From “cyborg” lobsters to a pacemaker powered by implantable biofuel cells†
Kevin
MacVittie
a,
Jan
Halámek
*a,
Lenka
Halámková
ab,
Mark
Southcott
c,
William D.
Jemison
*c,
Robert
Lobel
d and
Evgeny
Katz
*a
aDepartment of Chemistry and Biomolecular Science, Clarkson University, Potsdam, NY 13699, USA. E-mail: ekatz@clarkson.edu; jhalamek@clarkson.edu
bDepartment of Biology, Clarkson University, Potsdam, NY 13699, USA
cDepartment of Electrical and Computer Engineering, Clarkson University, Potsdam, NY 13699, USA. E-mail: wjemison@clarkson.edu
dFletcher Allen Cardiology, University of Vermont College of Medicine, Medical Center Campus, McClure 1, 111 Colchester Avenue, Burlington, VT 05401-1473, USA
First published on 25th September 2012
Abstract
Enzyme-based biofuel cells implanted into living lobsters or designed as fluidic systems mimicking human blood circulation were used for powering electronic devices. Two lobsters with implanted biofuel cells connected in series were able to generate open circuit voltage (Voc) up to 1.2 V and an electrical watch, selected as a model electronic device, was activated by the power extracted from the “living battery”. The fluidic system composed of five cells filled with human serum solution connected in series generated Voc of ca. 3 V and was able to power a pacemaker. Sustainable operation of the pacemaker was achieved with the system closely mimicking human physiological conditions characteristic of normal and pathophysiological glucose concentrations with the fluidic rate typical for a blood circulation upon resting or performing physical exercises. While the “cyborg” lobsters demonstrate a model system with future possible military, homeland security and environmental monitoring applications, the system activating a pacemaker presents practicality for biomedical applications. The first demonstration of the pacemaker activated by the physiologically produced electrical energy shows promise for future electronic implantable medical devices powered by electricity harvested from the human body.
Broader contextBiofuel cells converting chemical energy into electricity upon biocatalyzed chemical reactions have recently emerged as promising alternative sources of sustainable electrical energy. Implantable biofuel cells suggested as micro-power sources operating in living organisms are still exotic and very challenging to design within bioelectronic systems. Very few examples of enzyme-based biofuel cells operating in animals in vivo have been reported and the present work is another step in this research. Enzyme-modified electrodes were implanted in living lobsters resulting in biocatalytic oxidation of glucose and reduction of oxygen in the biofluid inside the lobster's body thus producing electrical power from the biological source. Integration of the “electrified” lobsters with the biofuel cells connected in series allowed for the activation of a digital watch as a model electronic device. Various sensing, information processing and wireless transmission devices for future military, homeland security and environmental monitoring applications are feasible based on the present example-device with the power produced by a “cyborg” creature. Another fluidic device with implanted bioelectrodes assembled in series and mimicking the human blood circulatory system was used to activate a pacemaker. Future implantable medical devices such as cardiac defibrillators/pacemakers, deep brain neurostimulators, spinal cord stimulators, gastric stimulators, foot drop implants, cochlear implants, insulin pumps, etc. powered by implanted biofuel cells extracting electrical energy directly from a human body are possible, resulting in bionic human hybrids. The present study is a step on the long path to the design of bioelectronic self-powered “cyborgs” which can autonomously operate using power from biological sources. |
Biofuel cells1,2 harvesting electrical power from biological fuels by means of biocatalytic activity of microbial cells,3–5 enzymes,6–12 or biological organelles, such as mitochondria,13 have received high attention in the last two decades, rapidly moving from basic scientific research to applied engineering and development. While microbial biofuel cells are typically designed as middle-to-large scale energy sources powering electronic devices,14 robotic systems15,16 and even households,5 enzyme-based biofuel cells have always been considered micro-scale energy devices,17,18 mostly for biomedical applications, more specifically the powering of implantable bioelectronic devices. Despite the fact that this application of micro-biofuel cells was proposed a long time ago,19 very few practical realizations of implanted biofuel cells operating in vivo under physiological conditions have been achieved to date;20–27 some of which are based on non-enzymatic (abiotic) electrodes with inorganic catalytic species.20,21 Most researchers, even if they claim to be doing potentially “implantable” biofuel cells, perform experiments in model solutions with little correlation with actual implanted devices for in vivo operation.28 It should be noted that implanted biofuel cells operating in vivo were never successfully used for powering real electronic or biomedical devices. The obtained data for biofuel cells implanted in the retroperitoneal space of a freely moving rat,22 in a blood vessel in a rabbit ear,23 in the abdomen of an insect,24 or in the hemolymph of snails,25 or clams26 were limited to the analysis of the current–voltage output with no attempts to use the produced electrical power for activating real electronic devices (except very preliminary tests with electrical motors powered by clams or lobsters26,27). Despite the fact that many papers demonstrated power release from biofuel cells which may potentially be enough for activating electronic devices, real interfacing of electronics with implanted biofuel cells has yet to be demonstrated. The major problem is the low voltage produced by biofuel cells, which is thermodynamically limited by the redox potentials of the biological fuel (usually glucose) and oxygen. In most of the reported biofuel cells the open circuit voltage hardly exceeds 0.5 V being decreased upon consuming current from the cells;1,6,7 at best the voltage was measured as high as 0.78 V while operating under non-physiological conditions.29 However, this voltage is still not enough for most electronic devices, which usually require several volts for their operation. Improving efficiency of biofuel cells includes mostly increasing their current production and results in a very little effect on the voltage which is thermodynamically limited. It should be noted that most papers on biofuel cells do not discuss the problem of their interfacing with electronics, while being concentrated on resolving internal problems of the biofuel cells, such as current efficiency, stability, etc. Two approaches have been applied to resolve the low voltage problem: (i) assembling biofuel cells in series electrically, thus increasing the total output voltage,26,30–32 and (ii) collecting produced electrical energy in capacitors/charge pumps for the burst release in short pulses.14,27,33–35 The latter approach has already been applied for activating a wireless transmitting electronic device, however, using non-implantable enzyme-based35 or microbial biofuel cells.14
The present paper is the first attempt to address the problem of interfacing electronic devices with implanted biofuel cells operating in vivo in lobsters and in a model system closely mimicking human physiology. Two different examples illustrate two different future applications of implantable biofuel cells: (i) military, homeland security, and environmental monitoring applications,36 where microelectronic sensing and wireless transmitting devices could be carried by small animals being powered by implanted biofuel cells taking energy from the physiologically produced metabolites (e.g. glucose), or (ii) biomedical applications where implanted biomedical devices (e.g. pacemakers)37 could be powered taking energy directly from the human body, thus operating without batteries.38 While the former way of using implanted biofuel cells looks like science fiction reminiscent of “cyborg” creatures,39 the latter is an extremely important reality for medicine. Millions of patients with implanted pacemakers must undergo surgery replacing the battery when it is depleted,40 whereas implanted biofuel cells could potentially operate as long as the physiological fuel is available, in other words while the patient is alive, thus reducing the health risk and cost of frequent surgery.
Based on our recently published reports on biofuel cells implanted in snails25 and clams,26 we implanted the biocatalytic electrodes in the American lobster (Homarus americanus). Lobsters are large marine crustaceans (Crustacea) with a segmented body covered by a hard chitinous exoskeleton. They have a main body cavity (hemocoel) that is a part of the hemal system. Lobsters have an open circulatory system consisting of a single-chambered heart, several large arteries derived from the heart and a venous system that is made of large sinuses (cavities) freely communicating with each other. The hemolymph passes through the heart and arteries and then is released into the sinuses directly to the various organs, which are bathed in the hemolymph. Hemolymph glucose serves as an immediate source of energy; its level is ca. 900 μM,41 but the level varies with different factors (temperature, handling, stress, etc.).42 In addition to oxygen carried by hemocyanin (oxygen-carrying pigment in hemolymph), there is free dissolved oxygen with the concentration of ca. 250–400 μM, depending on the temperature of the body.43 Thus, at a low temperature (ca. 4–10 °C) which is the most natural for lobsters, the glucose and soluble O2 in hemolymph are approximately at the stoichiometric (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) ratio.
1) ratio.
The biocatalytic electrodes (geometrical area exposed to hemolymph ca. 0.25 cm2) included a PQQ-dependent glucose dehydrogenase-modified anode for glucose oxidation and laccase-modified cathode for oxygen reduction immobilized on a buckypaper conductive support,44–47 similarly to our previous work25,26 (see Experimental details in the ESI†). The enzyme-modified anode and cathode were implanted in a lobster. Two lateral sagittal rectangular slits were excised from the exoskeleton of the lobster cephalothorax. These slits were approximately 2 mm wide by 1 cm in length and cut through to the dorsal sinus of the venous system. The electrodes were then placed into the hemolymph between exoskeleton and stomach. The implanted electrodes performing the bioelectrocatalytic reactions in a lobster were connected in a circuit configuration, Fig. 1A, composed of an external variable load resistance, RL, where the voltage and current were measured during the biofuel cell operation in vivo, resulting in the polarization data, Fig. 2. In a typical experiment open circuit voltage, Voc, and short circuit current, Isc, achieved in the biofuel cell in vivo were ca. 540 mV (up to 600 mV in the best experiments) and ca. 1 mA (4 mA cm−2 for the geometrical electrode area), respectively. The maximum power, Pmax, produced in the typical experiment by the implanted biofuel cell at the optimum resistance, RL,o, of 500 Ω was ca. 0.16 mW (ca. 0.64 mW cm−2), Fig. 2, inset. We selected a digital watch (Timex IronMan, Triathalon Men's Watch) as a simple example-model device to be activated with the implanted biofuel cell. In preliminary experiments, when the watch was powered from an external variable power source, we found that the minimum voltage required for its operation is 700 mV, which is not achievable from the lobster-biofuel cell. Thus, we attempted to assemble a stack of two couples of biocatalytic anodes–cathodes with a series connection to enhance the output voltage similar to our previous experiments with biofuel cells implanted in clams.26 Hoping that a larger animal could accommodate two biofuel cells in its body, we implanted both biofuel cells in one lobster locating them close to each other in its back. However, the series connection of two biofuel cells, Fig. 1B, showed only a minor (50–100 mV) increase in the produced voltage, which is much less than the expected double voltage. This negative experimental result was easily explained by the electrical resistance of the lobster's body tissue, RT (the resistance between the electrodes implanted in the lobster's back at a distance of 2 cm was ca. 180 kΩ), which formed a low impedance path (jumper) between the anodes and cathodes, Fig. 1C, thus preventing the desired series operation of the two fuel cells. While trying to solve the problem, we relocated the biocatalytic electrodes to different claws (not shown in Fig. 1) hoping that the bigger distance between the interconnected biocathode and bioanode and thus bigger tissue resistance, RT, would allow the increased voltage from the cells. Unfortunately, even in this case the series connection was showing only ca. 0.5–0.6 V, thus the low impedance was still formed in the lobster's body (the resistance between the electrodes located in the left and right claws was ca. 200 kΩ, being almost the same as in the case of positioning the electrodes in the lobster's back). The unsuccessful experiments were very important to learn that biofuel cells cannot be interconnected in series while operating in the same body due to biotissue conductivity (eventually, parallel connections of biocatalytic electrodes are possible, but they would only increase the current output proportionally to the increase of the electrode area). Therefore, returning back to the original design of the biofuel batteries demonstrated on clams (where three separate clams with implanted bioelectrodes were used in series connections),26 we implanted the biocatalytic electrodes in two lobsters locating them in the back and connecting the biofuel cells in series, Fig. 1D and 3A and B. As can be easily expected, we obtained the double voltage (Voc) of ca. 1 V (in the best experiment it was up to 1.2 V) compared with a single lobster-biofuel cell. Applying the output from this biofuel battery to the watch, we successfully activated the model electronic device, Fig. 3B and C. It should be noted that in a typical experiment, upon extracting current for powering the watch, the voltage produced by the biofuel battery was decreasing only by ca. 10% compared with Voc. The watch was active over an hour until the voltage was decreased because of the local glucose consumption near the electrode surface. After a certain period of time (tens of minutes), when the glucose level was restored similarly to our previous experiments with snails,25 the watch could be activated again. The electronic watch was powered by the implanted biofuel cell during the whole life-time of the lobsters (several hours), which was only limited by our ability to maintain them alive in the laboratory conditions. (Note that lobsters are marine creatures and their life-time in the laboratory conditions is much shorter than in the case of snails used in our previous experiments.25)
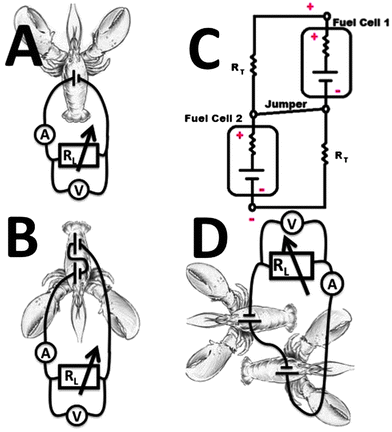 | ||
| Fig. 1 The cartoons showing schematically different wirings of the biocatalytic electrodes implanted in lobsters: (A) A single pair of the biocatalytic cathode–anode implanted in the lobster. (B) Two pairs of the biocatalytic cathodes–anodes implanted into the same lobster and connected in series. (C) The electrical circuitry equivalent to the wiring scheme shown in B. (D) Two pairs of the biocatalytic cathodes–anodes implanted into two different lobsters and connected in series. | ||
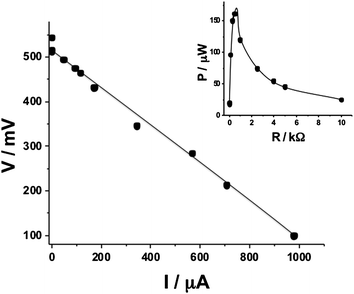 | ||
| Fig. 2 Polarization curve of a single biofuel cell implanted in a lobster and operating in vivo (corresponding to the wiring scheme shown in Fig. 1A). Inset: power generated on a variable load resistance. | ||
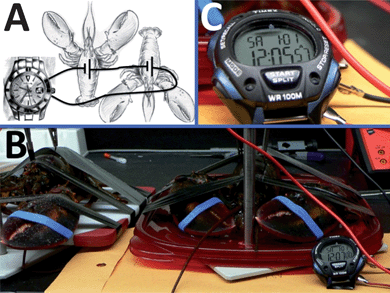 | ||
| Fig. 3 The biofuel cell composed of two pairs of the biocatalytic cathodes–anodes implanted in two lobsters wired in series and used for powering an electronic watch. (A) The wiring scheme. (B) The photo of the setup. (C) The operating watch – close view. | ||
In the second example, attempting to demonstrate the biomedical applications, we had to mimic the human blood circulatory system, which is significantly different from the poorly circulating lobster's hemolymph. Thus, we designed a fluidic system mimicking a human capillary blood vessel. The liquid used in the model fluidic system was human serum solution spiked with various concentrations of glucose. It should be noted that the initial natural concentration of glucose in serum, prior to the glucose additions, was found to be ca. 3.7 mM. Thus, glucose concentrations of 3.7, 6.4 and 10.3 mM were realized, corresponding to physiological conditions of a “hungry” healthy person with a low glucose level, a “normal” healthy person and a diabetic person with the elevated level of glucose, respectively.48 For all three glucose concentrations mimicking various physiological conditions we applied two different flow rates of the serum solution, 58.9 and 235.6 μL min−1, mimicking blood circulation (typical for 8 μm diameter blood capillary vessel) when a person is resting or running (see Experimental details in the ESI†).49 The purpose of this experiment was to show the biofuel flow-cell operation under different physiological conditions mimicking different glucose concentrations in blood and different blood circulation rates. The biocatalytic electrodes (geometrical area exposed to the serum solution ca. 0.75 cm2) installed in the flow-cell were connected to a variable load resistance and polarization was measured, Fig. 4, bottom-left inset. Surprisingly, we found almost identical polarization curves for all six combinations of the model physiological conditions that differed by the glucose concentration and flow rate, thus indicating that the biofuel cell operated under saturation conditions. A typical polarization curve is shown in Fig. 4, demonstrating the Voc of ca. 630 mV, Isc of ca. 600 μA and Pmax of ca. 97 μW at the optimum load resistance of 1 kΩ, Fig. 4, top-right inset, which is not much different from the values found for the biofuel cell implanted in the lobster.
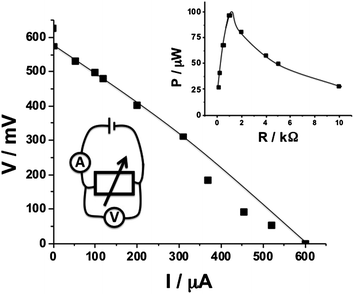 | ||
| Fig. 4 Polarization curve of a single biofuel cell operating in a fluidic setup with a serum solution. Note that the polarization function was almost the same for glucose concentrations of 3.7–10.3 mM corresponding to the broad range of physiological concentrations and for the liquid rates of 58.9 and 235.6 μL min−1 mimicking blood circulation for a resting person and a person performing physical activity. Insets: bottom-left: the wiring scheme; top-right: power generated on a variable load resistance. | ||
In order to illustrate the interfacing of the biofuel flow-cell with an implantable biomedical device we selected a pacemaker (Affinity DR 5330L, St Jude Medical)37 as an example. A sealed pacemaker was cut open and the internal battery was removed allowing for wiring of the pacemaker to the biofuel cell or any other external source of electricity, Fig. 5A. Prior to the experiments with the biofuel cell we tested the pacemaker operation applying electrical power from a variable electrical power source (Tenma 72-8700). In this preliminary experiment we found that the used pacemaker needs at least 1.4 V to generate output pulse signals registered by an oscilloscope (Agilent DSO-3000), however, the standard battery (WG9438) used in the pacemaker produces 2.8 V required for the correct operation of the circuitry. The estimated power consumption by the pacemaker was ca. 90 μW. Thus, the required power is certainly achievable from the biofuel flow-cell, however, the voltage for activating the pacemaker is much higher than the voltage produced by the cell. In order to increase the output voltage produced by the biofuel cell we assembled a flow-cell circuit composed of 5 individual flow-cells connected to the same fluidic channel filled with the serum solution and with series electrode connection. As might be expected from our unsuccessful experiments described above for the lobster with two implanted biofuel cells, the biofuel circuitry in this design did not show significant increase of voltage compared with a single cell because of the short circuit connection between the biocatalytic electrodes through the resistance of the fluidic channel. Therefore, we disconnected the fluidic channels and organized the liquid flow separately for each individual biofuel flow-cell keeping the series electrical connections of the electrodes, Fig. 5B. In this design the formation of the short circuit connection between the cathode–anode pairs through the liquid phase was prevented and the Voc of ca. 2.8 V was obtained being already sufficient to start experiments with the pacemaker. When the pacemaker was connected to the biofuel flow-battery, Fig. 5B, it started to generate pulses as expected. The pulses being registered by the oscilloscope, Fig. 5C, demonstrated voltage spikes of ca. 5 V with the duration of ca. 0.6 ms separated by the time-gaps of ca. 1 s, which correspond to the normal performance of the pacemaker.50 Particularly important is that the shape of the generated pulses, Fig. 5D, was characteristic of the normal behavior of the pacemaker.50,51 The biofuel cell produced electrical power which was sufficient for the pacemaker operation for at least 5 hours (longer stability test was not performed in this preliminary study).
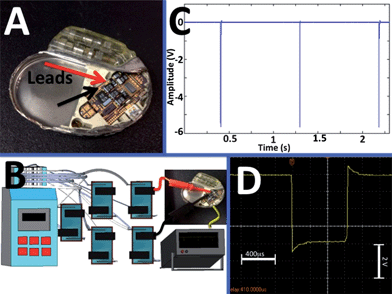 | ||
| Fig. 5 The biofuel cell battery composed of five pairs of the biocatalytic cathodes–anodes operating in a fluidic setup with a serum solution and used for powering a pacemaker. (A) Open pacemaker – a close view showing the microscheme and wiring leads for the battery. Note the empty space (left part of the device) from which the original battery was removed. (B) The experimental setup showing the multi-channel peristaltic pump, five individual fluidic tubings providing serum solution to five biofuel cells connected in series electrically and used for powering the pacemaker. (C) The electrical signals generated by the pacemaker recorded by an oscilloscope. (D) The individual pulse produced by the pacemaker powered by the biofuel cell. | ||
It should be noted in conclusion that the activation of the pacemaker with the biofuel cells closely mimicking human physiological conditions was achieved for the first time. Importantly, similar electrical output generated by the biofuel cells for various glucose concentrations and flow rates corresponding to different physiological conditions allows stable operation of the pacemaker regardless of the physiological changes. Using the present experiment with the pacemaker as an example, one can foresee in the future various medical electronic implants, including cardiac defibrillators/pacemakers, deep brain neurostimulators, gastric stimulators, foot drop implants, cochlear implants, insulin pumps, etc. powered by implanted biofuel cells and resulting in bionic human–machine hybrids.52 On the other hand, activating a watch by the biofuel cells implanted in the lobster and operating in vivo opens the way for designing various integrated electronic and biological systems operating together in this concerted way, thus leading to “cyborg” organisms for various biotechnological applications.
In the next step of this research, which is underway in the lab, we will apply a charge pump to reduce the number of the electrodes/cells required for the activation of the bioelectronic devices. We will also demonstrate the implantable biofuel cell use for activating different biomedical implantable devices, e.g. for powering a spinal cord stimulator, thus extending to possible biomedical applications.
All experiments were performed in compliance with the guidelines set forth by Clarkson University’s Institutional Animal Care and Use Committee (IACUC).
Acknowledgements
This research was supported by the National Science Foundation (Award CBET-1066397) and by the Semiconductor Research Corporation (Award 2008-RJ-1839G). The authors acknowledge several helpful suggestions from Professor Todd Wey.References
- F. Davis and S. P. J. Higson, Biosens. Bioelectron., 2007, 22, 1224–1235 CrossRef CAS.
- E. Katz, A. N. Shipway and I. Willner, in Handbook of Fuel Cells - Fundamentals, Technology, Applications, ed. W. Vielstich, H. Gasteiger and A. Lamm, Wiley, 2003, vol. 1, Part 4, ch. 21, pp. 355–381 Search PubMed.
- F. Scholz and U. Schröder, Nat. Biotechnol., 2003, 21, 1151–1152 CrossRef CAS.
- M. H. Osman, A. A. Shah and F. C. Walsh, Biosens. Bioelectron., 2010, 26, 953–963 CrossRef CAS.
- B. E. Logan, Microbial Fuel Cells, John Wiley & Sons, Inc., Hoboken, NJ, 2008 Search PubMed.
- J. A. Cracknell, K. A. Vincent and F. A. Armstrong, Chem. Rev., 2008, 108, 2439–2461 CrossRef CAS.
- M. J. Moehlenbrock and S. D. Minteer, Chem. Soc. Rev., 2008, 37, 1188–1196 RSC.
- N. Mano, F. Mao and A. Heller, ChemBioChem, 2004, 5, 1703–1705 CrossRef CAS.
- V. Soukharev, N. Mano and A. Heller, J. Am. Chem. Soc., 2004, 126, 8368–8369 CrossRef CAS.
- P. Kar, H. Wen, H. Z. Li, S. D. Minteer and S. C. Barton, J. Electrochem. Soc., 2011, 158, B580–B586 CrossRef CAS.
- M. J. Moehlenbrock, T. K. Toby, A. Waheed and S. D. Minteer, J. Am. Chem. Soc., 2010, 132, 6288–6289 CrossRef CAS.
- R. A. Rincon, C. Lau, H. R. Luckarift, K. E. Garcia, E. Adkins, G. R. Johnson and P. Atanassov, Biosens. Bioelectron., 2011, 27, 132–136 CrossRef CAS.
- D. Bhatnagar, S. A. Xu, C. Fischer, R. L. Arechederra and S. D. Minteer, Phys. Chem. Chem. Phys., 2011, 13, 86–92 RSC.
- A. Meehan, H. Gao and Z. Lewandowski, IEEE Trans. Power Electron., 2011, 26, 176–181 CrossRef.
- I. A. Ieropoulos, J. Greenman, C. Melhuish and I. Horsfield, ChemSusChem, 2012, 5, 1020–1026 CrossRef CAS.
- S. Wilkinson, Auton. Rob., 2000, 9, 99–111 CrossRef.
- A. Heller, Phys. Chem. Chem. Phys., 2004, 6, 209–216 RSC.
- S. C. Barton, J. Gallaway and P. Atanassov, Chem. Rev., 2004, 104, 4867–4886 CrossRef CAS.
- R. F. Drake, B. K. Kusserow, S. Messinger and S. Matsuda, Trans. - Am. Soc. Artif. Intern. Organs, 1970, 16, 199–205 CAS.
- T. Sharma, Y. Hu, M. Stoller, M. Feldman, R. S. Ruoff, M. Ferrari and X. Zhang, Lab Chip, 2011, 11, 2460–2465 RSC.
- S. Kerzenmacher, J. Ducrée, R. Zengerle and F. von Stetten, J. Power Sources, 2008, 182, 1–17 CrossRef CAS.
- P. Cinquin, C. Gondran, F. Giroud, S. Mazabrard, A. Pellissier, F. Boucher, J.-P. Alcaraz, K. Gorgy, F. Lenouvel, S. Mathé, P. Porcu and S. Cosnier, PLoS One, 2010, 5, e10476 Search PubMed.
- T. Miyake, K. Haneda, N. Nagai, Y. Yatagawa, H. Onami, S. Yoshino, T. Abe and M. Nishizawa, Energy Environ. Sci., 2011, 4, 5008–5012 CAS.
- M. Rasmussen, R. E. Ritzmann, I. Lee, A. J. Pollack and D. Scherson, J. Am. Chem. Soc., 2012, 134, 1458–1460 CrossRef CAS.
- L. Halámková, J. Halámek, V. Bocharova, A. Szczupak, L. Alfonta and E. Katz, J. Am. Chem. Soc., 2012, 134, 5040–5043 CrossRef.
- A. Szczupak, J. Halámek, L. Halámková, V. Bocharova, L. Alfonta and E. Katz, Energy Environ. Sci., 2012, 5, 8891–8895 CAS.
- Preliminary video highlight, G4 TV, USA 2012: http://www.g4tv.com/videos/58223/how-to-harvest-electricity-from-lobsters/.
- B. I. Rapoport, J. T. Kedzierski and R. Sarpeshkar, PLoS One, 2012, 7, e38436 CAS.
- N. Mano, F. Mao, W. Shin, T. Chen and A. Heller, Chem. Commun., 2003, 518–519 RSC.
- I. Ieropoulos, J. Greenman and C. Melhuish, Bioelectrochemistry, 2010, 78, 44–50 CrossRef CAS.
- I. Ieropoulos, J. Greenman and C. Melhuish, Int. J. Energy Res., 2008, 32, 1228–1240 CrossRef CAS.
- P. Aelterman, K. Rabaey, H. T. Pham, N. Boon and W. Verstraete, Environ. Sci. Technol., 2006, 40, 3388–3394 CrossRef CAS.
- I. Ieropoulos, J. Greenman, C. Melhuish and I. Horsfield, Proc. of the Alife XII Conference, Odense, Denmark, 2010, pp. 733–740 Search PubMed.
- T. Hanashi, T. Yamazaki, W. Tsugawa, S. Ferri, D. Nakayama, M. Tomiyama, K. Ikebukuro and K. Sode, Biosens. Bioelectron., 2009, 24, 1837–1842 CrossRef CAS.
- T. Hanashi, T. Yamazaki, W. Tsugawa, K. Ikebukuro and K. Sode, J. Diabetes Sci. Technol., 2011, 5, 1030–1035 Search PubMed.
- W. Gellett, M. Kesmez, J. Schumacher, N. Akers and S. D. Minteer, Electroanalysis, 2010, 22, 727–731 CrossRef CAS.
- K. A. Ellenbogen, G. N. Kay, C.-P. Lau and B. L. Wilkoff, Clinical Cardiac Pacing, Defibrillation, and Resynchronization Therapy, Elsevier, Philadelphia, PA, 4th edn, 2011 Search PubMed.
- D. Bhatia, S. Bairagi, S. Goel and M. Jangra, J. Pharm. BioAllied Sci., 2010, 2, 51–54 CrossRef.
- U. Schröder, Angew. Chem., Int. Ed., 2012, 51, 2–5 CrossRef.
- A. M. Crespi, S. K. Somdahl, C. L. Schmidt and P. M. Skarstad, J. Power Sources, 2001, 96, 33–38 CrossRef CAS.
- E. S. Chang, Integr. Comp. Biol., 2005, 45, 43–50 CrossRef CAS.
- S. Lorenzon, P. G. Giulianini, M. Martinis and E. A. Ferrero, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 2007, 147, 94–102 CrossRef CAS.
- CRC Handbook of Chemistry and Physics, ed. D. R. Lide, CRC Press, Boca Raton, 86th edn, 2005 Search PubMed.
- C. W. N. Villarrubia, R. A. Rinćon, V. K. Radhakrishnan, V. Davis and P. Atanassov, ACS Appl. Mater. Interfaces, 2011, 3, 2402–2409 Search PubMed.
- A. Zebda, C. Gondran, A. Le Goff, M. Holzinger, P. Cinquin and S. Cosnier, Nat. Commun., 2011, 2, 370 CrossRef.
- L. Hussei, S. Rubenwolf, F. von Stetten, G. Urban, R. Zengerle, M. Krueger and S. Kerzenmacher, Biosens. Bioelectron., 2011, 26, 4133–4138 CrossRef.
- G. Strack, H. R. Luckarift, R. Nichols, K. Cozart, E. Katz and G. R. Johnson, Chem. Commun., 2011, 47, 7662–7664 RSC.
- D. L. Nelson, A. L. Lehninger and M. M. Cox, Lehninger - Principles of Biochemistry, W.H. Freeman and Company, New York, 2008 Search PubMed.
- P. Vennemann, R. Lindken and J. Westerweel, Exp. Fluids, 2007, 42, 495–511 CrossRef.
- S. S. Barold, R. X. Stroobandt and A. F. Sinnaeve, Cardiac Pacemakers and Resynchronization Step-by-Step, Wiley-Blackwell, Chichester, UK, 2010, p. 23 Search PubMed.
- Manufacturer Specifications - Affinity DR 5330, St Jude Medical: http://www.medwow.com/med/pacemaker/st-jude-medical/affinity-dr-5330/7305.model-spec.
- The Bionic Human: Health Promotion for People with Implanted Prosthetic Devices, ed. F. E. Johnson and K. S. Virgo, Humana Press, Totowa, NJ, 2006 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and additional illustrating photos. See DOI: 10.1039/c2ee23209j |
| This journal is © The Royal Society of Chemistry 2013 |
