.6 kg CO2eq. per GJLHV) and natural gas combined cycle plant electricity (101.3 kg CO2eq. per GJ) will also be determined. If electricity is input to the BTL refinery, then the associated GHG emissions with electricity production is added to the lifecycle GHG emissions for the refinery. If electricity is output from the BTL refinery, the avoided GHG emissions is subtracted from the total lifecycle GHG emissions. The results of twenty-four additional case studies designed to target a maximum diesel or maximum kerosene output are included for reference as ESI.†The cost parameters used for the BTL refinery are listed in Table 3. The costs for feedstocks (i.e., biomass, freshwater, and butanes) include all costs associated with delivery to the plant gate. The products (i.e., electricity and propane) are assumed to be sold from the plant gate, and do not include the costs expected for transport to the end consumer. The cost of CO2 capture and compression will be included in the investment cost of the BTL refinery while the cost for transportation, storage, and monitoring of the CO2 is shown in Table 3.
Once the global optimization algorithm has completed, the resulting process topology provides (i) the operating conditions and working fluid flow rates of the heat engines, (ii) the amount of electricity produced by the engines, (iii) the amount of cooling water needed for the engines, and (v) the location of the pinch points denoting the distinct subnetworks. Given this information, the minimum number of heat exchanger matches necessary to meet specifications (i), (ii), (iii), and (iv) are calculated as previously described.14,15,117,122 Upon solution of the minimum matches model, the heat exchanger topology with the minimum annualized cost can be found using the superstructure methodology.13,117,122 The investment cost of the heat exchangers is added to the investment cost calculated within the process synthesis model to obtain the final investment cost for the superstructure.
3.1 Optimal process topologies
The major topological selections within the BTL refinery for all case studies are shown in Table 4. For all case studies, a raw synthesis gas will be produced via gasification of the biomass feedstock. The gasifier may input either only biomass or a combination of biomass and recycle gases. The recycle gases may contain CO2 which can be consumed via the reverse water–gas-shift reaction at the high operating temperatures within the gasifier. Each of the twenty-four case studies contain a gasifier that only inputs the solid feedstock. The H2/CO ratio exiting a biomass gasifier is generally less than the ratio required for Fischer–Tropsch or methanol synthesis, so a forward water–gas-shift reaction will need to occur downstream of the gasifier. The generation of additional CO2 from this reaction cannot be recycled back to the gasifier without subsequently decreasing the H2/CO ratio in the effluent. Thus, a process design that recycles CO2 to the gasifier is not practical. If a non-carbon based source of H2 was available to the refinery (e.g., electrolysis of water), then the recycle of CO2 to the gasifier may be economically feasible since the reduced H2/CO ratio can be balanced by the additional supply of H2. However, current capital and operating costs for non-carbon based H2 are prohibitively high and significantly reduce the economic viability of a BTL plant.| Topological design | U-1 | U-5 | U-10 | U-50 | R-1 | R-5 | R-10 | R-50 |
|---|---|---|---|---|---|---|---|---|
| Case study – forest residues | ||||||||
| Biomass conv. | S | S | S | S | S | S | S | S |
| Biomass temp. | 1100 | 1100 | 1000 | 1000 | 1100 | 1100 | 1000 | 1000 |
| WGS/RGS temp. | — | — | — | — | — | — | — | — |
| Min wax FT | — | — | — | — | — | — | — | — |
| Nom. wax FT | — | — | — | — | — | Co-LTFT | Co-LTFT | Co-LTFT |
| FT upgrading | — | — | — | — | — | ZSM-5 | ZSM-5 | ZSM-5 |
| MTG usage | Y | Y | Y | Y | Y | Y | Y | Y |
| MTOD usage | — | — | — | — | Y | — | — | — |
| CO2SEQ usage | — | — | — | — | — | — | — | — |
| GT usage | — | — | — | — | — | — | — | — |
| Case study – perennial crops | ||||||||
| Biomass conv. | S | S | S | S | S | S | S | S |
| Biomass temp. | 900 | 900 | 900 | 900 | 900 | 900 | 900 | 900 |
| WGS/RGS temp. | — | — | — | — | — | — | — | — |
| Min wax FT | — | — | — | — | — | — | — | — |
| Nom. wax FT | — | — | — | — | — | Co-LTFT | Co-LTFT | Co-LTFT |
| FT upgrading | — | — | — | — | — | ZSM-5 | ZSM-5 | ZSM-5 |
| MTG usage | Y | Y | Y | Y | Y | Y | Y | Y |
| MTOD usage | — | — | — | — | Y | — | — | — |
| CO2SEQ usage | — | — | — | — | — | — | — | — |
| GT usage | — | — | — | — | — | — | — | — |
| Case study – agricultural residues | ||||||||
| Biomass conv. | S | S | S | S | S | S | S | S |
| Biomass temp. | 1100 | 1100 | 1000 | 1000 | 1100 | 1100 | 1000 | 1000 |
| WGS/RGS temp. | — | — | — | — | — | — | — | — |
| Min wax FT | — | — | — | — | — | — | — | — |
| Nom. wax FT | — | — | — | — | — | Co-LTFT | Co-LTFT | Co-LTFT |
| FT upgrading | — | — | — | — | — | ZSM-5 | ZSM-5 | ZSM-5 |
| MTG usage | Y | Y | Y | Y | Y | Y | Y | Y |
| MTOD usage | — | — | — | — | Y | — | — | — |
| CO2SEQ usage | — | — | — | — | — | — | — | — |
| GT usage | — | — | — | — | — | — | — | — |
Three possible temperature options were considered for the gasifier (900 °C, 1000 °C, or 1100 °C). The gasifier temperature was selected to operate at the high end of the range (1100 °C) for low capacities (1 kBD and 5 kBD) using perennial crops and agricultural residues. For these feedstocks, the 1000 °C gasifier was selected to operate at the higher capacities of 10 kBD and 50 kBD. A temperature of 900 °C was used in the gasifier for hardwood residues at all capacity levels. An important trade-off in utility cost and capital cost for the biomass gasifier and other process units is illustrated from the case studies. The high moisture content of the hardwood residues requires additional energy from the refinery to dry the biomass, so the availability of waste heat for utility generation is smaller than the other two feedstocks. The use of a lower operating temperature within the gasifier will reduce the oxygen requirement which will therefore reduce the steam and electricity requirement from the utility system.
To increase the H2/CO ratio for syngas conversion, a dedicated water–gas-shift unit operating at 300 °C is utilized by all case studies. A fraction of the synthesis gas will be directed to this unit such that the H2/CO ratio of the effluent and the bypass syngas will be equal to the desired value for syngas conversion. The bypass syngas will be combined with the effluent of the water–gas-shift unit prior to further processing (i.e., wet scrubbing and acid gas removal). The water–gas-shift unit will operate isothermally and all excess heat is utilized to generate low-pressure steam for use throughout the BTL refinery.
The type of fuels desired from the BTL refinery has a strong impact on the selection of the hydrocarbon conversion and upgrading units. Methanol synthesis and methanol-to-gasoline (MTG) were consistently utilized when an unrestricted fuel composition was allowed. The reduction in capital costs associated with methanol synthesis, the MTG reactor, and the subsequent gasoline upgrading versus the alternative processes is clearly reflected in these twelve case studies. These case studies produce 100% gasoline out of the C5+ hydrocarbon products. Gasoline could also be produced using FT synthesis and ZSM-5 catalytic conversion of the FT hydrocarbons, but this process requires a higher capital investment and a higher overall fuels cost. It is important to note that both the MTG and the FT/ZSM-5 processes produce a significant amount of byproduct liquefied petroleum gas (LPG), which accounts for 9 vol% of the total liquid product. The twelve case studies that enforced a product composition equal to that of the United States demand show an interesting topological trade-off at the various capacity levels. At the 1 kBD capacity, no FT units were utilized and all synthesis gas was directed to methanol generation. A combination of the MTG process and the methanol-to-olefins (MTO) process was used to convert the methanol to hydrocarbon species. The Mobil olefins-to-gasoline/distillate (MOGD) process oligomerized the olefin species to mostly distillate range hydrocarbons and some gasoline. At higher BTL capacity levels, a low-temperature cobalt-based FT process was utilized to produce a sufficient quantity of wax/distillate that are upgraded to diesel and kerosene. Some gasoline is generated from reforming and hydrotreating of naphtha, but most of the gasoline requirement is satisfied by methanol synthesis and MTG.
The methane and ethane-rich gases generated from the process upgrading units and the FT synthesis effluent are partially recycled to an auto-thermal reformer (ATR) to produce additional CO and H2. Three possible temperature options were considered for the ATR (800 °C, 900 °C, 1000 °C), though the optimal topology for all twenty-four case studies incorporated a 1000 °C unit. This high temperature helps to increase both (i) methane conversion in the unit via steam reforming and (ii) CO2 conversion via the reverse water–gas-shift reaction. Selection of the high-temperature units also suggests that the higher species conversions within the reformer due to higher temperature outweights the increased operating costs with a higher temperature. The balance of the light gases that are not directed to the ATR are split to the fuel combustor for process heating. No gas turbine was used in any of the twenty-four case studies. CO2 sequestration was also not utilized in any of the twenty-four case studies. The lifecycle greenhouse gas emissions from the BTL refinery will consistently be less than that of fossil-fueled processes (see Table 10), so the environmental benefit of sequestration is not typically required.
As an illustrative example, the process flow diagram for the U-1 case study using agricultural residues is shown in Fig. 4. This diagram shows the key points for biomass conversion, syngas handling, syngas conversion, liquid fuel production, and light gas handling. Several process units including compressors, turbines, heat exchangers, and separation units are not shown in the figure though they are included in the BTL refinery. Biomass is input to the gasifier with oxygen and steam to generate a raw syngas which is partially passed over a water–gas-shift unit to produce a H2/CO ratio of 2/1. The syngas is then passed over a dual-capture physical absorption acid gas unit to remove both the CO2 and H2S from the gas. The CO2 is mostly vented from the refinery while the sulfur is recovered using a Claus plant. The clean syngas is sent to a methanol synthesis unit where a high level of internal recycle is utilized to increase the overall conversion of CO. The crude methanol exiting the synthesis unit is directed to a methanol-to-gasoline (MTG) unit to produce gasoline and LPG. Light gases from the MTG unit are partially split to an auto-thermal reactor to recover additional syngas. The balance of the light gases from the MTG unit and the purge gas from the methanol synthesis unit are sent to the fuel combustor to provide high-temperature heating for the BTL refinery.
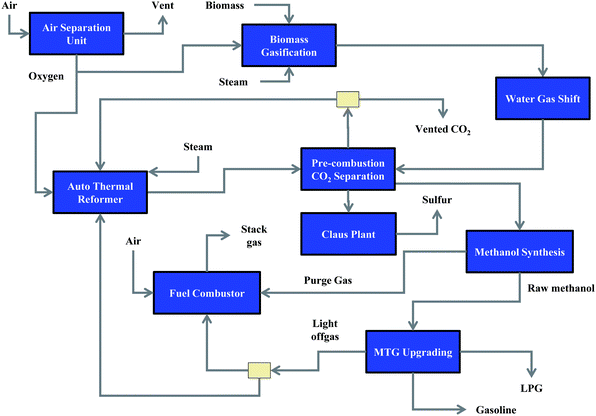 | ||
| Fig. 4 Process flow diagram for case study U-1 using agricultural residues. | ||
3.2 Overall costs of liquid fuels
Table 5 shows the overall cost of liquid fuel production for all twenty-four case studies. The contribution of feedstock cost, capital investment, operation and maintenance, and CO2 transportation/storage/monitoring (TS&M) are shown in $ per GJ of liquid products (lower-heating value). Feedstock costs for biomass, butanes, or freshwater are based on the as-delivered price for each of the raw materials. Butanes may be included for C4 isomerization to increase the isobutane/isobutene ratio necessary for alkylation12,14,105,110 and freshwater is needed to make-up for process losses.15 The sum of each individual contribution provides the overall cost, which is then converted to a break-even oil price (BEOP) in $ per barrel (bbl). The BEOP represents the price of crude oil at which the BTL process becomes economically competitive with petroleum-based processes and is calculated using the refiner's margin for gasoline, diesel, and kerosene.10,14Table 5 also includes the lower bound on the objective value (in $ per GJ) for each cast study along with the corresponding optimality gap. The global optimization framework29 was consistently able to identify high-quality lower and upper bounds, and was able to reduce the optimality gap below 7% for all studies.| Contribution to cost($ per GJ of products) | U-1 | U-5 | U-10 | U-50 | R-1 | R-5 | R-10 | R-50 |
|---|---|---|---|---|---|---|---|---|
| Case study – forest residues | ||||||||
| Biomass | 8.32 | 8.43 | 8.44 | 8.19 | 8.09 | 8.12 | 8.23 | 8.18 |
| Butane | 0.61 | 0.61 | 0.61 | 0.61 | 0.41 | 0.41 | 0.41 | 0.41 |
| Water | 0.02 | 0.02 | 0.03 | 0.02 | 0.02 | 0.03 | 0.03 | 0.03 |
| CO2 TS&M | — | — | — | — | — | — | — | — |
| Investment | 12.00 | 7.54 | 6.99 | 5.90 | 13.08 | 8.32 | 6.66 | 5.74 |
| O&M | 3.17 | 1.99 | 1.85 | 1.56 | 3.45 | 2.20 | 1.76 | 1.52 |
| Electricity | −0.61 | −0.74 | −0.57 | −0.57 | −0.91 | −0.60 | −0.71 | −0.83 |
| LPG | −2.25 | −1.57 | −1.57 | −1.12 | −0.34 | −0.24 | −0.24 | −0.17 |
| Total ($ per GJ) | 21.27 | 16.28 | 15.77 | 14.58 | 23.81 | 18.24 | 16.15 | 14.87 |
| BEOP ($ per bbl) | 108.43 | 79.98 | 77.11 | 70.30 | 122.89 | 91.15 | 79.25 | 71.96 |
| Lower bound ($ per GJ) | 19.92 | 15.46 | 14.74 | 13.86 | 22.50 | 17.25 | 15.33 | 14.12 |
| Gap | 6.35% | 4.99% | 6.57% | 4.95% | 5.48% | 5.44% | 5.07% | 5.08% |
| Case study – perennial crops | ||||||||
| Biomass | 5.81 | 5.71 | 5.76 | 5.78 | 5.80 | 5.59 | 5.56 | 5.75 |
| Butane | 0.61 | 0.61 | 0.61 | 0.61 | 0.41 | 0.41 | 0.41 | 0.41 |
| Water | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.03 | 0.02 | 0.02 |
| CO2 TS&M | — | — | — | — | — | — | — | — |
| Investment | 12.01 | 7.50 | 6.91 | 5.95 | 13.29 | 8.20 | 6.68 | 5.69 |
| O&M | 3.17 | 1.98 | 1.83 | 1.57 | 3.51 | 2.17 | 1.77 | 1.50 |
| Electricity | 0.28 | 0.39 | 0.29 | 0.40 | 0.35 | 0.28 | 0.28 | 0.41 |
| LPG | −2.25 | −1.57 | −1.57 | −1.12 | −0.34 | −0.24 | −0.24 | −0.17 |
| Total ($ per GJ) | 19.66 | 14.64 | 13.85 | 13.20 | 23.04 | 16.44 | 14.48 | 13.61 |
| Total ($ per bbl) | 99.26 | 70.66 | 66.15 | 62.44 | 118.51 | 80.90 | 69.73 | 64.78 |
| Lower bound ($ per GJ) | 18.56 | 13.82 | 12.89 | 12.59 | 21.85 | 15.39 | 13.73 | 12.84 |
| Gap | 5.60% | 5.58% | 6.93% | 4.63% | 5.14% | 6.39% | 5.18% | 5.63% |
| Case study – agricultural residues | ||||||||
| Biomass | 10.06 | 10.13 | 9.98 | 10.05 | 9.66 | 9.97 | 9.74 | 9.70 |
| Butane | 0.61 | 0.61 | 0.61 | 0.61 | 0.41 | 0.41 | 0.41 | 0.41 |
| Water | 0.02 | 0.02 | 0.02 | 0.02 | 0.03 | 0.02 | 0.02 | 0.02 |
| CO2 TS&M | — | — | — | — | — | — | — | — |
| Investment | 11.87 | 7.52 | 6.91 | 5.89 | 13.30 | 8.18 | 6.76 | 5.75 |
| O&M | 3.14 | 1.99 | 1.83 | 1.56 | 3.51 | 2.16 | 1.79 | 1.52 |
| Electricity | −0.91 | −0.88 | −0.79 | −0.71 | −0.75 | −0.59 | −0.77 | −0.69 |
| LPG | −2.25 | −1.57 | −1.57 | −1.12 | −0.34 | −0.24 | −0.24 | −0.17 |
| Total ($ per GJ) | 22.54 | 17.81 | 17.00 | 16.29 | 25.82 | 19.92 | 17.72 | 16.54 |
| Total ($ per bbl) | 115.68 | 88.72 | 84.08 | 80.05 | 134.39 | 100.75 | 88.22 | 81.46 |
| Lower bound ($ per GJ) | 21.39 | 16.73 | 16.05 | 15.51 | 24.52 | 18.76 | 16.77 | 15.71 |
| Gap | 5.08% | 6.09% | 5.58% | 4.79% | 5.03% | 5.83% | 5.36% | 4.99% |
The BEOP ranges from $99–138 per bbl for a 1 kBD plant, $71–101 per bbl for a 5 kBD plant, $66 per bbl to $91 per GJ per bbl for a 10 kBD plant, and $62–84 per bbl for a 50 kBD plant. The two major components that contribute to the overall cost are the biomass feedstock and the costs related to the capital investment (i.e., capital charges, operation, and maintenance). The relative contribution of these two components will change with increasing capacity due to the economy of scale that is anticipated within the BTL refinery. For the 1 kBD capacity, the capital charges represent approximately 60% of the overall cost. As the capacity increases to 50 kBD, the capital charges reduce to about 40% of the cost while the biomass purchase price rises to about 55% of the overall cost. Note that for any given feedstock and product combination, the absolute contribution of the biomass purchase price remains relatively constant while the absolute contribution of the capital/O&M charges becomes smaller. The decrease in the investment/O&M charges is very pronounced at the lower capacities due to the high economy of scale benefit that is realized that these low capacity levels. All of the major sections within the BTL refinery will require only one unit to operate, so there is a large benefit to increase the capacity of these units (and therefore the refinery) to the largest possible operating capacity. However, as the refinery capacity grows beyond 5–10 kBD, several sections of the BTL refinery will require multiple units (i.e., two or more biomass gasifiers) to handle the additional throughput. Multiple units within the same train can share auxiliary equipment and the operation/maintenance requirement generally has less than a linear increase, so some capital cost savings may be expected with higher refinery capacity.10 Thus, the scaling factor for BTL refineries will begin to approach 0.9 as the capacity increases above 10 kBD.
Table 5 shows that the overall fuels cost is highly dependent on the biomass feedstock and the type/composition of liquid fuels that are produced. The case studies placing no restriction on the liquid products generally provide the lowest overall cost, which is aided by the sale of byproduct LPG. The LPG could alternatively be converted to aromatic naphtha via the Cyclar process123 if no market for the LPG could be found. The hardwood feedstock typically provides the lowest overall cost of the three biomass feeds. This is partially due to the lower purchase price of the hardwood residues with respect to the other biomass feedstocks. For instance, the U-5 case studies show a $71 per bbl BEOP for hardwood, $80 per bbl for perennial crops, and $89 per bbl for agricultural residues. The nominal purchase prices for these feedstocks was assumed to be $70 per dry ton, $100 per dry ton, and $120 per dry ton respectively (Table 3). Parametric analysis on the biomass purchase price from $80–120 per dry ton for each of the three feedstocks reveals an important trend between the three feedstocks, and is shown in Fig. 5. Note that at $80 per dry ton, the BEOP of the perennial crops and the agricultural residues is $70 per bbl and $69 per bbl respectively while the BEOP of the hardwood is $75 per bbl. If all biomass feedstocks are valued equally on a bone-dry basis, then the BTL refinery that processes higher moisture feedstocks will have a higher capital and utility costs due to the additional drying of the biomass. This trend is consistently seen at all biomass purchase prices. Note that biomass with a higher moisture content should typically be valued less because the added pre-processing costs associated with drying. The BEOP differences between the perennial crops and the agricultural residues is minimal for all biomass purchase prices since these two feedstocks have very similar elemental compositions and moisture. However, the perennial crops are assumed to be grown on degraded lands and will therefore sequester carbon in the soil during growth. Thus, these crops have a significantly better emissions lifecycle than the agricultural crops. No economic value was imposed (e.g., carbon tax) for the added environmental benefit that perennial crops would have, so these crops will have similar BEOP values to agricultural crops for a given feedstock cost. If such a value was imposed, then the perennial crops would have a lower BEOP than the agricultural residues at a given feedstock cost.
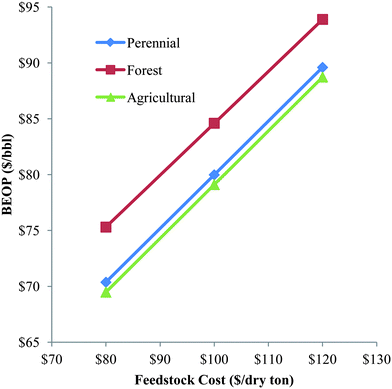 | ||
| Fig. 5 Parametric analysis of biomass purchase price on U-5 case studies. | ||
3.3 Investment costs
The total plant cost (TPC) for all twenty-four case studies is shown in Table 6. The seven major sections of the refinery include syngas generation, syngas cleaning, hydrocarbon production, hydrocarbon upgrading, hydrogen/oxygen production, heat and power integration, and wastewater treatment. The TPC (in 2011 MM $) is shown for each section along with the combined total for the BTL refinery. Syngas generation and syngas cleaning consists of gasifying the biomass and producing a syngas that is ready for conversion (e.g., tar, acid gases, water removed) and represents 55–65% of the overall cost of the plant. The costs for hydrogen/oxygen production, hydrocarbon production, and heat/power generation require relatively equal amounts of capital investment ranging from 10–15% of the overall cost. The cost of hydrocarbon upgrading and wastewater treatment are the smallest two fractions and typically require about 5% of the overall cost. Note that the values in Table 6 may be converted into a “total overnight capital” by adding the inventory capital, financing costs, preproduction costs, and other owner's costs. These combined costs typically range from 10–15% of the TPC.10,104 The “total as-spent capital” figure, which incorporates the interest on the debt/equity during construction, can be calculated using the real discount rate and the construction time. This information has been accounted for when determining the levelized capital charge rate used in the process synthesis model.| Contribution to cost($ MM) | U-1 | U-5 | U-10 | U-50 | R-1 | R-5 | R-10 | R-50 |
|---|---|---|---|---|---|---|---|---|
| Case study – forest residues | ||||||||
| Syngas generation | 41 | 124 | 275 | 1173 | 42 | 138 | 214 | 945 |
| Syngas cleaning | 31 | 99 | 178 | 748 | 31 | 94 | 152 | 644 |
| Hydrocarbon production | 22 | 67 | 143 | 570 | 25 | 79 | 122 | 498 |
| Hydrocarbon upgrading | 6 | 20 | 18 | 79 | 11 | 36 | 64 | 262 |
| Hydrogen/oxygen production | 16 | 56 | 94 | 391 | 19 | 60 | 98 | 446 |
| Heat and power integration | 16 | 51 | 96 | 423 | 17 | 50 | 82 | 358 |
| Wastewater treatment | 7 | 22 | 10 | 45 | 8 | 25 | 43 | 184 |
| Total (MM $) | 140 | 438 | 813 | 3430 | 152 | 484 | 775 | 3336 |
| Total ($ per bpd) | 139![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 562 562 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 659 659 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 333 333 | 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 591 591 | 152![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 060 060 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 761 761 | 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 501 501 | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 717 717 |
| Case study – perennial crops | ||||||||
| Syngas generation | 41 | 127 | 263 | 1153 | 44 | 135 | 216 | 921 |
| Syngas cleaning | 31 | 96 | 181 | 767 | 31 | 96 | 156 | 663 |
| Hydrocarbon production | 21 | 67 | 139 | 593 | 25 | 77 | 119 | 506 |
| Hydrocarbon upgrading | 6 | 20 | 18 | 82 | 11 | 35 | 61 | 260 |
| Hydrogen/oxygen production | 17 | 54 | 95 | 406 | 19 | 58 | 101 | 429 |
| Heat and power integration | 16 | 50 | 98 | 412 | 16 | 51 | 83 | 352 |
| Wastewater treatment | 7 | 21 | 10 | 43 | 8 | 24 | 42 | 177 |
| Total (MM $) | 140 | 436 | 804 | 3457 | 155 | 477 | 777 | 3307 |
| Total ($ per bpd) | 139![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 652 652 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 270 270 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 399 399 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 149 149 | 154![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 558 558 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 367 367 | 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 696 696 | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 146 146 |
| Case study – agricultural residues | ||||||||
| Syngas generation | 41 | 125 | 251 | 1133 | 44 | 129 | 222 | 920 |
| Syngas cleaning | 31 | 100 | 189 | 754 | 31 | 97 | 162 | 659 |
| Hydrocarbon production | 21 | 69 | 142 | 578 | 25 | 80 | 118 | 504 |
| Hydrocarbon upgrading | 6 | 20 | 18 | 79 | 12 | 36 | 58 | 272 |
| Hydrogen/oxygen production | 17 | 52 | 96 | 413 | 19 | 60 | 100 | 448 |
| Heat and power integration | 15 | 50 | 99 | 425 | 16 | 49 | 86 | 360 |
| Wastewater treatment | 7 | 21 | 10 | 45 | 8 | 25 | 40 | 178 |
| Total (MM $) | 138 | 437 | 804 | 3426 | 155 | 476 | 787 | 3342 |
| Total ($ per bpd) | 138![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 048 048 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 465 465 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 410 410 | 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 519 519 | 154![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 706 706 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 178 178 | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 654 654 | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 834 834 |
The value of TPC ranges from $138–157 MM for 1 kBD plants, $425–494 MM for 5 kBD plants, $775–813 MM for 10 kBD plants, and $3307–3468 MM for 50 kBD plants. To illustrate the economy of scale for each capacity level, the TPC is normalized by the total amount of liquid fuels. The normalized cost range from $138 k to $157 k per bpd for 1 kBD plants, $85 k to $99 k per bpd for 5 kBD plants, $78 k to $81 k per bpd for 10 kBD plants, and $66 k to $69 k per bpd for 50 kBD plants. Note that the significant difference in normalized cost between 1 kBD plants and 5 kBD plants is consistent with the results from Table 5. The case studies with the unrestricted topology have lower investment costs than the cases that satisfy the United States demand ratios. The additional refinery complexity that is necessary to produce all three liquid fuels versus producing only gasoline ultimately adds about 10% to the capital cost of the BTL refinery. This increase in capital cost is shared between both the hydrocarbon production and the hydrocarbon upgrading sections. The inner recycle loop for methanol synthesis can be higher than that for Fischer–Tropsch synthesis since the build-up of inert species is less pronounced during methanol synthesis. The production of C1–C2 hydrocarbons during FT synthesis requires a more complex reaction/separation scheme to remove these species from the inner recycle loop.
3.4 Material and energy balances
The material and energy balances for all case studies are displayed in Tables 7 and 8, respectively. In Table 7, biomass is shown in dry tons per hour (dt per h) and liquid feedstocks/products (i.e., butanes, LPG, water, gasoline, diesel, and kerosene) are shown in thousand barrels per day (kBD). For a given refinery capacity, the amount of biomass feedstock needed for the refinery is relatively similar for all three feedstocks types and both product compositions. Each biomass feedstock has a similar carbon content on a dry basis, so this result is indicative of similar carbon conversion efficiencies across refinery types. While the amount of freshwater input to the refineries is similar, the variability between feedstock type and product composition is more substantial than the biomass feed. For example, the agricultural crops require the smallest amount of freshwater (1.50 kBD) for the U-1 case study, but the largest amount (1.82 kBD) for the R-1 case study. Most of the carbon dioxide that is generated in the refinery is vented from the system. A portion is recycled within the refinery to act as a carrier gas for the biomass and as a recycle to the auto-thermal reactor. No CO2 sequestration was utilized in any of the case studies. The liquid fuels produced for each case study is dependent on the product composition requirements for the study. The case studies that enforce the United States demand ratios output products with approximately 67 vol% gasoline, 22 vol% diesel, and 11 vol% kerosene. Byproduct LPG (2 vol%) is also produced during hydrocarbon upgrading. The case studies that place no restriction on the product composition all utilize methanol-to-gasoline technology to produce 100% gasoline from the C5+ products. No diesel or kerosene are produced in these case studies, and the byproduct LPG is approximately 9 vol% of the total gasoline.| Material balances | U-1 | U-5 | U-10 | U-50 | R-1 | R-5 | R-10 | R-50 |
|---|---|---|---|---|---|---|---|---|
| Case study – forest residues | ||||||||
| Biomass (dt per h) | 21.74 | 110.10 | 220.38 | 1069.56 | 21.14 | 106.05 | 214.91 | 1068.16 |
| Butane (kBD) | 0.05 | 0.23 | 0.45 | 2.26 | 0.03 | 0.15 | 0.30 | 1.52 |
| Water (kBD) | 1.63 | 6.34 | 18.18 | 75.05 | 1.43 | 9.42 | 19.51 | 102.57 |
| Gasoline (kBD) | 1.00 | 5.00 | 10.00 | 50.00 | 0.67 | 3.36 | 6.72 | 33.60 |
| Diesel (kBD) | 0.00 | 0.00 | 0.00 | 0.00 | 0.22 | 1.08 | 2.15 | 10.77 |
| Kerosene (kBD) | 0.00 | 0.00 | 0.00 | 0.00 | 0.11 | 0.56 | 1.13 | 5.63 |
| LPG (kBD) | 0.10 | 0.49 | 0.99 | 4.95 | 0.01 | 0.07 | 0.15 | 0.74 |
| Seq. CO2 (tonne per h) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Vented CO2 (tonne per h) | 14.72 | 75.55 | 151.35 | 711.48 | 13.94 | 70.16 | 144.27 | 712.37 |
| Case study – perennial crops | ||||||||
| Biomass (dt per h) | 21.69 | 106.44 | 215.05 | 1077.31 | 21.62 | 104.25 | 207.29 | 1073.12 |
| Butane (kBD) | 0.05 | 0.23 | 0.45 | 2.26 | 0.03 | 0.15 | 0.30 | 1.52 |
| Water (kBD) | 1.70 | 7.84 | 14.51 | 74.21 | 1.30 | 9.67 | 15.18 | 72.55 |
| Gasoline (kBD) | 1.00 | 5.00 | 10.00 | 50.00 | 0.67 | 3.36 | 6.72 | 33.60 |
| Diesel (kBD) | 0.00 | 0.00 | 0.00 | 0.00 | 0.22 | 1.08 | 2.15 | 10.77 |
| Kerosene (kBD) | 0.00 | 0.00 | 0.00 | 0.00 | 0.11 | 0.56 | 1.13 | 5.63 |
| LPG (kBD) | 0.10 | 0.49 | 0.99 | 4.95 | 0.01 | 0.07 | 0.15 | 0.74 |
| Seq. CO2 (tonne per h) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Vented CO2 (tonne per h) | 14.64 | 70.43 | 143.89 | 722.32 | 14.61 | 67.64 | 133.60 | 719.31 |
| Case study – agricultural residues | ||||||||
| Biomass (dt per h) | 21.89 | 110.23 | 217.27 | 1093.34 | 21.02 | 108.44 | 212.06 | 1055.47 |
| Butane (kBD) | 0.05 | 0.23 | 0.45 | 2.26 | 0.03 | 0.15 | 0.30 | 1.52 |
| Water (kBD) | 1.50 | 8.01 | 17.84 | 80.05 | 1.82 | 8.26 | 14.18 | 83.39 |
| Gasoline (kBD) | 1.00 | 5.00 | 10.00 | 50.00 | 0.67 | 3.36 | 6.72 | 33.60 |
| Diesel (kBD) | 0.00 | 0.00 | 0.00 | 0.00 | 0.22 | 1.08 | 2.15 | 10.77 |
| Kerosene (kBD) | 0.00 | 0.00 | 0.00 | 0.00 | 0.11 | 0.56 | 1.13 | 5.63 |
| LPG (kBD) | 0.10 | 0.49 | 0.99 | 4.95 | 0.01 | 0.07 | 0.15 | 0.74 |
| Seq. CO2 (tonne per h) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Vented CO2 (tonne per h) | 14.93 | 75.72 | 147.00 | 744.76 | 13.76 | 73.51 | 140.28 | 694.61 |
| Energy balances (MW) | U-1 | U-5 | U-10 | U-50 | R-1 | R-5 | R-10 | R-50 |
|---|---|---|---|---|---|---|---|---|
| Case study – forest residues | ||||||||
| Biomass | 122 | 620 | 1242 | 6027 | 119 | 598 | 1211 | 6019 |
| Butane | 3 | 14 | 27 | 137 | 2 | 9 | 18 | 92 |
| Gasoline | 64 | 319 | 637 | 3186 | 43 | 214 | 428 | 2141 |
| Diesel | 0 | 0 | 0 | 0 | 15 | 77 | 153 | 766 |
| Kerosene | 0 | 0 | 0 | 0 | 8 | 39 | 78 | 390 |
| LPG | 6 | 30 | 60 | 300 | 1 | 4 | 9 | 45 |
| Electricity | −2 | −13 | −19 | −98 | −3 | −10 | −24 | −141 |
| Efficiency (%) | 57.33% | 56.95% | 56.45% | 58.14% | 57.81% | 56.76% | 56.31% | 56.99% |
| Case study – perennial crops | ||||||||
| Biomass | 127 | 622 | 1256 | 6293 | 126 | 609 | 1211 | 6268 |
| Butane | 3 | 14 | 27 | 137 | 2 | 9 | 18 | 92 |
| Gasoline | 64 | 319 | 637 | 3186 | 43 | 214 | 428 | 2141 |
| Diesel | 0 | 0 | 0 | 0 | 15 | 77 | 153 | 766 |
| Kerosene | 0 | 0 | 0 | 0 | 8 | 39 | 78 | 390 |
| LPG | 6 | 30 | 60 | 300 | 1 | 4 | 9 | 45 |
| Electricity | 1 | 7 | 10 | 68 | 1 | 5 | 10 | 69 |
| Efficiency (%) | 53.14% | 53.82% | 53.55% | 53.16% | 51.23% | 53.29% | 53.59% | 51.45% |
| Case study – agricultural residues | ||||||||
| Biomass | 123 | 621 | 1224 | 6161 | 118 | 611 | 1195 | 5947 |
| Butane | 3 | 14 | 27 | 137 | 2 | 9 | 18 | 92 |
| Gasoline | 64 | 319 | 637 | 3186 | 43 | 214 | 428 | 2141 |
| Diesel | 0 | 0 | 0 | 0 | 15 | 77 | 153 | 766 |
| Kerosene | 0 | 0 | 0 | 0 | 8 | 39 | 78 | 390 |
| LPG | 6 | 30 | 60 | 300 | 1 | 4 | 9 | 45 |
| Electricity | −3 | −15 | −27 | −121 | −3 | −10 | −26 | −118 |
| Efficiency (%) | 57.74% | 57.28% | 57.84% | 57.28% | 57.69% | 55.49% | 57.22% | 57.27% |
The energy balances in Table 8 consistently show that biomass is the major source of input energy to the process. For the eight case studies that use forest residues, electricity is also added as an input to the BTL refinery. In all case studies, a steam turbine was used to generate electricity from the refinery waste heat. The additional energy requirement for biomass drying using forest residues restricted the amount of produced electricity and therefore additional purchased electricity was required to satisfy the balance. In the sixteen case studies using perennial crops or agricultural residues, the excess electricity was sold as a byproduct and is therefore included as output energy. The major source of output energy is the three liquid products gasoline, diesel, and kerosene. LPG consists of about 9% of the total energy for the unrestricted case studies and about 2% for the United States ratios case studies. In Table 8, the energy efficiency values are calculated by dividing the total energy output (i.e., fuel products, propane, or electricity) by the total energy input (i.e., biomass, natural gas, butane, or electricity). Electricity is listed as a negative value if it is output as a byproduct and positive if it is input to the refinery. The overall efficiency ranges from 51–58% for all of the case studies.
3.5 Carbon and greenhouse gas balances
The overall carbon balance (in kg s−1) is shown in Table 9 for the seven major point in the BTL refineries where carbons is input or output from the system. Biomass supplies over 99% of the input carbon, with the balance attributed to butanes input to the C4 isomerization unit. The trends evident from the mass balances in Table 7 are consistent with the associated carbon balances in Table 9. The flow of carbon associated with each product is consistent with the volumetric flow rate of each product since the percentage of carbon in the liquid products is roughly similar. The total amount of carbon output in the LPG, gasoline, diesel, and kerosene products is therefore approximately constant in each case study. The carbon conversion efficiency is around 51–55% for all case studies, which are slightly higher than those reported in previous BTL studies.9 The capture and recycle of some refinery CO2 to the auto-thermal reformer plays a critical role in the increase of the carbon conversion efficiency. This increased efficiency will require a decrease in the net electricity produced (or increase in net consumed) due to the increase compression costs for CO2 recycle. However, the BTL refinery designs that target this higher level of carbon conversion ultimately lead to reduced overall costs for the entire plant.| Carbon balances (kg s−1) | U-1 | U-5 | U-10 | U-50 | R-1 | R-5 | R-10 | R-50 |
|---|---|---|---|---|---|---|---|---|
| Case study – forest residues | ||||||||
| Biomass | 2.31 | 11.68 | 23.37 | 113.44 | 2.24 | 11.25 | 22.79 | 113.29 |
| Butane | 0.04 | 0.21 | 0.41 | 2.07 | 0.03 | 0.14 | 0.28 | 1.39 |
| Gasoline | 1.16 | 5.79 | 11.58 | 57.91 | 0.78 | 3.89 | 7.78 | 38.91 |
| Diesel | 0.00 | 0.00 | 0.00 | 0.00 | 0.28 | 1.42 | 2.85 | 14.24 |
| Kerosene | 0.00 | 0.00 | 0.00 | 0.00 | 0.14 | 0.70 | 1.40 | 6.98 |
| LPG | 0.07 | 0.37 | 0.73 | 3.66 | 0.01 | 0.05 | 0.11 | 0.55 |
| Vented CO2 | 1.12 | 5.73 | 11.47 | 53.93 | 1.06 | 5.32 | 10.94 | 54.00 |
| Seq. CO2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| % Conversion | 52.47% | 51.81% | 51.77% | 53.31% | 53.46% | 53.29% | 52.60% | 52.91% |
| Case study – perennial crops | ||||||||
| Biomass | 2.30 | 11.29 | 22.81 | 114.26 | 2.29 | 11.06 | 21.99 | 113.82 |
| Butane | 0.04 | 0.21 | 0.41 | 2.07 | 0.03 | 0.14 | 0.28 | 1.39 |
| Gasoline | 1.16 | 5.79 | 11.58 | 57.91 | 0.78 | 3.89 | 7.78 | 38.91 |
| Diesel | 0.00 | 0.00 | 0.00 | 0.00 | 0.28 | 1.42 | 2.85 | 14.24 |
| Kerosene | 0.00 | 0.00 | 0.00 | 0.00 | 0.14 | 0.70 | 1.40 | 6.98 |
| LPG | 0.07 | 0.37 | 0.73 | 3.66 | 0.01 | 0.05 | 0.11 | 0.55 |
| Vented CO2 | 1.11 | 5.34 | 10.91 | 54.75 | 1.11 | 5.13 | 10.13 | 54.53 |
| Seq. CO2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| % Conversion | 52.59% | 53.56% | 53.03% | 52.93% | 52.28% | 54.20% | 54.51% | 52.67% |
| Case study – agricultural residues | ||||||||
| Biomass | 2.32 | 11.69 | 23.04 | 115.96 | 2.23 | 11.50 | 22.49 | 111.94 |
| Butane | 0.04 | 0.21 | 0.41 | 2.07 | 0.03 | 0.14 | 0.28 | 1.39 |
| Gasoline | 1.16 | 5.79 | 11.58 | 57.91 | 0.78 | 3.89 | 7.78 | 38.91 |
| Diesel | 0.00 | 0.00 | 0.00 | 0.00 | 0.28 | 1.42 | 2.85 | 14.24 |
| Kerosene | 0.00 | 0.00 | 0.00 | 0.00 | 0.14 | 0.70 | 1.40 | 6.98 |
| LPG | 0.07 | 0.37 | 0.73 | 3.66 | 0.01 | 0.05 | 0.11 | 0.55 |
| Vented CO2 | 1.13 | 5.74 | 11.14 | 56.46 | 1.04 | 5.57 | 10.63 | 52.65 |
| Seq. CO2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| % Conversion | 52.12% | 51.75% | 52.50% | 52.17% | 53.78% | 52.13% | 53.30% | 53.54% |
The lifecycle greenhouse gas (GHG) emissions for the major sources in the BTL refinery are shown in Table 10. No GHG emissions target was enforced for the case studies because a refinery utilizing solely a biomass feedstock will inherently have reduced GHG emissions with respect to fossil-fueled processes. For each study, the total lifecycle GHG emissions (LGHG) is defined as the sum of the total emissions from each stage of the process. The GHG emission rates (in kg CO2 eq. per s) include (a) acquisition and transportation of the biomass and butane feeds, (b) transportation and use of the gasoline, diesel, kerosene, and LPG, (c) transportation and sequestration of any CO2, (d) venting of any process emissions, and (e) atmospheric sequestration of CO2 during growth of biomass that occurs due to photosynthesis or soil-storage. The GREET model for well-to-wheel emissions124 is used to calculate the GHG emissions for feedstock acquisition and transportation in (a), product transportation in (b), and CO2 transportation in (c). Transportation distances of 50 miles are assumed for feedstocks, 100 miles for products, and 50 miles for CO2. The final liquid products are assumed to be completely combusted to generate CO2 that is released into the atmosphere. The amount of atmospheric CO2 that is removed during photosynthesis in part (e) is based on the carbon content of the biomass and the perennial crops are also assumed to store 0.3 g of carbon in degraded lands per g of as-received biomass over the lifetime of the crop.9 The total GHG emissions avoided from liquid fuels (GHGAF) is equivalent to the total energy of fuels produced times a typical petroleum-based emissions level of 91.6 kg CO2eq. per GJLHV. The emissions avoided from electricity (GHGAE) is equivalent to the energy produced by electricity times a typical natural gas-based emissions level of 101.3 kg CO2eq. per GJ.104 Note that GHGAE will be equal to a negative value if electricity is input to the BTL refinery. The GHG emissions index (GHGI) for the refinery represents the division of LGHG by the sum of GHGAF and GHGAE. Values that are less than 1 are representative of processes that have superior lifecycle emissions to fossil-based processes. Values that are less than 0 indicate that the net CO2 emissions is negative and that more CO2 is extracted from the atmosphere than is released by the refinery.
| GHG balances (kg CO2 eq. per s) | U-1 | U-5 | U-10 | U-50 | R-1 | R-5 | R-10 | R-50 |
|---|---|---|---|---|---|---|---|---|
| Case study – forest residues | ||||||||
| Biomass | −11.23 | −56.88 | −113.85 | −552.53 | −10.92 | −54.78 | −111.02 | −551.81 |
| Butane | 0.01 | 0.03 | 0.05 | 0.25 | 0.00 | 0.02 | 0.03 | 0.17 |
| Gasoline | 4.24 | 21.22 | 42.44 | 212.20 | 2.85 | 14.26 | 28.52 | 142.59 |
| Diesel | 0.00 | 0.00 | 0.00 | 0.00 | 1.04 | 5.22 | 10.43 | 52.17 |
| Kerosene | 0.00 | 0.00 | 0.00 | 0.00 | 0.51 | 2.56 | 5.12 | 25.58 |
| LPG | 0.27 | 1.34 | 2.69 | 13.43 | 0.04 | 0.20 | 0.40 | 2.01 |
| Vented CO2 | 4.09 | 20.98 | 42.04 | 197.63 | 3.87 | 19.49 | 40.07 | 197.88 |
| Seq. CO2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| LGHG | −2.62 | −13.30 | −26.63 | −129.02 | −2.60 | −13.04 | −26.45 | −131.41 |
| GHGAF | 6.39 | 31.93 | 63.87 | 319.34 | 6.12 | 30.61 | 61.22 | 306.08 |
| GHGAE | 0.21 | 1.27 | 1.95 | 9.88 | 0.31 | 1.04 | 2.43 | 14.31 |
| GHGI | −0.40 | −0.40 | −0.40 | −0.39 | −0.40 | −0.41 | −0.42 | −0.41 |
| Case study – perennial crops | ||||||||
| Biomass | −7.13 | −35.00 | −70.72 | −354.28 | −7.11 | −34.28 | −68.17 | −352.90 |
| Butane | 0.01 | 0.03 | 0.05 | 0.25 | 0.00 | 0.02 | 0.03 | 0.17 |
| Gasoline | 4.24 | 21.22 | 42.44 | 212.20 | 2.85 | 14.26 | 28.52 | 142.59 |
| Diesel | 0.00 | 0.00 | 0.00 | 0.00 | 1.04 | 5.22 | 10.43 | 52.17 |
| Kerosene | 0.00 | 0.00 | 0.00 | 0.00 | 0.51 | 2.56 | 5.12 | 25.58 |
| LPG | 0.27 | 1.34 | 2.69 | 13.43 | 0.04 | 0.20 | 0.40 | 2.01 |
| Vented CO2 | 4.07 | 19.56 | 39.97 | 200.65 | 4.06 | 18.79 | 37.11 | 199.81 |
| Seq. CO2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| LGHG | 1.45 | 7.15 | 14.42 | 72.24 | 1.40 | 6.76 | 13.45 | 69.42 |
| GHGAF | 6.39 | 31.93 | 63.87 | 319.34 | 6.12 | 30.61 | 61.22 | 306.08 |
| GHGAE | −0.10 | −0.67 | −1.00 | −6.90 | −0.12 | −0.48 | −0.96 | −6.96 |
| GHGI | 0.23 | 0.23 | 0.23 | 0.23 | 0.23 | 0.22 | 0.22 | 0.23 |
| Case study – agricultural residues | ||||||||
| Biomass | −6.78 | −34.17 | −67.34 | −338.89 | −6.51 | −33.61 | −65.73 | −327.15 |
| Butane | 0.01 | 0.03 | 0.05 | 0.25 | 0.00 | 0.02 | 0.03 | 0.17 |
| Gasoline | 4.24 | 21.22 | 42.44 | 212.20 | 2.85 | 14.26 | 28.52 | 142.59 |
| Diesel | 0.00 | 0.00 | 0.00 | 0.00 | 1.04 | 5.22 | 10.43 | 52.17 |
| Kerosene | 0.00 | 0.00 | 0.00 | 0.00 | 0.51 | 2.56 | 5.12 | 25.58 |
| LPG | 0.27 | 1.34 | 2.69 | 13.43 | 0.04 | 0.20 | 0.40 | 2.01 |
| Vented CO2 | 4.15 | 21.03 | 40.83 | 206.88 | 3.82 | 20.42 | 38.97 | 192.95 |
| Seq. CO2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| LGHG | 1.88 | 9.46 | 18.66 | 93.86 | 1.76 | 9.06 | 17.74 | 88.32 |
| GHGAF | 6.39 | 31.93 | 63.87 | 319.34 | 6.12 | 30.61 | 61.22 | 306.08 |
| GHGAE | 0.31 | 1.52 | 2.71 | 12.28 | 0.26 | 1.01 | 2.63 | 11.90 |
| GHGI | 0.28 | 0.28 | 0.28 | 0.28 | 0.28 | 0.29 | 0.28 | 0.28 |
The GHGI is between 0.21 and 0.23 for the forest residues, between 0.26 and 0.29 for the agricultural residues, and between −0.44 and −0.40 for the perennial crops. All twenty-four case studies represent a significant reduction in GHG emissions from fossil-fueled processes, and imply that the introduction of economic legislation (e.g., carbon tax) will immediately benefit the economics of the BTL refineries. However, such legislation is not necessary for the BTL refineries to be economically competitive (see Table 5). Table 10 shows that one of the major components of lifecycle emissions are attributed to the combustion of the liquid fuels. Approximately 40% of the total emissions will result from the use of the three major liquid products and the LPG byproduct. Venting of CO2 from the process accounts for an additional 40% of the total process emissions. The use of CO2 sequestration could be incorporated into the refinery design, but it would increase the overall cost of the BTL refinery and is not necessary to reduce emissions. The balance of the lifecycle emissions is due to acquisition and transportation of the feedstocks and products. The lifecycle GHG emissions is strongly mitigated by the total amount of carbon that is input to the biomass from the atmosphere. Approximately 70–75% of the CO2 that is emitted during within the BTL refinery lifecycle is captured during photosynthesis and stored back in the biomass feed. In the cases of crops planted on degraded lands, the additional carbon captured and stored in the soil changes the GHG balance to a net-negative process that absorbs CO2 over the refinery lifecycle.
4 Conclusions
A rigorous optimization-based framework for the process synthesis and simultaneous heat/power/water integration of a BTL refinery was proposed in this study. The framework includes a deterministic global optimization branch-and-bound scheme to identify high-quality feasible solutions (i.e., process designs) and also provide a tight lower bound on the quality of each solution. The process synthesis model incorporates several existing technologies including biomass gasification, acid gas cleanup, Fischer–Tropsch synthesis, methanol synthesis, methanol-to-gasoline, methanol-to-olefins, and hydrocarbon upgrading units in one combined superstructure. Detailed mathematical modeling of all process units is incorporated in a large-scale mixed-integer nonlinear optimization model which can be solved efficiently to determine the process design that has the lowest overall cost. The framework was demonstrated using twenty-four case studies, and the economic viability of each case study was presented.The price of crude oil for which the BTL refineries will be competitive ranges from $99–138 per bbl for a 1 kBD plant, $71–101 per bbl for a 5 kBD plant, $66 per bbl to $91 per GJ per bbl for a 10 kBD plant, and $62–84 per bbl for a 50 kBD plant. Key differences between the refineries at a given capacity level are attributed to the purchase price of the biomass and the desired liquid fuel products. For a given refinery design, there exists a threshold price of biomass above which the BTL refinery will no longer be economically competitive with crude oil refining. This threshold level for biomass purchase is dependent on the desired refinery capacity, and will be higher as the capacity increases. At crude oil prices above $80 per bbl, the process synthesis framework has identified all BTL refineries as economically viable if the biomass purchase price is below an approximate threshold of $120 per dry ton. The implementation of environmental legislation that penalizes greenhouse gas emissions will improve the economics of the BTL refinery for all capacity levels. Given the Energy Information Administration estimates for crude oil over the next two decades, it seems clear that BTL refineries at or above 5 kBD can be economically viable throughout the nation. Moreover, these BTL refineries use existing technology that has been demonstrated on a variety of scales including pilot plant level and full-scale commercial level. Demonstration of the refinery designs proposed in this study at a level of 1 kBD will lend credibility to the operating characteristics that are outlined in this study and can serve as a fundamental and rigorous basis toward the national pursuit for clean and sustainable energy.
Acknowledgements
The authors acknowledge partial financial support from the National Science Foundation (NSF EFRI-0937706 and NSF CBET-1158849).References
- W. Zittel and J. Schindler, Crude Oil: The Supply Outlook, Document Number: EWG-Series No. 3/2007, Report to the Energy Watch Group, October 2007, 2007 Search PubMed.
- I. S. Nashawi, A. Malallah and M. Al-Bisharah, Energy Fuels, 2010, 24, 1788–1800 CrossRef.
- Energy Information Administration, Annual Energy Outlook 2013 with Projections to 2035, Document Number: DOE/EIA-0383(2012), http://www.eta.doe.gov/oiaf/aeo/, 2011 Search PubMed.
- Water Science and Technology Board, Water Implications of Biofuels Production in the United States, 2008 Search PubMed.
- L. R. Lynd, E. Larson, N. Greene, M. Laser, J. Sheehan, B. E. Dale, S. McLaughlin and M. Wang, Biofuels, Bioprod. Biorefin., 2009, 3, 113–123 Search PubMed.
- National Academy of Sciences, National Academy of Engineering and National Research Council, Liquid Transportation Fuels from Coal and Biomass: Technological Status, Costs, and Environmental Issues, EPA, Prepublication, Washington, DC, 2009 Search PubMed.
- Department of Energy, Biomass as Feedstock for a Bioenergy and Bioproducts Industry: The Technical Feasibility of a Billion-Ton Annual Supply, Document Number: DOE/GO-102005-2135, http://www1.eere.energy.gov/biomass/publications.html, 2005 Search PubMed.
- Energy Information Administration, Annual Energy Outlook 2011 with Projections to 2035, Document Number: DOE/EIA-0383(2011), http://www.eta.doe.gov/oiaf/aeo/, 2011 Search PubMed.
- E. D. Larson, H. Jin and F. E. Celik, Biofuels, Bioprod. Biorefin., 2009, 3, 174–194 Search PubMed.
- T. G. Kreutz, E. D. Larson, G. Liu and R. H. Williams, Fischer–Tropsch Fuels from Coal and Biomass, Proceedings of the 25th International Pittsburg Coal Conference, 2008 Search PubMed.
- R. Agrawal, N. R. Singh, F. H. Ribeiro and W. N. Delgass, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 4828–4833 CrossRef CAS.
- R. C. Baliban, J. A. Elia and C. A. Floudas, Ind. Eng. Chem. Res., 2010, 49, 7343–7370 Search PubMed.
- J. A. Elia, R. C. Baliban and C. A. Floudas, Ind. Eng. Chem. Res., 2010, 49, 7371–7388 Search PubMed.
- R. C. Baliban, J. A. Elia and C. A. Floudas, Comput. Chem. Eng., 2011, 35, 1647–1690 Search PubMed.
- R. C. Baliban, J. A. Elia and C. A. Floudas, Comput. Chem. Eng., 2012, 37, 297–327 Search PubMed.
- J. A. Elia, R. C. Baliban, C. A. Xiao and X. Floudas, Comput. Chem. Eng., 2011, 35, 1399–1430 Search PubMed.
- J. A. Elia, R. C. Baliban and C. A. Floudas, AIChE J., 2012, 58, 2142–2154 Search PubMed.
- E. D. Larson, G. Fiorese, G. Liu, R. H. Williams, T. G. Kreutz and S. Consonni, Energy Environ. Sci., 2010, 3, 28–42 RSC.
- Y. Chen, T. A. Adams, II and P. I. Barton, Ind. Eng. Chem. Res., 2011, 50, 5099–5113 CrossRef CAS.
- Y. Chen, T. A. Adams, II and P. I. Barton, Ind. Eng. Chem. Res., 2011, 50, 4553–4566 Search PubMed.
- P. Liu, A. Whitaker, E. N. Pistikopoulos and Z. Li, Comput. Chem. Eng., 2011, 35, 1359–1373 Search PubMed.
- A. Warren and M. El-Halwagi, Fuel Process. Technol., 1996, 49, 157–166 Search PubMed.
- G. Liu, E. D. Larson, R. H. Williams, T. G. Kreutz and X. Guo, Energy Fuels, 2011, 25, 415–437 CrossRef CAS.
- R. H. Williams, G. Liu, T. G. Kreutz and E. D. Larson, Energy Procedia, 2011, 4, 1843–1850 Search PubMed.
- G. Liu, R. H. Williams, E. D. Larson and T. G. Kreutz, Energy Procedia, 2011, 4, 1989–1996 Search PubMed.
- R. H. Borgwardt, Biomass Bioenergy, 1997, 12, 333–345 Search PubMed.
- H. Li, H. Hong, H. Jin and R. Cai, Appl. Energy, 2010, 87, 2846–2853 Search PubMed.
- Y. Dong and M. Steinberg, Int. J. Hydrogen Energy, 1997, 22, 971–977 Search PubMed.
- R. C. Baliban, J. A. Elia, R. Misener and C. A. Floudas, Comput. Chem. Eng., 2012, 42, 64–86 Search PubMed.
- R. C. Baliban, J. A. Elia, V. W. Weekman and C. A. Floudas, Comput. Chem. Eng., 2012, 47, 29–56 Search PubMed.
- C. A. Floudas, J. A. Elia and R. C. Baliban, Comput. Chem. Eng., 2012, 41, 24–51 Search PubMed.
- M. Laser, H. Jin, K. Jayawardhana, B. E. Dale and L. R. Lynd, Biofuels, Bioprod. Biorefin., 2009, 3, 231–246 Search PubMed.
- L. Tock, M. Gassner and F. Maréchal, Biomass Bioenergy, 2010, 34, 1838–1854 CrossRef CAS.
- C. Hamelinck, A. Faaij, H. Uil and H. Boerrigter, Energy, 2004, 29, 1743 CrossRef CAS.
- A. L. V. Perales, C. R. Valle, P. Ollero and A. G. Barea, Energy, 2011, 36, 4097–4108 Search PubMed.
- M. J. A. Tijmensen, A. P. C. Faaij, C. N. Hamelinck and M. R. M. Hardeveld, Biomass Bioenergy, 2002, 23, 129–152 CrossRef CAS.
- A. V. Bridgwater and J. M. Double, Fuel, 1991, 70, 1209–1224 Search PubMed.
- R. M. Swanson, A. Platon, J. A. Satrio and R. C. Brown, Fuel, 2010, 89, S11–S19 CrossRef.
- L. R. Clausen, B. Elmegaard and N. Houbak, Energy, 2010, 35, 4831–4842 Search PubMed.
- P. Sharma, B. R. Sarker and J. A. Romagnoli, Comput. Chem. Eng., 2011, 35, 1767–1781 Search PubMed.
- E. Henrich, N. Dahmen and E. Dinjus, Biofuels, Bioprod. Biorefin., 2009, 3, 28–41 Search PubMed.
- K. Sunde, A. Brekke and B. Solberg, Energies, 2011, 4, 845–877 Search PubMed.
- B. Bao, D. K. S. Ng, D. H. S. Tay, A. J. Gutiérrez and M. M. El-Halwagi, Comput. Chem. Eng., 2011, 35, 1374–1383 Search PubMed.
- D. H. S. Tay, D. K. S. Ng, N. E. Sammons and M. R. Eden, Ind. Eng. Chem. Res., 2011, 50, 1652–1665 Search PubMed.
- S. Giarola, A. Zamboni and F. Bezzo, Comput. Chem. Eng., 2011, 35, 1782–1797 Search PubMed.
- Y. Bai, T. Hwang, S. Kang and Y. Ouyang, Transportation Research Part B: Methodological, 2011, 45, 162–175 Search PubMed.
- I. M. Bowling, J. M. P. Ortega and M. M. El-Halwagi, Ind. Eng. Chem. Res., 2011, 50, 6276–6286 Search PubMed.
- N. Parker, P. Tittmann, Q. Hart, R. Nelson, K. Skog, A. Schmidt, E. Gray and B. Jenkins, Biomass Bioenergy, 2010, 34, 1597–1607 Search PubMed.
- Y. Huang, C. W. Chen and Y. Fan, Transport. Res. E Logist. Transport. Rev., 2010, 46, 820–830 Search PubMed.
- B. Aksoy, H. T. Cullinan, N. E. Sammons Jr and M. R. Eden, Environ. Prog., 2008, 27, 515–523 Search PubMed.
- R. H. Williams, E. D. Larson, G. Liu and T. G. Kreutz, Energy Procedia, 2009, 1, 4379–4386 Search PubMed.
- K. Kumabe, S. Fujimoto, T. Yanagida, M. Ogata, T. Fukuda, A. Yabe and T. Minowa, Fuel, 2008, 87, 1422–1427 Search PubMed.
- F. Ju, H. Chen, X. Ding, H. Yang, X. Wang, S. Zhang and Z. Dai, Biotechnol. Adv., 2009, 27, 599–605 Search PubMed.
- C. N. Hamelinck and A. P. C. Faaij, J. Power Sources, 2002, 111, 1–22 CrossRef CAS.
- L. R. Clausen, N. Houbak and B. Elmegaard, Energy, 2010, 35, 2338–2347 Search PubMed.
- H. Kim, K. Han and E. S. Yoon, J. Chem. Eng. Jpn., 2010, 43, 671–681 Search PubMed.
- J. He and W. Zhang, Appl. Energy, 2011, 88, 1224–1232 CrossRef CAS.
- K. S. Ng and J. Sadhukkan, Biomass Bioenergy, 2011, 35, 1153–1169 Search PubMed.
- J. Xiao, L. Shen, Y. Zhang and J. Gu, Ind. Eng. Chem. Res., 2009, 48, 9999–10007 Search PubMed.
- M. Erturk, Renewable Sustainable Energy Rev., 2011, 15, 2766–2771 CrossRef.
- O. Vliet, A. Faaij and W. Turkenburg, Energy Convers. Manage., 2009, 50, 855–876 CrossRef CAS.
- M. Dal-Mas, S. Giarola, A. Zamboni and F. Bezzo, Biomass Bioenergy, 2011, 35, 2059–2071 Search PubMed.
- B. Amigun, J. Gorgens and H. Knoetze, Energy Policy, 2010, 38, 312–322 Search PubMed.
- S. Leduc, D. Schwab, E. Dotzauer, E. Schmid and M. Obersteiner, Int. J. Energy Res., 2008, 32, 1080–1091 Search PubMed.
- S. Leduc, E. Schmid, M. Obersteiner and K. Riahi, Biomass Bioenergy, 2009, 33, 745–751 Search PubMed.
- S. Leduc, J. Lundgren, O. Franklin and E. Dotzauer, Appl. Energy, 2010, 87, 68–75 Search PubMed.
- D. Mignard and C. Pritchard, Chem. Eng. Res. Des., 2008, 86, 473–487 Search PubMed.
- R. H. Williams, E. D. Larson, R. E. Katofsky and J. Chen, Energy Sustainable Dev., 1995, 1, 18–34 Search PubMed.
- S. Sarkar, A. Kumar and A. Sultana, Energy, 2011, 36, 6251–6262 Search PubMed.
- N. Kou and F. Zhao, Biomass Bioenergy, 2011, 35, 608–616 Search PubMed.
- L. Cucek, M. Martin, I. E. Grossmann and Z. Kravanja, Comput. Chem. Eng., 2011, 35, 1547–1557 Search PubMed.
- W. A. Marvin, L. D. Schmidt, S. Benjaafar, D. G. Tiffany and P. Daoutidis, Chem. Eng. Sci., 2012, 67, 68–79 Search PubMed.
- J. Terrados, G. Almonacid and P. P. Higueras, Renewable Sustainable Energy Rev., 2009, 13, 2022–2030 Search PubMed.
- O. Akgul, A. Zamboni, F. Bezzo, N. Shah and L. G. Papageorgiou, Ind. Eng. Chem. Res., 2011, 50, 4927–4938 Search PubMed.
- S. van Dyken, B. H. Bakken and H. I. Skjelbred, Energy, 2010, 35, 1338–1350 Search PubMed.
- A. A. Rentizelas and I. P. Tatsiopoulos, Int. J. Prod. Econ., 2010, 123, 196–209 Search PubMed.
- A. A. Rentizelas, I. P. Tatsiopoulos and A. Tolis, Biomass Bioenergy, 2009, 33, 223–233 Search PubMed.
- S. D. Eksioglu, A. Acharya, L. E. Leightley and S. Arora, Comput. Ind. Eng., 2009, 57, 1342–1352 Search PubMed.
- S. D. Eksioglu, S. Li, S. Zhang, S. Sokhansanj and D. Petrolia, Transp. Res. Rec., 2010, 2191, 144–151 Search PubMed.
- A. Zamboni, N. Shah and F. Bezzo, Energy Fuels, 2009, 23, 5121–5133 Search PubMed.
- A. J. Dunnett, C. S. Adjiman and N. Shah, Biotechnol. Biofuels, 2008, 1, 13–40 Search PubMed.
- B. V. Marti and E. F. Gonzalez, Renewable Energy, 2010, 35, 2136–2142 Search PubMed.
- L. Panichelli and E. Gnansounou, Biomass Bioenergy, 2008, 32, 289–300 Search PubMed.
- F. Zhang, D. M. Johnson and J. W. Sutherland, Biomass Bioenergy, 2011, 35, 3951–3961 Search PubMed.
- H. An, W. E. Wilhelm and S. W. Searcy, Bioresour. Technol., 2011, 102, 7860–7870 Search PubMed.
- J. Gan and C. T. Smith, Biomass Bioenergy, 2011, 35, 3350–3359 Search PubMed.
- S. Leduc, F. Starfelt, E. Dotzauer, G. Kindermann, I. McCallum, M. Obersteiner and J. Lundgren, Energy, 2010, 35, 2709–2716 Search PubMed.
- K. Hacatoglu, P. J. McLellan and D. B. Layzell, Bioresour. Technol, 2011, 102, 1087–1094 Search PubMed.
- F. Cherubini, Renewable Energy, 2010, 35, 1565–1573 CrossRef CAS.
- M. Kocoloski, W. M. Griffin and H. S. Matthews, Energy Policy, 2011, 39, 47–56 Search PubMed.
- L. Cucek, H. L. Lam, J. J. Klemes, P. S. Varbanov and Z. Kravanja, Clean Technol. Environ. Policy, 2010, 12, 635–645 Search PubMed.
- R. W. R. Zwart and H. Boerrigter, Energy Fuels, 2005, 19, 591–597 CrossRef CAS.
- R. C. Baliban, J. A. Elia and C. A. Floudas, AIChE J., 2012 Search PubMed , submitted.
- M. Martin and I. E. Grossmann, Ind. Eng. Chem. Res., 2011, 50, 13485–13499 Search PubMed.
- J. Ellepola, N. Thijssen, J. Grievink, G. Baak, A. Avhale and J. van Schijndel, Comput. Chem. Eng., 2012, 42, 2–14 Search PubMed.
- M. A. Duran and I. E. Grossmann, AIChE J., 1986, 32, 123–138 Search PubMed.
- R. Karuppiah and I. E. Grossmann, Comput. Chem. Eng., 2006, 30, 650–673 Search PubMed.
- E. Ahmetovic and I. E. Grossmann, Ind. Eng. Chem. Res., 2010, 49, 7972–7982 Search PubMed.
- I. E. Grossmann and M. Martín, Chin. J. Chem. Eng., 2010, 18, 914–922 Search PubMed.
- E. Ahmetovic and I. E. Grossmann, AIChE J., 2010, 57, 434–457 Search PubMed.
- A. van der Drift and J. van Doorn, Analysis of Biomass Data in ECN Database Phyllis, http://www.ecn.nl/phyllis/, 2002 Search PubMed.
- National Renewable Energy Laboratory, Gasoline from Wood via Integrated Gasification, Synthesis, and Methanol-to-Gasoline Technologies, USDOE contract DE-AC36–08GO28308, 2011 Search PubMed.
- National Renewable Energy Laboratory, Process Design and Economics for Conversion of Lignocellulosic Biomass to Ethanol: Thermochemical Pathway by Indirect Gasification and Mixed Alcohol Synthesis, USDOE contract DE-AC36–08GO28308, 2011 Search PubMed.
- National Energy Technology Laboratory, Cost and Performance Baseline for Fossil Energy Plants. Volume 1: Bituminous Coal and Natural Gas to Electricity Final Report, Document Number: DOE/NETL-2007/1281, http://www.netl.doe.gov/energy-analyses/baseline_studies.html, 2007 Search PubMed.
- A. de Klerk, Fischer–Tropsch Refining, Wiley-VCH Verlag & Co. KGaA, 2011 Search PubMed.
- Mobil R. and D. Corporation, Slurry Fischer–Tropsch/Mobil Two Stage Process of Converting Syngas to High Octane Gasoline, USDOE contract DE-AC22–80PC30022, 1983 Search PubMed.
- Mobil R. and D. Corporation, Two-Stage Process For Conversion of Synthesis Gas to High Quality Transportation Fuels, USDOE contract DE-AC22–83PC60019, 1985 Search PubMed.
- A. R. Steynberg and M. E. Dry, Stud. Surf. Sci. Catal., 2004, 152, 64–195 Search PubMed.
- Bechtel, Aspen Process Flowsheet Simulation Model of a Battelle Biomass-Based Gasification, Fischer–Tropsch Liquefaction and Combined-Cycle Power Plant, Contract Number: DE-AC22-93PC91029, http://www.fischer–tropsch.org/, 1998 Search PubMed.
- Bechtel, Baseline Design/Economics for Advanced Fischer–Tropsch Technology, Contract No. DE-AC22-91PC90027, 1992 Search PubMed.
- S. A. Tabak and S. Yurchak, Catal. Today, 1990, 6, 307–327 CrossRef CAS.
- Mobil R. and D. Corporation, Research Guidance Studies to Assess Gasoline from Coal by Methanol-to-Gasoline and Sasol-Type Fischer–Tropsch Technologies, USDOE contract EF-77-C-O1-2447, 1978 Search PubMed.
- National Energy Technology Laboratory, Assessment of Hydrogen Production with CO2 Capture Volume 1: Baseline State-of-the-Art Plants, DOE/NETL-2010/1434, 2010 Search PubMed.
- Chemical Engineering Magazine, Chemical Engineering Plant Cost Index, http://www.che.com/pci/, 2012.
- CPLEX, ILOG CPLEX C++ API 12.1 Reference Manual, 2009 Search PubMed.
- A. Drud, Math. Program., 1985, 31, 153–191 Search PubMed.
- C. A. Floudas, Nonlinear and Mixed-Integer Optimization, Oxford University Press, New York, 1995 Search PubMed.
- C. A. Floudas, Deterministic Global Optimization: Theory, Methods and Applications, Kluwer Academic Publishers, Dordrecht, Netherlands, 2000 Search PubMed.
- C. A. Floudas and P. M. Pardalos, J. Global Optim., 1995, 7, 113 Search PubMed.
- C. A. Floudas, I. G. Akrotirianakis, S. Caratzoulas, C. A. Meyer and J. Kallrath, Comput. Chem. Eng., 2005, 29, 1185–1202 Search PubMed.
- C. A. Floudas and C. E. Gounaris, J. Global Optim., 2009, 45, 3–38 Search PubMed.
- C. A. Floudas, A. R. Ciric and I. E. Grossmann, AIChE J., 1986, 32, 276–290 Search PubMed.
- J. H. Gregor, C. D. Gosling and H. E. Fullerton, Upgrading Fischer–Tropsch LPG with the Cyclar Process, Contract No. DE-AC22–86PC90014, 1989 Search PubMed.
- Argonne National Laboratory, GREET 1.8b, The Greenhouse Gases, Regulated Emissions, and Energy Use in Transportation (GREET) Model, 2007, Release September 2008 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed process flow diagrams and all relevant nomenclature and equations for the process synthesis mathematical model. See DOI: 10.1039/c2ee23369j |
| This journal is © The Royal Society of Chemistry 2013 |
