Comparison of the catalytic activity of MOFs and zeolites in Knoevenagel condensation†
Maksym
Opanasenko
ab,
Amarajothi
Dhakshinamoorthy
c,
Mariya
Shamzhy
ab,
Petr
Nachtigall
d,
Michal
Horáček
a,
Hermenegildo
Garcia
c and
Jiří
Čejka
*a
aJ. Heyrovský Institute of Physical Chemistry, Academy of Sciences of Czech Republic, v.v.i. Dolejškova 3, 182 23 Prague 8, Czech Republic. E-mail: jiri.cejka@jh-inst.cas.cz; Fax: +420 286 582 307; Tel: +420 286 583 014
bL.V. Pisarzhevskiy Institute of Physical Chemistry, National Academy of Sciences of Ukraine, pr. Nauky, 31 Kyiv 03028, Ukraine. Fax: +380 445 256 216; Tel: +380 445 254 196
cInstituto de Tecnología Química CSIC-UPV, Universita Politecnica de Valencia, 46022 Valencia, Spain
dCharles University in Prague, Celetná 13, 116 36, Prague 1, Czech Republic
First published on 4th October 2012
Abstract
The catalytic behavior of metal–organic-frameworks (MOFs) CuBTC and FeBTC was investigated in Knoevenagel condensation of cyclohexane carbaldehyde and benzaldehyde with active methylene compounds and compared with zeolites BEA and TS-1. High yields were achieved over the CuBTC catalyst in the Knoevenagel condensation involving malonitrile, especially at a relatively low reaction temperature (80 °C); no leaching of the active phase was evidenced. In contrast, zeolites were not active under such reaction conditions. We propose an activation of malonitrile on a pair of adjacent Cu ions to explain the high catalytic activity of CuBTC with respect to conventional catalysts. Compared with CuBTC, zeolites exhibited usually lower selectivities, which is ascribed to a high acid strength of their active sites promoting consecutive reactions.
1. Introduction
The Knoevenagel condensation of aldehydes with active methylene compounds (carrying two electron withdrawing groups) is one of the most useful carbon–carbon bond forming reactions,1 having wide applications in the synthesis of fine chemicals,2 biologically active substances,3,4 or hetero Diels–Alder reactions.5 The condensation is usually catalysed by bases,6–8 or Lewis acids,9 and it requires high reaction temperatures10,11 or microwave irradiation.11,12Various homogeneous and heterogeneous catalysts are used in Knoevenagel condensation, namely TiCl4,13 ZnCl2,14 MgF2,15 HClO4–SiO2,16 Ni–SiO2,17 surfactants,18 phosphates,19 zeolites,10,20–23 clays,24 organic-functionalized molecular sieves and silicate–organic composite materials.25 Heterogeneous catalysts provide a number of significant advantages over homogeneous ones. They are easily recoverable, reusable and minimize the undesired waste. Thus, the development of new environmentally friendly, selective catalysts for the Knoevenagel condensation is of great interest.
In this contribution, we compare the catalytic behavior of representative zeolites and metal–organic-frameworks (MOFs) in Knoevenagel condensation. While zeolites are mature catalysts with a high number of large-scale industrial applications,26,27 they find limitations in liquid phase reactions due to narrow pore sizes available in conventional microporous zeolites.28 In this regard, it has been proposed that MOFs can overcome this limitation in pore sizes due to their flexibility in design easy synthesis,29 adjustable chemical functionality,30 and extra-high porosity,31 therefore, MOFs appear as promising solid catalysts for liquid phase reactions. Thus, comparison of the performance of MOFs and zeolites as heterogeneous catalysts in a liquid phase reaction is a topic of interest aiming to show the potential of MOFs in this area. MOFs are highly active in various organic reactions,32–39 including the Knoevenagel condensation.40–42
Our goal was to evaluate the catalytic activity of various catalysts in the Knoevenagel condensation of aldehydes (cyclohexane carbaldehyde and benzaldehyde) with active methylene compounds (ethyl acetoacetate, methyl cyanoacetate and malononitrile, Scheme 1). The following catalysts were investigated: (i) acid form of zeolite BEA where both Brønsted and Lewis acid sites exists in 12-ring pores, (ii) a medium pore size zeolite TS-1 where only Lewis acid sites exist in 10-ring pores, (iii) large pore CuBTC MOF where the structural details of active Cu2+ Lewis sites are known, and (iv) FeBTC MOF, structure of which is not fully understood. The selection of zeolites and MOFs catalysts was made based on their wide availability, ample use as solid catalysts and the presence of the required type of acid sites.
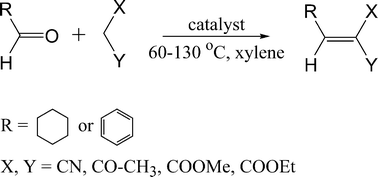 | ||
| Scheme 1 Schematic representation of liquid phase Knoevenagel condensation of aldehydes with active methylene compounds. | ||
2. Experimental section
2.1. Materials
H-Beta (Si/Al = 12.5) was purchased from Zeolyst. Ti-MFI (Ti-ZSM-5, Si/Ti = 35) was synthesized using a standard method with tetrapropylammonium hydroxide as a template.43 CuBTC (Basolite C300) and FeBTC (Basolite F300) were purchased from Sigma Aldrich. Cyclohexane carbaldehyde (97%), benzaldehyde (≥99.0%), ethyl acetoacetate (≥99.0%), methyl cyanoacetate (99%), malononitrile (≥99.0%) were used as substrates, mesitylene (≥99%) as the internal standard and p-xylene (≥99%) as the solvent in catalytic experiments. All reactants and solvents were obtained from Sigma Aldrich and used as received without any further treatment.2.2. Characterization
The crystallinity of the samples under study was determined by powder X-ray diffraction on a Bruker AXS D8 Advance diffractometer with a Vantec-1 detector in the Bragg–Brentano geometry using CuKα radiation. All samples were gently grinded to limit the effect of preferential orientation of individual crystals. The shape and size of crystals were determined by scanning electron microscopy (SEM; Jeol, JSM-5500LV).Adsorption isotherms of nitrogen at −196 °C were determined using an ASAP 2020 (Micromeritics) static volumetric apparatus. Before adsorption experiments the samples were degassed under turbomolecular pump vacuum at the temperature of 150 °C for MOFs and 250 °C for zeolites. This temperature was maintained for 8 h.
The concentrations of Brønsted and Lewis acid sites in zeolites were determined by pyridine adsorption followed by FTIR spectroscopy (Nicolet 6700) according to the methodology reported previously.44 Generally, all samples were activated in a form of self-supporting wafers (ca. 8.3–12.5 mg cm−3) at 400 °C under vacuum for 2 h prior to the adsorption of probe molecules. The adsorption temperature was 150 °C. The adsorption of probe molecules was investigated with a resolution of 2 cm−1. All measured spectra were recalculated to a “normalized” wafer of 10 mg. For a quantitative characterization of acid sites, the following bands and adsorption coefficients were used: PyH+ band at 1545 cm−1, ε = 1.67 cm μmol−1, pyL band at 1454 cm−1, ε = 2.22 cm μmol−1. The determination of Lewis acid sites in CuBTC is discussed in detail elsewhere.34 CuBTC was prepared as a self-supported wafer and activated at 150 °C. The adsorption temperature was 100 °C.
2.3. Catalysis
The Knoevenagel condensation was performed in the liquid phase under atmospheric pressure and a temperature of 60–130 °C in a multi-experiment work station StarFish (Radley's Discovery Technologies UK). Prior to use, 200 mg of the catalyst was activated at 150 °C (for MOFs) or 450 °C (for zeolites) for 90 min with a temperature rate 10 °C min−1 in a stream of air. Typically, 6.0 mmol of aldehyde, 9.0 mmol of active methylene compound, 0.4 g of mesitylene (internal standard) and 200 mg of catalyst were added to the 3-necked vessel, equipped with a condenser and a thermometer, stirred and heated. Aliquots of the reaction mixture were sampled at the interval time of 0–24 h in order to determine the equilibrium of the reaction. The zero point of conversion corresponds to the concentration of aldehyde in the starting solution in the presence of catalyst (to neglect the contribution of adsorption).To evaluate a potential influence of leaching of active species from the heterogeneous catalysts, a part of the reaction mixture was filtered at the reaction temperature and the obtained liquid phase was further investigated in the Knoevenagel condensation under the same reaction conditions.
For the 2nd and 3rd catalytic run catalysts were separated by centrifugation and washed with acetone several times. Then the samples were activated at 150 °C.
2.4. Reaction product analysis
The reaction products were analyzed by gas chromatography (GC) using an Agilent 6850 with a FID detector equipped with a nonpolar HP1 column (diameter 0.25 mm, thickness 0.2 μm and length 30 m). Reaction products were indentified using GC-MS analysis (ThermoFinnigan, FOCUS DSQ II Single Quadrupole GC/MS).3. Results and discussion
3.1. Characterization of catalysts
All catalysts used are pure phases as evidenced by X-ray diffraction patterns corresponding well with the literature data (Fig. S1, ESI†). In order to confirm the incorporation of Ti in the framework of TS-1, UV-Vis spectra of synthesized zeolite were recorded (Fig. S2, ESI†). We observed a strong band in the region of 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–50
000–50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1, ascribed to the ligand to metal charge transfer from oxygen to tetrahedral titanium(IV) in the zeolite framework. Since UV-Vis spectra of TS-1 did not contain a band in the region of 30
000 cm−1, ascribed to the ligand to metal charge transfer from oxygen to tetrahedral titanium(IV) in the zeolite framework. Since UV-Vis spectra of TS-1 did not contain a band in the region of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–38
000–38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 we can exclude the presence of hexacoordinated titanium species. While CuBTC and zeolites BEA and TS-1 are found to be highly crystalline materials, FeBTC belongs to less ordered materials, the crystal structure of FeBTC still remains unknown. The known frameworks of the catalysts under investigation are shown in Fig. 1. Experimentally, nitrogen isothermal adsorption indicates that FeBTC exhibits an average pore size of 2.1 nm and has a BET surface area of 840 m2 g−1. The morphology of the particles and the average crystallite size was determined by SEM (Fig. S3, ESI†).
000 cm−1 we can exclude the presence of hexacoordinated titanium species. While CuBTC and zeolites BEA and TS-1 are found to be highly crystalline materials, FeBTC belongs to less ordered materials, the crystal structure of FeBTC still remains unknown. The known frameworks of the catalysts under investigation are shown in Fig. 1. Experimentally, nitrogen isothermal adsorption indicates that FeBTC exhibits an average pore size of 2.1 nm and has a BET surface area of 840 m2 g−1. The morphology of the particles and the average crystallite size was determined by SEM (Fig. S3, ESI†).
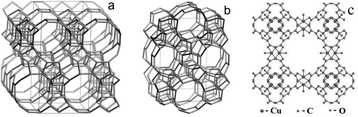 | ||
| Fig. 1 Frameworks of BEA (a) TS-1 (b) and CuBTC (c). | ||
Acidic properties of zeolites, namely type and concentrations of Brønsted and Lewis acid sites, were assessed using adsorption of pyridine followed by FTIR spectroscopy (Table 1). For zeolite, absorption bands around 1546 cm−1 (which is due to the interaction of pyridine with Brønsted acid sites) and 1453–1455 cm−1 (which is due to the interaction of pyridine with Lewis acid sites) were chosen as characteristic.
| Catalyst | D micro/nm | S BET /m2 g−1 | V micro /cm3 g−1 | Brønsted acid sitesb/mmol g−1 | Lewis acid sitesb/mmol g−1 |
|---|---|---|---|---|---|
| a According to nitrogen adsorption experiments. b According to FTIR of adsorbed pyridine. | |||||
| CuBTC | 0.90; 0.50; 0.35 | 1500 | 0.64 | — | 2.3 |
| FeBTC | 0.86a | 1060 | 0.33 | — | nd |
| BEA | 0.66 × 0.56 | 670 | 0.31 | 0.21 | 0.32 |
| TS-1 | 0.56 × 0.54 | 290 | 0.15 | — | 0.19 |
The Lewis acidity of CuBTC by analogy to zeolites may be determined quantitatively by the adsorption of basic probe molecules. The band at 1069 cm–1 assigned to C–C out-of-plane vibrations in the pyridine molecule was used for quantitative analysis, following the protocol described elsewhere.45 As it was shown, the concentrations of Lewis acid centers in CuBTC determined using pyridine and CO as probe molecules were practically identical (2.3 mmol g−1).34
Nitrogen adsorption isotherms of individual catalysts are depicted in Fig. S4 (ESI†). In all cases, the isotherms were of the type I according to the IUPAC classification,46 which is typical for microporous solids. Structural and acidic properties of the catalysts under investigation are summarized in Table 1.
As can be seen, all catalysts have a 3D pore system but they differ in pore sizes. Conventional BEA (HBeta) and MFI (TS-1) are the solid catalysts with the smallest pore dimensions. As commented in the introduction, the range of pore sizes in conventional zeolites is limited, while MOFs have pores with larger dimensions. Concerning the nature of the acid sites, CuBTC, FeBTC and TS-1 are typical Lewis solid acids, and zeolite BEA has both Brønsted and Lewis acid centers.
3.2. Knoevenagel condensation
Knoevenagel condensation proceeds according to the reaction shown in Scheme 1. In addition to the main product, the reaction mixture may contain some byproducts from the consecutive reaction of the primary product. A summary of the results of the catalytic experiments is presented in Table 2.| Aldehyde | Methylene component | Catalyst | T/°C | t/h | Conversion (%) | Selectivity (%) |
|---|---|---|---|---|---|---|

|
|
BEA | 130 | 25 | 92 | 26 |
| TS-1 | 24 | 100 | 29 | |||
| CuBTC | 24 | 89 | 58 | |||
| FeBTC | 25 | 98 | 16 | |||
|

|
BEA | 130 | 25 | 82 | 45 |
| TS-1 | 24 | 41 | 21 | |||
| CuBTC | 24 | 40 | 75 | |||
| FeBTC | 25 | 58 | 28 | |||

|
|
BEA | 130 | 24 | 97 | 27 |
| TS-1 | 99 | 10 | ||||
| CuBTC | 97 | 73 | ||||
| FeBTC | 100 | 61 | ||||
|

|
BEA | 130 | 24 | 66 | 94 |
| TS-1 | 39 | 100 | ||||
| CuBTC | 67 | 41 | ||||
| FeBTC | 75 | 47 | ||||

|

|
BEA | 130 | 4 | 100 | 98 |
| 80 | 5 | 44 | 49 | |||
| TS-1 | 130 | 6 | 89 | 88 | ||
| 80 | 5 | 25 | 0 | |||
| CuBTC | 130 | 1 | 98 | 100 | ||
| 80 | 5 | 87 | 93 | |||
| FeBTC | 130 | 6 | 93 | 100 | ||
| 80 | 5 | 55 | 100 | |||
|

|
BEA | 130 | 6 | 100 | 100 |
| 80 | 5 | 4 | 100 | |||
| 60 | 24 | 0 | 0 | |||
| TS-1 | 130 | 6 | 96 | 100 | ||
| 80 | 4 | 0 | 0 | |||
| 60 | 24 | 0 | 0 | |||
| CuBTC | 130 | 1 | 100 | 100 | ||
| 80 | 0.5 | 100 | 100 | |||
| 60 | 6 | 88 | 100 | |||
| FeBTC | 130 | 3 | 100 | 99 | ||
| 80 | 5 | 35 | 100 | |||
| 60 | 24 | 8 | 100 |
For the condensation of cyclohexane carbaldehyde and benzaldehyde with ethyl acetoacetate, the highest yields of target products were obtained over CuBTC and BEA, respectively (Fig. 2). It should be noted that the highest conversions were achieved for both aldehydes over zeolites (see Table 2) containing stronger acid sites. However, the selectivity of the product of Knoevenagel condensation was higher for CuBTC (Table 2) most probably due to the presence of milder acid sites preventing competitive transformations of substrates and consecutive reactions of products. The lowest yields were obtained over TS-1, which can be connected with the smallest pore size.
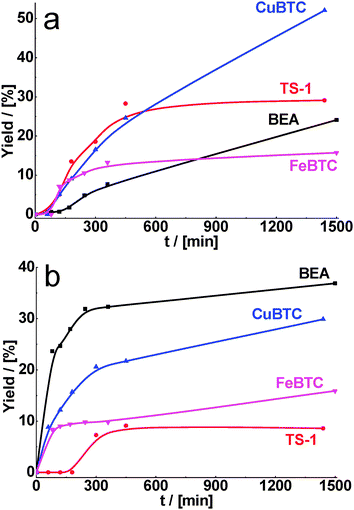 | ||
| Fig. 2 Yield of the product of cyclohexane carbaldehyde (a) and benzaldehyde (b) in condensation with ethyl acetoacetate over BEA, TS-1, CuBTC, FeBTC (reaction temperature – 130 °C). | ||
Fig. 3a provides the time-on-stream dependence of yield of the product of cyclohexane carbaldehyde condensation with methyl cyanoacetate. As can be seen from Table 2, the conversions of substrate increased in comparison with ethyl acetoacetate for all used catalysts. It is caused by a higher reactivity of methyl cyanoacetate in Knoevenagel condensation. While the maximum conversion of cyclohexane carbaldehyde, achieved within 24 h of TOS, was 97 and 100% over CuBTC and FeBTC, respectively, the corresponding conversions over zeolites BEA and TS-1 were also close to 100%. However, the selectivity to the target product was higher for MOFs (Table 2).
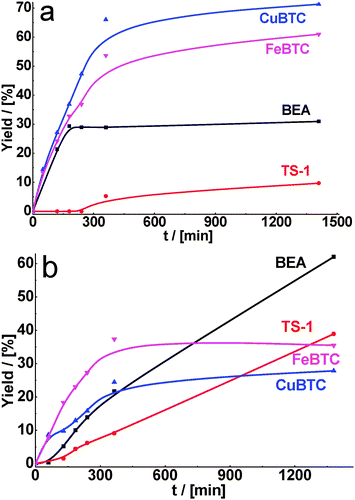 | ||
| Fig. 3 Yield of the product of cyclohexane carbaldehyde (a) and benzaldehyde (b) condensation with methyl cyanoacetate over BEA, TS-1, CuBTC, FeBTC (reaction temperature – 130 °C). | ||
As for malonitrile, having the most active methylene group, total conversion of cyclohexane carbaldehyde was reached within 1 h of reaction time for CuBTC. FeBTC and zeolites were less active, but in 6 h of TOS practically total conversion of cyclohexane carbaldehyde was also achieved with 90–100% selectivity (Fig. 4a, Table 2).
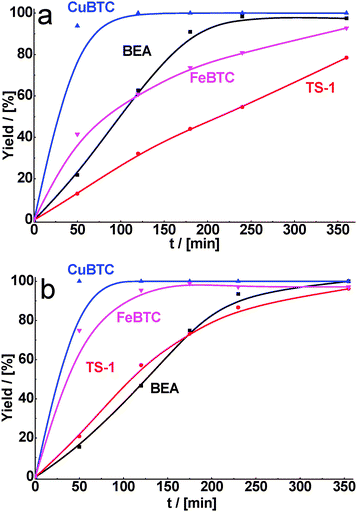 | ||
| Fig. 4 Yield of the product of cyclohexane carbaldehyde (a) and benzaldehyde (b) in condensation with malonitrile over BEA, TS-1, CuBTC, FeBTC (reaction temperature – 130 °C). | ||
We interpret this change in the order of relative catalytic activity considering that for more reactive substrates weaker acid sites are sufficient to promote the condensation and once high acid strength is not required, the performance is determined by site accessibility and open porosity favoring MOFs instead of zeolites.
In fact, we observed that MOFs were most active even after decreasing the reaction temperature from 130 to 80 °C. Under these reaction conditions, the differences in activity of MOFs and zeolites are especially pronounced at the beginning of the experiment (30 min). While the yield of the product formed on CuBTC was about 50%, the reaction still did not proceed over both zeolites (Fig. 5a).
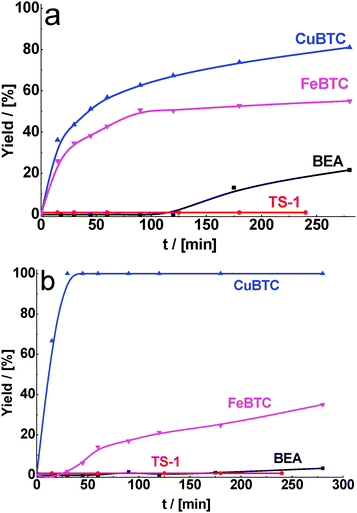 | ||
| Fig. 5 Yield of the product of cyclohexane carbaldehyde (a) and benzaldehyde (b) in condensation with malonitrile over BEA, TS-1, CuBTC, FeBTC (reaction temperature – 80 °C). | ||
Similar trends as reported above for cyclohexane carbaldehyde were also found for the condensation of benzaldehyde as a carbonyl substrate. We observed the increase in the activity of all catalysts in benzaldehyde condensation with methyl cyanoacetate (Fig. 3b), especially with malonitrile (Fig. 4b) in comparison with ethyl acetoacetate (Fig. 2b). The conversion of benzaldehyde in condensation with malonitrile over zeolites (15 and 21% for BEA and TS-1, respectively) was much lower in comparison with MOFs (100 and 75% for CuBTC and FeBTC, respectively) after 1 h of TOS under the same reaction conditions. Unexpectedly for us, in most cases conversion of benzaldehyde was less than the conversion of cyclohexane carbaldehyde (Table 2) under comparable conditions (time, temperature, type of active methylene compound). This can be explained by the fact that contrary to cyclohexane carbaldehyde, benzaldehyde can interact with Cu2+via the aromatic ring. It may result in some electron density flow from benzaldehyde to Cu and therefore in lower activity of benzaldehyde (compared to cyclohexane carbaldehyde).
In the case of benzaldehyde condensation with malonitrile at 80 °C, the difference in the conversion over CuBTC and zeolites reaches its maximum value (Table 2). In this way, benzaldehyde is completely converted into the desired product over CuBTC after 1 h of TOS while the reaction practically does not proceed over zeolites. Even at the reaction temperatures of 60 °C, the conversion of benzaldehyde over MOF catalysts was 88% after 6 h of TOS (Table 2). It should be noted that the reaction over zeolites under these conditions does not proceed at all, most probably due to a strong interaction of reactants/products with active sites embedded in 10- or 12-ring pores.
As evidenced by X-ray powder diffraction (Fig. 6), the crystal structure of CuBTC does not change during cyclohexane carbaldehyde and benzaldehyde condensation with malonitrile at 130 °C.
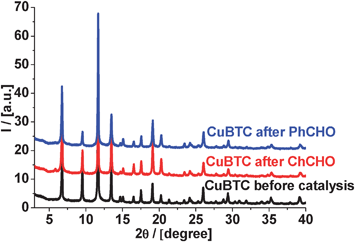 | ||
| Fig. 6 XRD patterns of CuBTC before and after the reaction (condensation with malonitrile at 130 °C). | ||
The X-ray patterns confirmed no changes in the structure and we can expect that most of the active sites of CuBTC should remain unchanged during the liquid-phase Knoevenagel condensation of all substrates under investigation. This is in contrast to our studies of MOF's framework stability in Pechmann condensation,39 when we observed some dissolution of CuBTC during the liquid phase reaction of pyrogallol with ethyl acetoacetate.
The last statement was also in accordance with the results of the leaching test, obtained for CuBTC (Fig. 7). Thus, separation of the solid by filtration of the “hot” reaction mixture followed by centrifugation at about 65% conversion stops the condensation reaction, indicating that no leaching of the active species takes place.
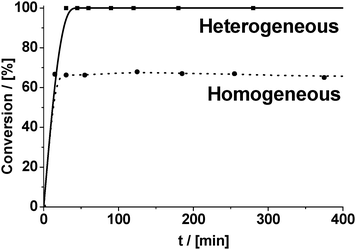 | ||
| Fig. 7 Leaching test of Cu2+ ions from the catalyst (CuBTC) under conditions of Knoevenagel condensation of benzaldehyde with malonitrile (reaction temperature – 80 °C). | ||
We observed that MOFs were quite active after the second and third catalytic run (Fig. 8). Despite a lower initial rate of the reaction over reactivated CuBTC and FeBTC, the yield of the product of cyclohexane carbaldehyde condensation with malonitrile was comparable with results on fresh catalysts after 300 min of TOS. It should be pointed that the activity of both used MOFs was practically identical after first and second reactivation.
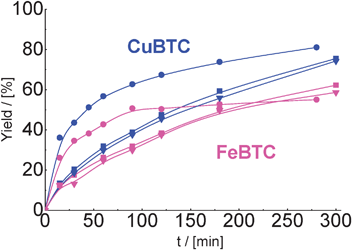 | ||
| Fig. 8 Yield of the product of cyclohexane carbaldehyde in condensation with malonitrile over CuBTC and FeBTC (reaction temperature – 80 °C) in the 1st (–●–), 2nd (–■–) and 3rd (–▼–) catalytic run. | ||
Moreover, a slight difference in the relative intensities of the bands in corresponding IR spectra also confirms that the structure of MOFs is almost unchanged (Fig. S5, ESI†). In the IR spectra of CuBTC and FeBTC after the reaction only additional bands in the region 2100–3300 cm−1 (corresponding to a small amount of adsorbed products of condensation) were observed.
Thus, MOFs can be considered as promising heterogeneous catalysts for Knoevenagel condensation of oxo compounds with active methylene-containing substrates providing the desired product under relatively mild conditions in comparison with zeolites.
It should be noted that the advantages of MOFs over zeolites are especially obvious in the condensation of less acid-strength demanding oxo-compounds such as for the reactions with malonitrile and methyl cyanoacetate with cyclohexane carbaldehyde. In the case of the condensation of cyclohexane carbaldehyde with ethyl acetoacetate, conversions achieved over MOFs and zeolites at 24 h reaction time were comparable but the selectivity towards the desired condensation product is much higher when using MOFs as solid catalysts.
In order to determine the role of the individual type of acid sites in the Knoevenagel reaction, zeolite BEA was calcined at 800 °C to remove all Brønsted acid sites. In this case, the conversion of benzaldehyde in the condensation reaction with the most active methylene component (malonitrile) decreased not significantly in comparison with the BEA sample activated at 450 °C (100% vs. 70% after 6 h). It is therefore likely that the diffusion becomes the rate-determining step and the concentration of acid sites does not significantly affect the rate of the catalytic process any longer. At the same time, using the ethyl acetoacetate and methyl cyanoacetate the conversion of benzaldehyde was significantly reduced (from 70 to 15 and from 22 to 5%, respectively). It can be explained by the fact that Brønsted acid sites efficiently catalyze Knoevenagel condensation and destruction of these centers leads to a significant decrease in the catalyst activity. The decrease in the total concentration of acid sites is apparent, however, some Brønsted acid sites are still present in the sample even after high-temperature treatment. The calcinations of BEA zeolite did not lead to any change in selectivity.
To explain the higher catalytic activity of CuBTC observed for malonitrile, we raise a possibility of a simultaneous interaction of two cyano-groups of malonitrile with two nearby Cu ions. Malonitrile is a V-shaped molecule and the distance between its two N atoms makes such simultaneous interaction possible. Such activation of malonitrile leads to the shift of the electronic density from nitrile groups to the copper ions (formation of donor–acceptor bonds), resulting in a decrease in the electron density of the methylene group. A similar effect of two close Cu sites on the reaction mechanism was recently described also for the Friedländer condensation.34
Unexpectedly for us, the yield of the corresponding product, formed in the presence of CuBTC in the cyclohexane carbaldehyde or benzaldehyde condensation with malonitrile, was only slightly dependent on the aldehyde/malonitrile ratio (Fig. 9). Probably, in the case of malonitrile reactions catalyzed by CuBTC the rate-determining step of the process is the interaction of activated malonitrile with aldehyde. Thus, the overall rate of the reaction does not significantly depend on the amount of the methylene-containing compound.
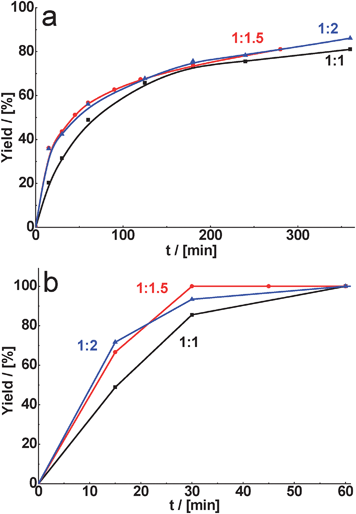 | ||
Fig. 9 Effect of substrate ratio (aldehyde/malonitrile) on yield of the corresponding product for condensation of cyclohexane carbaldehyde (a) and benzaldehyde (b) catalyzed by CuBTC at the reaction temperature – 80 °C (A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B = n(aldehyde) B = n(aldehyde)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(malonitrile)). n(malonitrile)). | ||
Conversion of benzaldehyde in Knoevenagel condensation decreases with decreasing amount of MOF used (Fig. 10). As an example, in the case of the condensation of benzaldehyde with malonitrile, 88% yield of the target product (TOS = 360 min) was achieved when using 0.2 g of CuBTC as the catalyst. At the same time, the yield of the corresponding product was equal to 56 and 14% in the presence of 0.1 and 0.05 g of CuBTC, respectively. The selectivity to the 2-benzylidenemalononitrile in the presence of CuBTC was almost 100%, independently either on the weight of the catalyst or the reaction temperature.
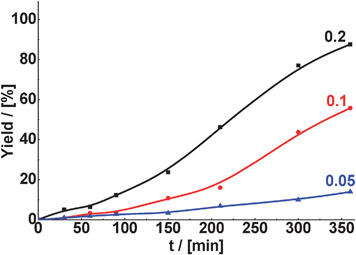 | ||
| Fig. 10 Effect of catalyst weight (g) with respect to aldehyde on the yield of 2-benzylidenemalononitrile in Knoevenagel condensation of benzaldehyde with malonitrile over CuBTC (reaction temperature – 60 °C, 6 mmol of aldehyde, 9 mmol of malonitrile). | ||
We notice in Fig. 10, and also in the case of the reactions catalyzed by zeolites, the temporal profile of the reaction product formation shows an induction period. In fact, this induction period is more pronounced for TS-1 (see Fig. 2b, 3a and b and 4b), this means with the catalyst having the smallest pores. Based on that, we can infer that the reaction products are adsorbed on the active sites and they are removed after some time when their concentration reaches the critical amount. At the same time some side products as benzoic or cyclohexanecarboxylic acids are formed and can be desorbed more easily than the products of the condensation reactions. In general, the smaller the pore size, the longer the induction period.
4. Conclusion
Compared with zeolites, MOFs appear as active and selective catalysts for the Knoevenagel condensation due to the absence of consecutive reactions of the primary condensation products and favorable diffusion of reactants and products due to their large pore size. As expected the reactivity of active methylene compounds decreased in the order malonitrile > methyl cyanoacetate > ethyl acetoacetate, but the reactivity of cyclohexane carbaldehyde was higher or equal to the reactivity of benzaldehyde. The selectivity changed in the order malonitrile > methyl cyanoacetate ≈ ethyl acetoacetate, benzaldehyde > cyclohexane carbaldehyde. In most cases the activity of catalysts decreased in the order CuBTC > FeBTC ≥ BEA ≥ TS-1. The active sites of CuBTC remained practically unchanged during the liquid-phase condensation of all substrates under investigation. CuBTC seems a good catalyst for Knoevenagel condensation, it gives high yield, high selectivity and it is not degraded during the course of the reaction. It is commercially available and it outperforms zeolites as catalyst.Acknowledgements
The research leading to these results has received funding from the European Community's Seventh Framework Programme (FP7/2007–2013) under grant agreement no. 228862. P.N., M.H., and J.Č. thank the Czech Grant Agency for the financial support (Centre of Excellence – P106/12/G015).References
- L. F. Tietze and U. Beifuss, Comprehensive Organic Synthesis, ed. B. M. Trost and I. Fleming, Pergamon Press, Oxford, 1991, vol. 2, p. 341 Search PubMed.
- F. Freeman, Chem. Rev., 1981, 80, 329 CrossRef.
- L. F. Tietze, Chem. Rev., 1996, 96, 115 CrossRef CAS.
- S. M. Lai, C. P. Ng, R. Martin-Aranda and K. L. Yeung, Microporous Mesoporous Mater., 2003, 66, 239 CrossRef CAS.
- H. N. Borah, M. L. Deb, R. C. Boruah and P. J. Bhuyan, Tetrahedron Lett., 2005, 46, 3391 CrossRef CAS.
- S. A.-E. Ayoubi, F. Texier-Boullet and J. Hamelin, Synthesis, 1994, 258 CrossRef.
- I. G. Binev, Y. G. Binev, B. A. Stamboliyska and I. N. Juchnovski, J. Mol. Struct., 1997, 435, 235 CrossRef CAS.
- G. Brufola, F. Fringuelli, O. Piermatti and F. Pizzo, Heterocycles, 1997, 45, 1715 CrossRef CAS.
- D. Prajapati, K. C. Lekhok, J. S. Sandhu and A. C. Ghosh, J. Chem. Soc., Perkin Trans. 1, 1996, 959 RSC.
- S. Saravanamurugan, M. Palanichamy, M. Hartmann and V. Murugesan, Appl. Catal., A, 2006, 298, 8 CrossRef CAS.
- S. Kantevari, R. Bantu and L. Nagarapu, J. Mol. Catal. A: Chem., 2007, 269, 53 CrossRef CAS.
- J. S. Yadav, B. V. Subba Reddy, A. K. Basak, B. Visali, A. V. Narsaiah and K. Nagaiah, Eur. J. Org. Chem., 2004, 546 CrossRef CAS.
- B. Green, R. I. Crane, I. S. Khaidem, R. S. Leighton, S. S. Newaz and T. E. Smyser, J. Org. Chem., 1985, 50, 640 CrossRef CAS.
- R. P. Shanthan and R. V. Venkataratnam, Tetrahedron Lett., 1991, 32, 5821 CrossRef.
- R. M. Kumbhare and M. Sridhar, Catal. Commun., 2008, 9, 403 CrossRef CAS.
- G. Bartoli, R. Beleggia, S. Giuli, A. Giuliani, E. Marcantoni, M. Massaccesi and M. Paletti, Tetrahedron Lett., 2006, 47, 6501 CrossRef CAS.
- V. S. R. R. Pullabhotla, A. Rahman and S. B. Jonnalagadda, Catal. Commun., 2009, 10, 365 CrossRef CAS.
- D. S. Bose and A. V. J. Narsaiah, J. Chem. Res., Synop., 2001, 36 CrossRef CAS.
- J. Bennazha, M. Zahouilly, A. Boukhari and E. A. Hol, J. Mol. Catal. A: Chem., 2003, 202, 247 CrossRef CAS.
- T. I. Reddy and R. S. Verma, Tetrahedron Lett., 1997, 38, 1721 CrossRef CAS.
- U. D. Joshi, P. N. Joshi, S. S. Tamhankar, V. V. Joshi, C. V. Rode and V. P. Shiralkar, Appl. Catal., A, 2003, 239, 209 CrossRef CAS.
- A. Corma, V. Fornes, R. M. Martin-Aranda, H. Garcia and J. Primo, Appl. Catal., 1990, 59, 237 CrossRef CAS.
- A. Corma and R. M. Martin-Aranda, Appl. Catal., A, 1993, 105, 271 CrossRef CAS.
- F. Bigi, L. Chesini, R. Maggi and G. Sartori, J. Org. Chem., 1999, 64, 1033 CrossRef CAS.
- Y. Kubota, Y. Nishizaki, H. Ikeya, M. Saeki, T. Hida, S. Kawazu, M. Yoshida, H. Fujii and Y. Sugi, Microporous Mesoporous Mater., 2004, 70, 135 CrossRef CAS.
- A. Corma, Chem. Rev., 1997, 97, 2373 CrossRef CAS.
- A. Corma, Chem. Rev., 1995, 95, 559 CrossRef CAS.
- A. Corma and M. E. Davis, ChemPhysChem, 2004, 5, 304 CrossRef CAS.
- A. Dhakshinamoorthy, M. Alvaro, A. Corma and H. Garcia, Dalton Trans., 2011, 40, 6344 RSC.
- M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O'Keeffe and O. M. Yaghi, Science, 2002, 295, 469 CrossRef CAS.
- H. K. Chae, D. Y. Siberio-Perez, J. Kim, Y. B. Go, M. Eddaoudi, A. J. Matzger, M. O'Keeffe and O. M. Yaghi, Nature, 2004, 427, 523 CrossRef CAS.
- E. Pérez-Mayoral and J. Čejka, ChemCatChem, 2011, 3, 157 CrossRef.
- A. Corma, H. García and F. X. Llabrés y Xamena, Chem. Rev., 2010, 110, 4606 CrossRef CAS.
- M. Thimmaiah, P. Li, S. Regati, B. Chen and J. C.-G. Zhao, Tetrahedron Lett., 2012, 53, 4870 CrossRef CAS.
- E. Pérez-Mayoral, Z. Musilová, B. Gil, B. Marszalek, M. Položij, P. Nachtigall and J. Čejka, Dalton Trans., 2012, 44, 4036 RSC.
- J. M. Roberts, B. M. Fini, A. A. Sarjeant, O. K. Farha, J. T. Hupp and K. A. Scheidt, J. Am. Chem. Soc., 2012, 134, 3334 CrossRef CAS.
- F. Vermoortele, R. Ameloot, A. Vimont, C. Serre and D. De Vos, Chem. Commun., 2011, 47, 1521 RSC.
- L. T. L. Nguyen, T. T. Nguyen, K. D. Nguyen and N. T. S. Phan, Appl. Catal., A, 2012, 425–426, 44 CrossRef CAS.
- M. Opanasenko, M. Shamzhy and J. Čejka, ChemCatChem DOI:10.1002/cctc.201200232.
- Y. K. Hwang, D. Y. Hong, J. S. Chang, S. H. Jhung, Y. K. Seo, J. Kim, A. Vimont, M. Daturi, C. Serre and G. Ferey, Angew. Chem., Int. Ed., 2008, 47, 4144 CrossRef CAS.
- J. Gascon, U. Aktay, M. D. Hernandez-Alonso, G. P. M. van Klink and F. Kapteijn, J. Catal., 2009, 261, 75 CrossRef CAS.
- F. X. Llabrés i Xamena, F. G. Cirujano and A. Corma, Microporous Mesoporous Mater., 2012, 157, 112 CrossRef.
- A. J. H. P. Van der Pol and J. H. C. van Hooff, Appl. Catal., A, 1992, 92, 93 CrossRef CAS.
- S. J. Gregg and K. S. W. Sing, Adsorption, Surface Area and Porosity, ed. S. J. Gregg and K. S. W. Sing, Academic Press Inc, London, 2nd edn, 1982, p. 303 Search PubMed.
- R. Ferwerda, J. H. van der Maas and F. B. van Duijnevelt, J. Mol. Catal. A: Chem., 1996, 104, 319 CrossRef CAS.
- N. Žilková, M. Bejblová, B. Gil, S. I. Zones, A. Burton, C. Y. Chen, Z. Musilová-Pavlačková, G. Košová and J. Čejka, J. Catal., 2009, 266, 79 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Figures showing the XRD patterns, SEM images and nitrogen adsorption isotherms for the catalysts under investigation. See DOI: 10.1039/c2cy20586f |
| This journal is © The Royal Society of Chemistry 2013 |
