Selective dehydrogenation of aromatic alcohols photocatalyzed by Pd-deposited CdS–TiO2 in aqueous solution using visible light†
Shinya
Higashimoto
*a,
Yoshimi
Tanaka
a,
Ryo
Ishikawa
a,
Shoko
Hasegawa
a,
Masashi
Azuma
a,
Hiroyoshi
Ohue
a and
Yoshihisa
Sakata
b
aCollege of Engineering, Osaka Institute of Technology, 5-16-1 Omiya, Asahi-ku, Osaka 535-8585, Japan. E-mail: higashimoto@chem.oit.ac.jp; Fax: +81 6 6957 2135; Tel: +81 6 6954 4283
bGraduate School of Science and Engineering, Yamaguchi University, 2-16-1 Tokiwadai, Ube 755-8611, Japan
First published on 26th October 2012
Abstract
A CdS/TiO2 photocatalyst modified with a Pd co-catalyst was applied to a photocatalytic system for the selective dehydrogenation of benzyl alcohol to benzaldehyde with high selectivity (>99%) accompanied by the formation of H2 in aqueous solution under visible-light irradiation.
The selective oxidation of alcohols to carbonyl compounds is one of the most crucial reactions for scientific and industrial applications. Particularly, heterogeneously catalyzed alcohol oxidations using molecular oxygen (O2) as the oxidant are attractive.1
In recent years, the selective photocatalytic oxidation of alcohol by O2 on titanium(IV) dioxide (TiO2) has been extensively studied in organic solvents,2 gas phases3 and aqueous solutions4 under UV-light irradiation. These photocatalytic systems can utilize only a small percentage of solar irradiation. Therefore, the development of a photocatalyst which works under visible light irradiation is strongly desired. We have previously reported that pure TiO2 exhibits the selective photocatalytic oxidation of benzylic alcohols to the corresponding aldehydes by O2 in acetonitrile solutions using visible light.5 However, this photocatalytic system does not work in aqueous solutions. Such green processes which work in water have been the focus of much attention since water is an abundant and eco-friendly solvent. It has been reported that benzyl alcohol in aqueous solutions suspended with Au/TiO2 or Au/CeO2 was converted to benzaldehyde by O2 under visible light irradiation.6
On the other hand, various photocatalysts which work in water splitting to produce H2 from aqueous methanol solutions have been extensively investigated,7 while oxidative products such as aldehyde, carboxylic acid and CO2 are formed unselectively. The oxidant-free dehydrogenation of alcohols to carbonyl compounds and hydrogen by thermal catalysts at high temperature (>373 K) has also received considerable attention.8 However, more fundamental research focused on the selective dehydrogenation of alcohol to carbonyl compounds using visible light-responsive photocatalysts seems necessary.
In this paper, we have studied the selective dehydrogenation of aromatic alcohols to the corresponding carbonyl compounds and hydrogen on TiO2-supported CdS (CdS/TiO2) photocatalysts in aqueous solutions at 298 K under an inert gas atmosphere using visible light.
Fig. 1 shows the reaction time profiles for the dehydrogenation of benzyl alcohol in aqueous solution suspended with Pd (0.4 wt%)/CdS (15 wt%)–TiO2 (hereinafter referred to as 0.4Pd/15CdS–TiO2) under visible light irradiation. This reaction does not proceed without Pd/CdS–TiO2 or irradiation. However, under irradiation, a decrease in the amount of benzyl alcohol was observed with an increase in the irradiation time, while an increase in benzaldehyde and hydrogen was observed. Moreover, neither oxidative products such as benzoic acid or CO2, nor coupling products such as benzoin were formed in this system. The yield of benzaldehyde reached ca. 98%, while that of hydrogen was >80% after photo-irradiation for 6 h, as shown in Fig. 1. Theoretically, the dehydrogenation of benzyl alcohol leads to the formation of benzaldehyde and H2 with an equivalent ratio. However, in fact, the ratio of H2 to benzaldehyde was not observed to be 1 since H2 and/or H-atoms may be partially adsorbed on the catalyst. Furthermore, this photo-assisted reaction was performed in a saturated solution of benzyl alcohol (0.30 mmol) suspended with 0.4Pd/15CdS–TiO2 (4 mg) under visible light irradiation for 48 h. As a consequence, the yields of benzaldehyde reached 70% and the turnover number (TON), i.e., total number of photo-formed benzaldehyde per Cd atom in 0.4Pd/15CdS–TiO2 exceeded 50, suggesting that this reaction system, in fact, takes place photocatalytically. It is known that the CdS photocatalyst photo-corrodes during the photochemical oxidation of water.9 However, the presence of benzyl alcohol as a hole scavenger was observed to retard the loss of CdS into the solution to less than 1%, as confirmed by ICP analysis. The anodic photo-corrosion of CdS was, thus, successfully suppressed.
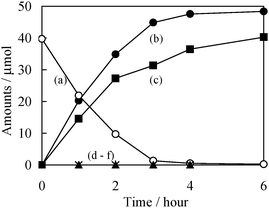 | ||
| Fig. 1 Reaction time profiles for the photocatalytic dehydrogenation of benzyl alcohol (50 μmol) on 0.4Pd/15CdS–TiO2 (50 mg) under visible light irradiation. Amounts of (a) benzyl alcohol, (b) benzaldehyde, (c) H2, (d) benzoic acid, (e) CO2, and (f) benzoin. The initial amount of benzyl alcohol (ca. 40 μmol) shown in (a) is due to equilibrium adsorption on the catalyst for 1 h under dark conditions. | ||
The photocatalytic activities of Pd-deposited CdS–TiO2 were optimized by examining the effect of the amount of CdS and Pd loaded (Fig. S1 and S2, ESI†). From these results, the photocatalytic activity was found to depend on the loadings of the CdS and Pd species, which were optimized with 0.4Pd/15CdS–TiO2. In order to understand the role of the components of the photocatalyst, various kinds of experiments were carried out and the results are listed in Table 1. Neither pure TiO2 nor the Pd-deposited TiO2 showed any activity for the reactions (runs 1 and 2). On the other hand, benzyl alcohol was converted to benzaldehyde with high selectivity (>99%) accompanied by H2 on the pure CdS photocatalyst (run 3), and its activity was enhanced remarkably by modification with the Pd species (run 4). Furthermore, it was observed that the CdS (15 wt%) dispersed on TiO2 showed higher activity than the pure CdS (run 5), and the activity of 15CdS–TiO2 was also significantly enhanced by the deposition of the Pd species (run 6). From these results, it was found that the dispersibility of CdS on TiO2 and the presence of the Pd species may work to favor improvement of the photocatalytic activities.
| Run | Catalysta | H2/μmol | Benzaldehyde /μmol | Sel.b/% |
|---|---|---|---|---|
| a Reaction conditions: catalyst (7.5 mg of CdS); benzyl alcohol (50 μmol); reaction time (4 h). b The selectivity to benzaldehyde was calculated by 100 × (amounts of benzaldehyde)/(amounts of benzaldehyde, carboxylic acid, CO2 and benzoin). | ||||
| 1 | TiO2 | 0 | 0 | — |
| 2 | 0.4Pd–TiO2 | 0 | 0 | — |
| 3 | CdS | 0.3 | 0.6 | >99 |
| 4 | 0.4Pd/CdS | 2.1 | 3.3 | >99 |
| 5 | 15CdS–TiO2 | 2.0 | 4.5 | >99 |
| 6 | 0.4Pd/15CdS–TiO2 | 36 | 48 | >99 |
Here, the dependence of the photo-irradiation wavelength on the activity of the dehydrogenation of benzyl alcohol was investigated in order to identify the photo-responsible center of the photocatalyst. Fig. 2 shows the UV-Vis absorption spectra of the CdS–TiO2 composites in the presence and absence of the Pd species, and the apparent quantum yields (AQY) for the dehydrogenation of benzyl alcohol on Pd/CdS–TiO2 under irradiation of monochromatic light. As can be seen in Fig. 2(a and b), 15CdS–TiO2 exhibits visible light absorption due to CdS, while 0.4Pd/15CdS–TiO2 exhibits absorption due to the CdS and Pd species. On the other hand, the AQY for 0.4Pd/15CdS–TiO2 was determined as follows: 3.5% at 480 nm, 1.4% at 540 nm, and ca. 0% above 600 nm. The AQY plots, thus, form the action spectrum for the photocatalytic reaction on 0.4Pd/15CdS–TiO2, as shown in Fig. 2(c). These results show the action spectrum to be in good agreement with the photo-absorption of 15CdS–TiO2. It could, thus, be concluded that CdS works as the photo-responsible center for the visible-light induced photocatalytic dehydrogenation of benzyl alcohol, while the Pd species work as the co-catalyst to improve the photocatalytic activities.
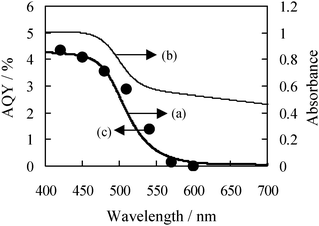 | ||
| Fig. 2 UV-Vis spectra of (a) 15CdS–TiO2, (b) 0.4Pd/15CdS–TiO2, and (c) AQY of the photocatalytic reactions on 0.4Pd/15CdS–TiO2. | ||
In order to characterize the photocatalysts, XRD and XPS analyses of Pd/CdS–TiO2 were performed. XRD analysis of 0.4Pd/15CdS–TiO2 confirmed that CdS and TiO2 form cubic and anatase structures, respectively, and no other phases due to the Pd species were detected (Fig. S3, ESI†). From XPS analysis of 0.4Pd/15CdS–TiO2, two kinds of Pd 3d5/2 peaks were observed at 336.3 and 335.3 eV, which were attributed to Pd(II) and Pd(0), respectively (Fig. S4, ESI†).10 Thus, the two kinds of Pd states in 0.4Pd/15CdS–TiO2 can be assigned as follows: one is Pd(II) probably originating from PdS and the other is metallic Pd. A plausible mechanism is proposed as follows: a hole–electron separation is generated on CdS under visible-light irradiation. Subsequently, the photo-produced holes efficiently transfer to the PdS sites,11 leading to the oxidation of benzyl alcohol to benzaldehyde, while the electrons participate in the reduction of protons to H2 on the metallic Pd sites. Details on the location of the Pd species and the effect of these species on the activities are currently under investigation.
The photocatalytic activities for various kinds of aromatic alcohol were also investigated and the results are shown in Table 2. It was observed that not only benzyl alcohol but also its derivatives substituted by electron-donating groups (p-OH, m-OCH3 and p-CH3) and an electron-withdrawing group (p-Cl) were converted to the corresponding carbonyl compounds with high selectivity (>99%), accompanied by H2 (Table 2, runs 2–5). Furthermore, 1-phenylethanol was also converted to acetophenone with high selectivity (>99%) (Table 2, run 6). These results indicate that this photocatalytic system is applicable to highly selective conversions of various benzylic alcohols on Pd-deposited CdS–TiO2 under visible light irradiation.
| Run | R1 | R2 | H2/μmol | Carbonyl compound/μmol | Conversiona/% | Selectivityb/% |
|---|---|---|---|---|---|---|
| a The conversion was calculated by 100 × (amounts of benzaldehyde)/(amounts of benzyl alcohol added). b The selectivity to benzaldehyde was calculated by 100 × (amounts of benzaldehyde)/(total amounts of benzaldehyde, carboxylic acid, CO2 and benzoin). | ||||||
| 1 | H | H | 36 | 48 | 96 | >99 |
| 2 | H | OH | 32 | 42 | 84 | >99 |
| 3 | H | OCH3 | 46 | 49 | 98 | >99 |
| 4 | H | CH3 | 43 | 48 | 96 | >99 |
| 5 | H | Cl | 44 | 47 | 94 | >99 |
| 6 | CH3 | H | 46 | 40 | 80 | >99 |
This work is the first to achieve the selective photocatalytic dehydrogenation of aromatic alcohol in aqueous solution using visible light at 298 K under unaerated conditions. The CdS–TiO2 photocatalyst was found to induce the highly selective formation of benzaldehyde from benzyl alcohol, and its activity was significantly enhanced by the presence of the Pd co-catalyst. These preliminary results are promising for various kinds of selective photocatalytic dehydrogenations in organic synthesis using visible light.
Experimental section
Materials
Commercially available TiO2 powder (100% anatase; BET surface area: 320 m2 g−1, ST-01, Ishihara Co., Ltd.), benzyl alcohol and para-benzylic alcohols substituted by –OCH3, –OH, –CH3, –Cl and phenyl ethanol, and their corresponding carbonyl compounds, purchased from Wako Pure Chemical Industries, Ltd., were used as received.Preparation of photocatalysts
The CdS-supported TiO2 (CdS–TiO2) was prepared by impregnation of TiO2 with aq. Cd(NO3)2 at the desired concentrations, followed by stirring in aq. Na2S (0.1 M) for 24 h at 298 K. Pure CdS was prepared as follows: 50 mL of aq. Cd(NO3)2 (0.1 M) was added to 50 mL of aq. Na2S (0.1 M). The solids were washed in distilled water, filtrated and dried at 298 K for 12 h. Pd deposition on the catalysts (1.0 g for each) was performed in an aqueous solution (50 mL) of methanol (0.5 mL) and the desired amounts of PdCl2 under UV-light (λmax = 365 nm, 0.8 mW cm−2) from black light for 4 h under N2 bubbling.Photocatalytic reactions
Photocatalytic reactions were performed in a Pyrex cell (20 mL in volume) with an aqueous suspension (10 mL) of the photocatalyst (50 mg) involving 50 μmol of benzylic alcohol under an argon (1 atm) atmosphere by blue light emitted from a LED lamp (λmax = 460 nm, ca. 10 mW cm−2). Photo-irradiation was performed after confirming the equilibrium adsorption of benzyl alcohol for 1 h under dark conditions. After the reactions, the catalysts were immediately separated from the suspension by filtration through a 0.20 μm membrane filter (Dismic-25, Advantec). The solution was then analyzed by high performance liquid chromatography: HPLC (Shimadzu LC10ATVP, UV-Vis detector, column: Chemcopak, mobile phase: a mixture of acetonitrile and 1.0% aqueous formic acid), while the gas phase (H2) was analyzed by GC (TCD; Model-802, Ohkura; column: Molecular sieve 5A).Apparent quantum yields
The apparent quantum yields (AQY) were measured in a quartz cell using a 100 W xenon lamp (Lax Cute II, Asahi Spectra Co., Ltd.) through appropriate band path filters with a FWHM of 10 ± 2 nm. The 0.4Pd/15CdS–TiO2 photocatalyst was suspended in an aqueous solution (2 mL) of benzyl alcohol (10 μmol). The apparent quantum yield (Φ) at each centered wavelength of light was calculated using the following equation (eqn (1)): | (1) |
Characterizations
Analyses of the optical properties of the samples were carried out in diffused reflectance mode using a UV-Vis spectrophotometer (UV-3100PC, Shimadzu). The UV-Vis reflectance spectra (R%) of the solids were obtained in diffused reflectance mode while these spectra were converted to absorbance (A) by the following equation: A = log (100/R). Powder X-ray diffraction (XRD) patterns were obtained with a RIGAKU RINT2000 diffractometer using Cu Kα radiation (λ = 1.5417 Å). The oxidation states of palladium were analyzed by X-ray photoelectron spectroscopy (XPS, ESCA 3200, Shimadzu). The binding energies were calibrated by using the containment carbon (C 1s = 284.6 eV). The loading amounts of Cd and Pd were analyzed by a multi-type ICP emission spectrometer (ICPE-9000, Shimadzu).Notes and references
- T. Mallat and A. Baiker, Chem. Rev., 2004, 104, 3037 CrossRef CAS; G. T. Brink, I. W. C. E. Arends and R. A. Sheldon, Science, 2000, 287, 1636 CrossRef; A. Abad, C. Almela, A. Corma and H. Garcia, Chem. Commun., 2006, 3178 RSC; T. Matsushita, K. Ebitani and K. Kaneda, Chem. Commun., 1999, 265 RSC; K. Yamaguchi and N. Mizuno, Angew. Chem., Int. Ed., 2002, 41, 4538 CrossRef; H. Tsunoyama, H. Sakurai, Y. Negishi and T. Tsukuda, J. Am. Chem. Soc., 2005, 127, 9374 CrossRef; Y. H. Ng, S. Ikeda, T. Harada, Y. Morita and M. Matsumura, Chem. Commun., 2008, 3181 RSC; B. Feng, Z. Hou, H. Yang, X. Wang, Y. Hu, H. Li, Y. Qiao, X. Zhao and Q. Huang, Langmuir, 2010, 26, 2505 CrossRef.
- Q. Wang, M. Zhang, C. Chen, W. Ma and J. Zhao, Angew. Chem., Int. Ed., 2010, 49, 7976 CrossRef CAS; M. Zhang, Q. Wang, C. Chen, L. Zang, W. Ma and J. Zhao, Angew. Chem., Int. Ed., 2009, 48, 6081 CrossRef; O. S. Mohamed, A. E. M. Gaber and A. A. Abdel-Wahab, J. Photochem. Photobiol., A, 2002, 148, 205 CrossRef; S. Farhadi, M. Afshari, M. Maleki and Z. Badazadeh, Tetrahedron Lett., 2005, 46, 8483 CrossRef; A. Z. G. G. Fattima, N. A. Ahmed and H. H. Falah, Desalination, 2009, 209, 342 Search PubMed; L. Samiolo, M. Valigi, D. Gazzoli and R. Amadelli, Electrochim. Acta, 2010, 55, 7788 CrossRef.
- U. R. Pillai and E. Sahle-Demessie, J. Catal., 2002, 211, 434 CAS.
- G. Palmisano, E. García-López, G. Marcì, V. Loddo, S. Yurdakal, V. Augugliaro and L. Palmisano, Chem. Commun., 2010, 46, 7074 RSC; S. Yurdakal, G. Palmisano, V. Loddo, O. Alagoez, V. Augugliaro and L. Palmisano, Green Chem., 2009, 11, 510 RSC; V. Augugliaro, V. Loddo, M. J. Lopez-Munoz, C. Marquez-Alvarez, G. Palmisano, L. Palmisano and S. Yurdakal, Photochem. Photobiol. Sci., 2009, 8, 663 Search PubMed; S. Yurdakal, G. Palmisano, V. Loddo, V. Augugliaro and L. Palmisano, J. Am. Chem. Soc., 2008, 130, 1568 CrossRef CAS.
- S. Higashimoto, N. Kitao, N. Yoshida, T. Sakura, M. Azuma, H. Ohue and Y. Sakata, J. Catal., 2009, 266, 279 CrossRef CAS; S. Higashimoto, K. Okada, T. Morisugi, M. Azuma, H. Ohue, T.-H. Kim, M. Matsuoka and M. Anpo, Top. Catal., 2010, 53, 578 CrossRef; S. Higashimoto, N. Suetsugu, M. Azuma, H. Ohue and Y. Sakata, J. Catal., 2010, 274, 76 CrossRef; S. Higashimoto, K. Okada, M. Azuma, H. Ohue, T. Terai and Y. Sakata, RSC Adv., 2012, 2, 669 RSC.
- S. Naya, A. Inoue and H. Tada, J. Am. Chem. Soc., 2010, 132, 6292 CrossRef CAS; A. Tanaka, K. Hashimoto and H. Kominami, Chem. Commun., 2011, 47, 10446 RSC.
- T. Kawai and T. Sakata, J. Chem. Soc., Chem. Commun., 1980, 694 RSC; A. Kudo and Y. Miseki, Chem. Soc. Rev., 2009, 38, 253 RSC.
- A. Friedrich and S. Schneider, ChemCatChem, 2009, 1, 72 CrossRef CAS; W. Fang, Q. Zhang, J. Chen, W. Deng and Y. Wang, Chem. Commun., 2010, 46, 1547 RSC; T. Mitsudome, Y. Mikami, H. Funai, T. Mizugaki, K. Jitsukawa and K. Kaneda, Angew. Chem., Int. Ed., 2008, 47, 138 CrossRef; T. Mitsudome, Y. Mikami, K. Ebata, T. Mizugaki, K. Jitsukawa and K. Kaneda, Chem. Commun., 2008, 4804 RSC.
- H. Kisch, M. Hopfner, in Electron transfer in chemistry, ed. V. Balzani, Wiley-VCH, Weinheim, 2001, vol. 4, p. 232 Search PubMed.
- Handbook of X-ray Photoelectron Spectroscopy, ed. C. D. Wagner, W. M. Riggs, L. E. Davis, J. F. Moulder and G. E.Mullenberg, Perkin-Elmer Corp., Eden Prairie, MN, USA, 1979 Search PubMed.
- H. Yan, J. Yang, G. Ma, G. Wu, X. Zong, Z. Lei, J. Shi and C. Li, J. Catal., 2009, 266, 165 CrossRef CAS.
- Y. Ohko, D. A. Tryk, K. Hashimoto and A. Fujishima, J. Phys. Chem. B, 1998, 102, 2699 CrossRef CAS; A. Sclafani, L. Palmisano and M. Schiavello, J. Phys. Chem., 1990, 94, 829 CrossRef; M. R. Hoffmann, S. T. Martin, W. Choi and W. Bahnemann, Chem. Rev., 1995, 95, 69 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2cy20607b |
| This journal is © The Royal Society of Chemistry 2013 |