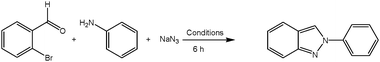
CuII–hydrotalcite catalyzed one-pot three component synthesis of 2H-indazoles by consecutive condensation, C–N and N–N bond formations†
Avvari N.
Prasad
,
Rapelli
Srinivas
and
Benjaram M.
Reddy
*
Inorganic and Physical Chemistry, CSIR - Indian Institute of Chemical Technology, Uppal Road, Hyderabad, 500607, India. E-mail: bmreddy@iict.res.in; mreddyb@yahoo.com; Fax: +91 40 2716 0921; Tel: +91 40 27191714
First published on 16th October 2012
Abstract
An efficient and straightforward synthesis of 2H-indazoles is achieved from 2-bromobenzaldehydes, primary amines and sodium azide through consecutive condensation, C–N and N–N bond formations, catalyzed by a novel heterogeneous CuII–HT catalyst. The recoverable heterogeneous CuII–HT catalyst exhibited an impressive activity for the title reaction without any additives (expensive ligands, etc.). Heterocyclization proceeds through C–N and N–N bond formation, which is the key step to deliver the desired 2H-indazole scaffold. A series of structurally diverse 2H-indazoles were prepared in good to excellent yields from easily accessible starting materials by employing this protocol.
1. Introduction
Over the past decades, there has been great interest in the synthesis of heterocyclic compounds. Among the numerous heterocycles, the indazole ring system has been receiving significant attention due to its pronounced biological activity. Captivatingly, in the field of drug discovery, the indazole scaffolds received a lot of interest because they act as efficient bioisosteres of indoles and benzimidazoles,1,2 and constitute the key subunit in various drug substances with a broad range of biological properties, including anti-inflammatory, antidepressant,3 antitumor,4 HIV protease inhibition,5 antimicrobial6 and contraceptive activities.7 In heterocyclic chemistry, the indazole unit has been recognized as a “privileged structure”, and it is an important pharmacophore in medicinal chemistry.8–16Although a number of methods were known for the preparation of indazoles, most of the existing approaches are targeted to 1H-indazoles or mixture of 1H- and 2H-indazoles.17 In addition, 2H-indazoles are less studied as compared to 1H-indazoles due to the difficulty in their preparation. Hence, the formation of 2H-indazoles still remains a challenging task.18 Recently, several promising synthetic routes have been devoted towards 2H-indazoles, such as (1) 2-halophenyl acetylenes with hydrazines by using a Pd-catalyst and palladium-catalyzed intramolecular amination of the corresponding N-aryl-N-(o-bromobenzyl) hydrazines,19 (2) Fe-catalyzed intramolecular N–N bond formation from aryl azides,20 (3) reaction of highly functionalized zinc reagents with aryldiazonium salts,21 (4) [3+2] dipolar cycloaddition of arynes and sydnones,8 (5) reaction of sodium hydride or DBU catalyzed 2-nitrobenzyl triphenylphosphonium bromide with aryl isocyanates,22 and (6) Baylis–Hillman adducts of 2-cyclohexen-1-one by using DDQ oxidation,23 and also the feasibility of further elaboration of 2H-indazoles using Suzuki–Miyaura and Sonogashira couplings.24
However, most of the existing methods exhibit several drawbacks, such as the formation of regioisomers, requirement of additives (expensive phosphine ligands, etc.) and low functional group tolerance, and also these methods require several steps to synthesize the starting materials. Furthermore, most of these methods are homogeneous in nature. Therefore, there is a need for novel, proficient and selective approaches to synthesize 2H-indazoles by using readily available starting materials or precursors. To the best of our knowledge, there is only one instance of the multicomponent reaction (MCR) for obtaining 2H-indazoles through a different approach.25 As part of our on-going research programme, stimulated by the development of MCRs by using heterogeneous catalysts, the present investigation was undertaken. The reported novel heterogeneous Cu–Al hydrotalcites (CuII–HTs) offer numerous advantages, such as being inexpensive and recoverable, and having a simple workup procedure.
2. Experimental section
2.1 Preparation of CuII–hydrotalcite (CuII–HT) catalysts
A series of CuII–HT catalysts were synthesized by adopting a coprecipitation method. In a typical procedure, a mixture solution containing the requisite quantities of Cu(NO3)2·6H2O and Al(NO3)3·9H2O (Cu/Al mole ratios = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2.5
1, 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 4
1, and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; all chemicals are Fluka, AR grade) precursors were dissolved separately and mixed together. A base mixture solution (1
1; all chemicals are Fluka, AR grade) precursors were dissolved separately and mixed together. A base mixture solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume of 2 M NaOH and 1 M Na2CO3) was also prepared separately in distilled water. Both mixture solutions were added slowly and simultaneously to a beaker containing double distilled water with vigorous stirring until the pH of the solution reached ∼9. The pH was maintained constantly throughout the addition. Later, the obtained precipitates were filtered off, washed thoroughly with distilled water until free from anion impurities (pH = 7) and then oven dried at 100 °C for 12 h. The synthesized catalysts were characterized by X-ray diffraction, FT-infrared, thermogravimetry and other techniques. There results could be found in the ESI.†
1 volume of 2 M NaOH and 1 M Na2CO3) was also prepared separately in distilled water. Both mixture solutions were added slowly and simultaneously to a beaker containing double distilled water with vigorous stirring until the pH of the solution reached ∼9. The pH was maintained constantly throughout the addition. Later, the obtained precipitates were filtered off, washed thoroughly with distilled water until free from anion impurities (pH = 7) and then oven dried at 100 °C for 12 h. The synthesized catalysts were characterized by X-ray diffraction, FT-infrared, thermogravimetry and other techniques. There results could be found in the ESI.†
2.2 Synthesis of 2H-indazoles
All chemicals employed in this study were commercially available and used without further purification. A mixture of 2-bromobenzaldehyde (1.5 mmol), primary amine (1.8 mmol), sodium azide (2 mmol) and catalytic amounts of CuII–HT (20 mg) in dimethylsulfoxide (DMSO) (1.5 mL) were stirred at 120 °C temperature for an appropriate time. After completion of the reaction, as indicated by thin layer chromatography (TLC), the reaction mixture was centrifuged to separate the catalyst. The reaction mixture was poured into EtOAc (30 mL) and washed with distilled water (3 × 20 mL), and the combined layers were dried over anhydrous Na2SO4, concentrated in vacuum and purified by column chromatography on 60–120 mesh silica gel, using ethyl acetate and hexane as the eluent (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) to afford the pure 2H-indazoles.
9) to afford the pure 2H-indazoles.
3. Results and discussion
From an ecological perspective, the MCRs arose as powerful strategies that allow multiple bond formation events taking place in a single vessel, and exhibit economy of steps and often atom economy, selectivity and low levels of by-product generation.26 Heterocyclic frameworks were well constructed by using these MCRs from readily available starting materials. Interestingly, the CuII–HTs exhibited good catalytic activity for various organic transformation reactions.27 Very recently, we have also reported the one-pot MCR for the synthesis of β-hydroxy 1,4-disubstituted 1,2,3-triazoles by using the CuII–HT heterogeneous catalyst.28 Herein, we report an MCR of 2-bromobenzaldehydes, primary amines and sodium azide with catalytic amounts of CuII–HT to produce 2H-indazoles through consecutive condensation, C–N and N–N bond formations (Scheme 1). In this the C–N and N–N bond formation is the crucial step for heterocyclization to deliver the 2H-indazole scaffold. | ||
| Scheme 1 Synthesis of 2H-indazoles using the CuII–HT catalyst. | ||
In the initial experiments, the reaction of 2-bromobenzaldehyde (1.5 mmol), aniline (1.8 mmol) and sodium azide (2 mmol) was investigated as the model reaction for optimization of reaction parameters such as, mole ratio of CuII–HTs, solvent and the temperature to synthesize 2H-indazoles; and the obtained results are summarized in Table 1. A preliminary reaction was also carried out to identify the best catalyst system using DMSO as the reaction medium at 120 °C temperature with catalytic amounts of CuII–HTs containing various Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al mole ratios. As can be seen from Table 1, the 3
Al mole ratios. As can be seen from Table 1, the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole ratio CuII–HT catalyst exhibited an impressive activity and enriched regioselectivity towards the desired 2H-indazole (Table 1, entry 5). Gratifyingly, the CuII–HTs with 4
1 mole ratio CuII–HT catalyst exhibited an impressive activity and enriched regioselectivity towards the desired 2H-indazole (Table 1, entry 5). Gratifyingly, the CuII–HTs with 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2.5
1 and 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Cu
1 (Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al) mole ratios also showed better yields of 82% and 76%, respectively (Table 1, entries 2 and 4). However, the yields were lower than that of the 3
Al) mole ratios also showed better yields of 82% and 76%, respectively (Table 1, entries 2 and 4). However, the yields were lower than that of the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole ratio CuII–HT catalyst. Trace amounts of products including some by-products and longer reaction times were noticed in the absence of the CuII–HT catalyst (Table 1, entry 6), and also we observed different spots in the TLC in the presence of catalyst under solvent-free conditions (Table 1, entry13).
1 mole ratio CuII–HT catalyst. Trace amounts of products including some by-products and longer reaction times were noticed in the absence of the CuII–HT catalyst (Table 1, entry 6), and also we observed different spots in the TLC in the presence of catalyst under solvent-free conditions (Table 1, entry13).
| Entry | Catalyst mole ratio (Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al) Al) |
Solvent | Temp. (°C) | Yieldb (%) |
|---|---|---|---|---|
| a Reagents and reaction conditions: 2-bromobenzaldehyde (1.5 mmol), aniline (1.8 mmol), sodium azide (2 mmol), catalyst (20 mg) and solvent (1.5 mL); unless otherwise mentioned, stirred at 120 °C temperature for 6 h. b Yields of isolated products. c Catalyst-free condition, stirred at 120 °C temperature for more than 24 h. d Solvent-free condition, stirred at 120 °C temperature for more than 24 h. | ||||
| 1 | CuII–HT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
DMSO | 120 | 45 |
| 2 | CuII–HT (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
DMSO | 120 | 82 |
| 3 | CuII–HT (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
DMSO | 120 | 62 |
| 4 | CuII–HT (2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
DMSO | 120 | 76 |
| 5 | CuII–HT (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
DMSO | 120 | 91 |
| 6 | — | DMSO | 120 | —c |
| 7 | CuII–HT (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
DMF | 120 | 78 |
| 8 | CuII–HT (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Methanol | 65 | 72 |
| 9 | CuII–HT (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Dichloromethane | 40 | 15 |
| 10 | CuII–HT (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Acetonitrile | 80 | 68 |
| 11 | CuII–HT (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
o-Xylene | 120 | 42 |
| 12 | CuII–HT (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Tetrahydrofuran | 65 | 64 |
| 13 | CuII–HT (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
— | 120 | —d |
| 14 | CuII–HT (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Toluene | 110 | 58 |
| 15 | CuII–HT (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Benzene | 80 | 28 |
| 16 | CuII–HT (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Water | 100 | 71 |
After identifying the most efficient catalyst (Table 1), we further screened different solvents in order to enhance the reaction rates. Among the various solvents we examined, the reaction performed well in DMSO, which was found to be the most appropriate solvent for synthesis of desired 2H-indazoles with excellent yields (Table 1, entry 5). We have also observed better results with various solvents namely, dimethylformamide (DMF), methanol (MeOH), acetonitrile, tetrahydrofuran (THF) and water (Table 1, entries 7, 8, 10, 12 and 16). Unexpectedly, much lower yields were observed with dichloromethane (Table 1, entry 9). Among different solvents that are screened, nonpolar solvents (Table 1, entries 11, 12 and 15) are found to be not suitable for the title reaction compared to polar solvents, and the polar solvents resulted in good to excellent yields of the desired products.
Having the optimized reaction conditions (catalyst, solvent, temperature and additive-free) in hand, we extended the scope of the reaction by using various 2-bromobenzaldehydes, structurally diverse amines (aliphatic, aromatic and heteroaromatic) and sodium azide as illustrated in Table 2. Furthermore, reactions of most of the substrates with several aromatic amines bearing electron neutral and -donating as well as electron-withdrawing groups were performed smoothly and the corresponding 2H-indazoles are attained in good to excellent yields. Interestingly, good yields were observed with heteroaromatic amines such as 2-aminopyridine (Table 2, entry 2). Fascinatingly, the reaction of 2-bromobenzaldehydes with sterically hindered aliphatic amines such as 1-adamantyl amine produced good yields (Table 2, entries 11 and 12). Remarkably, the substituted 2-bromobenzaldehydes reacted with amines (aliphatic and aromatic) resulting the corresponding 2H-indazoles in moderate to good yields. However, these yields were slightly lower than those attained from 2-bromobenzaldehyde.
| Entry | Aldehyde | Amine | Product | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reagents and reaction conditions: 2-bromobenzaldehyde (1.5 mmol), aniline (1.8 mmol), sodium azide (2 mmol), catalyst (20 mg) and solvent (1.5 mL); unless otherwise mentioned, stirred at 120 °C temperature for 6 h. b Yields of isolated products. | |||||
| 1 |

|

|

|
6 | 91 |
| 2 |

|
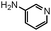
|

|
7 | 82 |
| 3 |
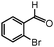
|

|
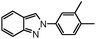
|
7 | 86 |
| 4 |

|

|

|
8 | 79 |
| 5 |

|

|

|
10 | 38 |
| 6 |

|

|
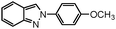
|
8 | 86 |
| 7 |

|

|

|
11 | 72 |
| 8 |

|

|

|
10 | 78 |
| 9 |

|

|

|
9 | 81 |
| 10 |
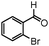
|
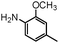
|

|
10 | 70 |
| 11 |
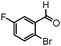
|

|

|
8 | 87 |
| 12 |
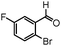
|

|

|
11 | 78 |
| 13 |
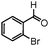
|

|
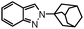
|
10 | 86 |
| 14 |

|

|

|
16 | 38 |
| 15 |

|

|
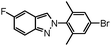
|
20 | 32 |
| 16 |

|

|
No product | 24 | 0 |
We studied a wide range of amines having both electron-donating and electron-withdrawing groups. In the case of aromatic amines having a methoxy group at the para position, there is insignificant effect and produced excellent yields, when compared to the methoxy group at the ortho position (Table 2, entries 6 and 10). This determines the ability of steric hindrance of ortho substituents. Conversely, 3,4-dimethylaniline provided better yields than p-toluidine (Table 2, entries 3 and 4). However, sterically hindered aromatic amines such as 4-bromo-2,6-dimethylaniline produced corresponding 2H-indazole yields of only 38% and 32% with 2-bromobenzaldehyde and 5-fluoro2-bromobenzaldehyde, respectively (Table 2, entries 14 and 15). Additionally, we carried out catalyst recycling experiments by using 2-bromobenzaldehyde, aniline and sodium azide as the model reaction. Interestingly, the used CuII–HT catalyst exhibited the same activity and selectivity in terms of the desired product up to three cycles since there is no metal leach.
On the basis of our investigation and earlier studies,20,25,28 the plausible mechanism is shown in Scheme 2. The one-pot multicomponent coupling reaction involves first the formation of the N-(2-bromobenzylidene)amine intermediate. In the next stage, the bromide is replaced with azide in the presence of the CuII–HT catalyst and results N-(2-azidobenzylidene)amine. In the resulting intermediate, the azide is activated by the CuII–HT catalyst and the desired 2H-indazole product is formed through intramolecular cyclization with N–N bond formation by simultaneous attack of the activated azide by the N-atom of benzylideneamine.
 | ||
| Scheme 2 A plausible reaction mechanism for 2H-indazoles formation with the CuII–HT catalyst. | ||
4. Conclusions
In conclusion, we report for the first time, to the best of our knowledge, that CuII–HT is an efficient heterogeneous catalyst for the synthesis of 2H-indazoles. We have developed a novel, simple and proficient protocol for three component (2-bromobenzaldehydes, primary amines and sodium azide) consecutive condensation, C–N and N–N bond formations as a one-pot MCR. Additionally, the CuII–HT catalyst can be readily recovered and reused for at least three runs without any significant loss of activity. It has the potential for large scale applications also.Acknowledgements
ANP and RS thank Council of Scientific and Industrial Research (CSIR), New Delhi, for Senior Research Fellowships. The authors thank the Department of Science and Technology (DST), New Delhi, Govt. of India (SERC Scheme: SR/S1/PC-63/2008), for financial support of this work.Notes and references
- (a) A. Schmidt, A. Beutler and B. Snovydovych, Eur. J. Org. Chem., 2008, 4073–4095 CrossRef CAS; (b) L. A. Clutterbuck, C. G. Posada, C. Visintin, D. R. Riddal, B. Lancaster, P. J. Gane, J. Garthwaite and D. L. Selwood, J. Med. Chem., 2009, 52, 2694–2707 CrossRef CAS.
- J. Elguero, in Comprehensive Heterocyclic Chemistry, ed. A. R. Katritzky and C. W. Rees, Pergamon Press, New York, 1984, vol. 5, p. 167 Search PubMed.
- Y. Ykeda, N. Takano, H. Matsushita, Y. Shiraki, T. Koide, R. Nagashima, Y. Fujimura, M. Shindo, S. Suzuki and T. Iwasaki, Arzneim. Forsch., 1979, 29, 511–520 Search PubMed.
- (a) G. A. Bistochi, G. De Meo, M. Pedini, A. Ricci, H. Brouilhet, S. Bucherie, M. Rabaud and P. Jacquignon, Farmaco, Ed. Sci., 1981, 36, 315–333 Search PubMed; (b) G. Picciola, F. Ravenna, G. Carenini, P. Gentili and M. Riva, Farmaco, Ed. Sci., 1981, 36, 1037–1056 CAS.
- B. K. Keppler and M. Hartmann, Met.-Based Drugs, 1994, 1(2–3), 145–149 CrossRef CAS.
- (a) J.-H. Sun, C. A. Teleha, J.-S. Yan, J. D. Rodgers and D. A. Nugiel, J. Org. Chem., 1997, 62, 5627–5629 CrossRef CAS; (b) J. D. Rodgers, B. L. Johnson, H. Wang, R. A. Greenberg, S. Erickson-Viitanen, R. M. Klabe, B. C. Cordova, M. M. Rayner, G. N. Lam and C.-H. Chang, Bioorg. Med. Chem. Lett., 1996, 6, 2919–2924 CrossRef CAS.
- G. Corsi, G. Palazzo, C. Germani, P. S. Barcellona and B. Silvestrini, J. Med. Chem., 1976, 19, 778–783 CrossRef CAS.
- C. Wu, Y. Fang, R. C. Larock and F. Shi, Org. Lett., 2010, 12, 2234–2237 CrossRef CAS.
- P. G. Wyatt, A. L Gill, G. Saxty and R. Apaya, WO 2005014554, 2005.
- J. Arnold, D. R. Artis, C. Hurt, P. N. Ibrahim, H. Krupka, J. Lin, M. V. Milburn, W. Wang and C. Zhang, WO 2005009958, 2005.
- M. H. Chen, J. B. Doherty, L. Liu, S. Natarajan and R. M. Tynebor, WO 2005026128, 2005.
- H.-C. Zhang, C. K. Derian, D. F. McComsey, K. B. White, H. Ye, L. R. Hecker, J. Li, M. F. Addo, D. Croll, A. J. Eckardt, C. E. Smith, Q. Li, W.-M. Cheung, B. R. Conway, S. Emanuel, K. T. Demarest, P. Andrade-Gordon, B. P. Damiano and B. E. Maryanoff, J. Med. Chem., 2005, 48, 1725–1728 CrossRef CAS.
- S. Andreonati, V. Sava, S. Makan and G. Kolodeev, Pharmazie, 1999, 54, 99–101 Search PubMed.
- M. D. Angelis, F. Stossi, K. A. Carlson, B. S. Katzenellenbogen and J. A. Katzenellenbogen, J. Med. Chem., 2005, 48, 1132–1144 CrossRef.
- X. Li, S. Chu, V. A. Feher, M. Khalili, Z. Nie, S. Margosiak, V. Nikulin, J. Levin, K. G. Sprankle, M. E. Tedder, R. Almassy, K. Appelt and K. M. Yager, J. Med. Chem., 2003, 46, 5663–5673 CrossRef CAS.
- H.-J. Boehm, M. Boehringer, D. Bur, H. Gmuender, W. Huber, W. Klaus, D. Kostrewa, H. Kuehne, T. Luebbers, N. Meunier-Keller and F. Mueller, J. Med. Chem., 2000, 43, 2664–2674 CrossRef CAS.
- (a) P. Lopez-Alvarado, C. Avendano and J. C. Menendez, J. Org. Chem., 1995, 60, 5678–5682 CrossRef CAS; (b) P. Y. S. Lam, C. G. Clark, S. Saubern, J. Adams, M. P. Winters, D. M. T. Chan and A. Combs, Tetrahedron Lett., 1998, 39, 2941–2944 CrossRef CAS; (c) A. Y. Fedorov and J.-P. Finet, Tetrahedron Lett., 1999, 40, 2747–2748 CrossRef CAS; (d) R. M. Claramunt, J. Elguero and R. Garceran, Heterocycles, 1985, 23, 2895–2906 CrossRef CAS; (e) For one successful example, see: D. J. Slade, N. F. Pelz, W. Bodnar, J. W. Lampe and P. S. Watson, J. Org. Chem., 2009, 74, 6331–6334 CrossRef CAS.
- N. Halland, M. Nazaré, O. R’kyek, J. Alonso, M. Urmann and A. Lindenschmidt, Angew. Chem., Int. Ed., 2009, 48, 6879–6882 CrossRef CAS.
- J. J. Song and N. K. Yee, Org. Lett., 2000, 2, 519–521 CrossRef CAS.
- B. J. Stokes, C. V. Vogel, L. K. Urnezis, M. Pan and T. G. Driver, Org. Lett., 2010, 12, 2884–2887 CrossRef CAS.
- B. Haag, Z. Peng and P. Knochel, Org. Lett., 2009, 11, 4270–4273 CrossRef CAS.
- A. Taher, S. Ladwa, S. T. Rajan and G. W. Weaver, Tetrahedron Lett., 2000, 41, 9893–9897 CrossRef CAS.
- K. Y. Lee, S. Gowrisankar and J. N. Kim, Tetrahedron Lett., 2005, 46, 5387–5391 CrossRef CAS.
- Y. Fang, C. Wu, R. C. Larock and F. Shi, J. Org. Chem., 2011, 76, 8840–8851 CrossRef CAS.
- M. R. Kumar, A. Park, N. Park and S. Lee, Org. Lett., 2011, 13, 3542–3545 CrossRef CAS.
- (a) B. M. Reddy and M. K. Patil, Chem. Rev., 2009, 109, 2185–2208 CrossRef CAS; (b) M. K. Patil, A. N. Prasad and B. M. Reddy, Curr. Org. Chem., 2011, 15, 3961–3985 CrossRef CAS; (c) B. Thirupathi, R. Srinivas, A. N. Prasad, J. K. P. Kumar and B. M. Reddy, Org. Process Res. Dev., 2010, 14, 1457–1463 CrossRef CAS.
- (a) M. L. Kantam, S. Laha, J. Yadav, P. R. Likhar, B. Sreedhar, S. Jha, S. Bhargava, M. Udayakiran and B. Jagadeesh, Org. Lett., 2008, 10, 2979–2982 CrossRef CAS; (b) K. Namitharan, M. Kumarraja and K. Pitchumani, Chem.–Eur. J., 2009, 15, 2755–2758 CrossRef CAS.
- A. N. Prasad, B. Thirupathi, G. Raju, R. Srinivas and B. M. Reddy, Catal. Sci. Technol., 2012, 2, 1264–1268 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional characterization studies supporting the results which include 1H NMR, 13C NMR, FTIR and MS data of isolated compounds. See DOI: 10.1039/c2cy20590d |
| This journal is © The Royal Society of Chemistry 2013 |