Aminopropyl coated on magnetic Fe3O4 and SBA-15 nanoparticles catalyzed mild preparation of chromeno[2,3-d]pyrimidines under ambient and solvent-free conditions
Hamid Reza
Shaterian
* and
Morteza
Aghakhanizadeh
Department of Chemistry, Faculty of Sciences, University of Sistan and Baluchestan, PO Box 98135-674, Zahedan, Iran. E-mail: hrshaterian@chem.usb.ac.ir; Fax: 0098-541-2431067; Tel: 0098-541-2446565
First published on 12th September 2012
Abstract
3-Aminopropyltriethoxysilane coated on magnetic Fe3O4 nanoparticles [APTES-MNPs] and 3-aminopropyltriethoxysilane coated on SBA-15 [APTES-(SBA-15)] catalyzed efficiently the one-pot pseudo four-component reaction of salicylaldehydes, malononitrile and secondary amines for preparation of chromeno[2,3-d]pyrimidines under solvent-free conditions at room temperature. The catalysts show environmentally benign character, which can be easily prepared, stored, and recovered without obvious significant loss of activity.
Introduction
The development of environmentally friendly solid catalysts for the synthesis of fine chemicals and pharmaceuticals is becoming an area of growing interest because the use of heterogeneous catalytic processes allows easier separation, recovery, and recycling of the catalysts from the reaction mixtures.1 Nanocatalysts bridge the gap between homogeneous and heterogeneous catalysis.2 These catalysts show high activity and selectivity (like a homogeneous system) and the ease of catalyst separation and recovery (like a heterogeneous system). One of the most stimulating features of nanotechnology is its potential use in almost any field.3 The discovery of nanoparticles (NPs) with varied size, shape, and composition has stretched the limits of technology in ways that scientists would never have dreamt of a century ago.4 The isolation and separation of nanocatalysts can be achieved using magnetically separable nanoparticles (MSNPs). They offer a promising option that can meet the requirements of high accessibility with improved reusability.5,6 Mesoporous silica is a form of silica and a recent development in nanotechnology. The most common types of mesoporous nanoparticles are MCM-41 and SBA-15.7 Research continues on these particles, which have applications in catalysis, drug delivery and imaging.8In continuation of our research on new synthetic methods in organic synthesis using green catalysts,9–12 we developed the synthesis of chromeno[2,3-d]pyrimidine derivatives via pseudo four component condensation of salicylaldehydes, malononitrile and secondary amines (Scheme 1), by using 3-aminopropyltriethoxysilane coated on magnetic Fe3O4 nanoparticles [APTES–MNPs] and 3-aminopropyltriethoxysilane coated on SBA-15 [APTES-(SBA-15)] (Fig. 1) as catalysts under mild, ambient, and solvent-free conditions.
![The preparation of chromeno[2,3-d]pyrimidine derivatives.](/image/article/2013/CY/c2cy20543b/c2cy20543b-s1.gif) | ||
| Scheme 1 The preparation of chromeno[2,3-d]pyrimidine derivatives. | ||
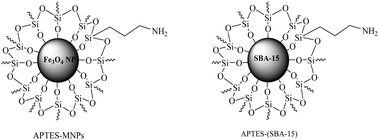 | ||
| Fig. 1 The structure of APTES-MNPs and APTES-(SBA-15). | ||
Results and discussion
3-Aminopropyltriethoxysilane coated on magnetic Fe3O4 nanoparticles [APTES-MNPs] was prepared13 according to Scheme 2. The loading of linker is 0.32 mmol g−1, which is according to the literature.13![The preparation of 3-aminopropyltriethoxysilane coated on magnetic Fe3O4 nanoparticles [APTES-MNPs].](/image/article/2013/CY/c2cy20543b/c2cy20543b-s2.gif) | ||
| Scheme 2 The preparation of 3-aminopropyltriethoxysilane coated on magnetic Fe3O4 nanoparticles [APTES-MNPs]. | ||
3-Aminopropyltriethoxysilane coated on SBA-15 [APTES-(SBA-15)] was prepared14 according to Scheme 3. The loading of linker is 2.28 mmol g−1, which is according to the literature.14
![The preparation of 3-aminopropyltriethoxysilane coated on SBA-15 [APTES-(SBA-15)].](/image/article/2013/CY/c2cy20543b/c2cy20543b-s3.gif) | ||
| Scheme 3 The preparation of 3-aminopropyltriethoxysilane coated on SBA-15 [APTES-(SBA-15)]. | ||
First, in order to carry out the preparation of chromeno[2,3-d]pyrimidine derivatives in a more efficient way, the reaction of salicylaldehyde (2 mmol), malononitrile (1 mmol), and morpholine (1 mmol) was selected as a model system under solvent-free conditions at room temperature to find the optimization amount of the catalysts. The preparation of 2-(4-morpholino-5H-chromeno [2,3-d]pyrimidin-2-yl)phenol using different amounts of nanoparticles as catalysts (2, 5, 10, 15, and 20 mg) (Table 1) was studied. The best result was obtained by using 5 mg of APTES-MNPs and 5 mg of [APTES-(SBA-15)] at room temperature (Table 1).
| Entry | Catalyst (mg) | Time (min) | Yielda (%) | ||
|---|---|---|---|---|---|
| A | B | A | B | ||
| a Yields refer to isolated pure products. Based on the reaction of salicylaldehyde (2 mmol), malononitrile (1 mmol), and morpholine at room temperature under solvent-free conditions. | |||||
| 1 | — | 120 | 120 | — | — |
| 2 | 2 | 27 | 24 | 84 | 86 |
| 3 | 5 | 9 | 7 | 86 | 89 |
| 4 | 10 | 8 | 8 | 85 | 84 |
| 5 | 15 | 10 | 9 | 80 | 84 |
| 6 | 20 | 12 | 11 | 78 | 80 |
Using these optimized catalysts, the scope and efficiency of the procedure were explored for the synthesis of chromeno[2,3-d]pyrimidine derivatives via pseudo four component condensation of salicylaldehydes, malononitrile and secondary amines at room temperature (Table 2). As shown in Table 2, salicylaldehydes with both electron-withdrawing and electron-donating substituents reacted efficiently with malononitrile and secondary amines in the presence of a catalytic amount of APTES-MNPs (5 mg) or APTES-(SBA-15) (5 mg) forming the corresponding chromeno[2,3-d]pyrimidine derivatives without the formation of any side products in good to high yields.
| Entry | 1 | 3 | Time (min) | Yielda (%) | Mp/Mpref (°C) | ||
|---|---|---|---|---|---|---|---|
| A | B | A | B | ||||
| a Yields refer to isolated pure products. Based on the reaction of salicylaldehyde (2 mmol), malononitrile (1 mmol) and secondary amine at room temperature under solvent-free conditions. All known products have been reported in the literature and they were characterized by comparing their melting point, IR and NMR spectra with authentic samples.15–17 | |||||||
| 1 | 1a | 3a | 9 | 8 | 88 | 91 | 181–183/177–17916 |
| 2 | 1a | 3b | 9 | 7 | 86 | 89 | 197–199/196–19716 |
| 3 | 1a | 3c | 11 | 9 | 85 | 87 | 167–179/168–17015 |
| 4 | 1b | 3a | 8 | 8 | 90 | 89 | 200–201/196–19815 |
| 5 | 1b | 3b | 7 | 6 | 89 | 87 | 216–218/21917 |
| 6 | 1b | 3c | 9 | 10 | 86 | 87 | 225/22617 |
| 7 | 1c | 3b | 8 | 7 | 90 | 92 | 249–251/25017 |
| 8 | 1c | 3c | 9 | 9 | 88 | 89 | 215–216/21617 |
| 9 | 1d | 3b | 10 | 8 | 91 | 93 | 231/23117 |
| 10 | 1d | 3c | 11 | 10 | 89 | 92 | 194–196/19517 |
| 11 | 1e | 3c | 14 | 12 | 85 | 88 | 180–182/181–18315 |
The suggested mechanism is presented according to the proposed mechanism in the literature.15 Initial Knoevenagel condensation of salicylaldehyde 1 and malononitrile 2 afforded 5 which upon subsequent Pinner reaction formed 6. Next, the cyano group of intermediate 6 can be attacked by the secondary amines 3 to produce intermediate 7. Finally, intermediate 7 reacts with another molecule of salicylaldehyde 1 followed by proton transfer to afford the product 4 (Scheme 4).
![The proposed mechanism for APTES-MNPs as a selected basic nanocatalyst for the preparation of chromeno[2,3-d]pyrimidine derivatives.](/image/article/2013/CY/c2cy20543b/c2cy20543b-s4.gif) | ||
| Scheme 4 The proposed mechanism for APTES-MNPs as a selected basic nanocatalyst for the preparation of chromeno[2,3-d]pyrimidine derivatives. | ||
We also compared the results of the present nanocatalysts with other catalysts reported in the literature such as lithium perchlorate (LiClO4),15 1-butyl-3 methylimidazolium tetrafluoroborate ([Bmim] BF4),16 and piperidine17 for the preparation of chromeno[2,3-d]pyrimidine derivatives (Table 3). Table 3 clearly demonstrates that APTES-MNPs and APTES-(SBA-15) are effective catalysts in terms of reaction time and yield of obtained products relative to other reported catalysts.
| Entry | Catalyst (mg) | Conditions | [Time (min)/Yielda (%)]ref |
|---|---|---|---|
| a Yields refer to isolated pure products. Based on the reaction of salicylaldehyde (2 mmol), malononitrile (1 mmol), and morpholine (1 mmol). | |||
| 1 | LiClO4 (21.1) | EtOH/rt | [1440/95]15 |
| 2 | [Bmim] BF4 (11.3) | rt | [20/76]16 |
| 3 | Piperidine (17.0) | 100 °C | [660/78]17 |
| 4 | Piperidine (17.0) | MW (180 W), 100 °C | [6/92]17 |
| 5 | APTES-MNPs (5) | Solvent-free, rt | [9/86] (this work) |
| 6 | APTES-(SBA-15) (5) | Solvent-free, rt | [7/89] (this work) |
In green organic synthesis, the recovery of the catalysts is more important. Thus, the reusability of APTES-MNPs and APTES-(SBA-15) as catalysts was studied in the synthesis of chromeno[2,3-d]pyrimidine derivatives. After the completion of the reaction, the solid product was dissolved in CH2Cl2 and then the catalyst was separated from the reaction mixture by an external magnet or centrifugation. To recover the catalyst, after the isolation of insoluble products, the catalyst was washed with CH2Cl2 (2 × 5 mL) and dried under reduced pressure. The recovered APTES-MNPs and APTES-(SBA-15) were tested for studying their catalytic activity in the subsequent run without adding the fresh catalyst. The APTES-MNPs and APTES-(SBA-15) were tested for 5 runs. It was seen that the APTES-MNPs and APTES-(SBA-15) as catalysts displayed very good reusability without any considerable loss of their activities (Fig. 2).
![Reusability of APTES-MNPs and APTES-(SBA-15) as catalysts in the preparation of chromeno[2,3-d]pyrimidine derivatives.](/image/article/2013/CY/c2cy20543b/c2cy20543b-f2.gif) | ||
| Fig. 2 Reusability of APTES-MNPs and APTES-(SBA-15) as catalysts in the preparation of chromeno[2,3-d]pyrimidine derivatives. | ||
Experimental
All reagents were purchased from Merck and Aldrich and used without further purification. All yields refer to isolated products after purification. 3-Aminopropyltriethoxysilane coated on magnetic Fe3O4 nanoparticles [APTES-MNPs]13 and 3-aminopropyltriethoxysilane coated on SBA-15 [APTES-(SBA-15)]14 were prepared according to literature procedure. The NMR spectra were recorded on a Bruker Avance DPX 500 MHz instrument. The spectra were measured in CDCl3 relative to TMS (0.00 ppm). Melting points were determined in open capillaries with a BUCHI 510 melting point apparatus. TLC was performed on silica-gel Poly Gram SIL G/UV 254 plates.General procedure for the synthesis of chromeno[2,3-d]pyrimidine derivatives (4)
A stirred mixture of salicylaldehydes (1) (2 mmol), malononitrile (2) (1 mmol), secondary amine (3) (1 mmol), and [APTES-MNPs] (5 mg) or [APTES-(SBA-15)] (5 mg) was reacted at room temperature for the appropriate times. After completion of the reaction as indicated by TLC, the solid product was dissolved in CH2Cl2 and then the catalyst was separated from the reaction mixture by an external magnet for [APTES-MNPs], or centrifugation for [APTES-(SBA-15)]. Then solvent was removed by evaporation and the crude solid product was purified by a recrystallization procedure in ethanol. To recover the catalyst, [APTES-MNPs] and [APTES-(SBA-15)] were washed with CH2Cl2 (2 × 5 mL) and dried under reduced pressure.All known products have been reported in the literature and they were characterized by comparing their melting point, IR and NMR spectra with authentic samples.15–17
Conclusions
We have described a rapid and highly efficient method for the green synthesis of chromeno[2,3-d]pyrimidine derivatives using APTES-MNPs and APTES-(SBA-15) as catalysts at room temperature under solvent-free conditions. With such successful results, this convenient and efficient protocol should provide a superior alternative to the existing methods because of its fast and clean reactions and high yields. Furthermore, it's simple work-up procedure will make the present methods useful and important for the mild synthesis of chromeno[2,3-d]pyrimidine derivatives. This methodology offers significant improvements such as simplicity in operation, and green aspects by avoiding expensive or corrosive catalysts.Acknowledgements
We are thankful to the University of Sistan and Baluchestan Research Council for the partial support of this research.Notes and references
- J. H. Clark and C. N. Rhodes, Clean Synthesis Using Porous Inorganic Solid Catalysts and Supported Reagents. RSC Clean Technology Monographs, ed. Clark, Royal Society of Chemistry, Cambridge, United Kingdom, 2000.
- K. Asakura, A. M. Balu and P. B. Balbuena, Catalysis, ed. J. J. Spivey and M. Gupta, P. B. Balbuena (Contributor), Royal Society of Chemistry, Cambridge, United Kingdom, 2012, vol. 24 Search PubMed.
- V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara and J. M. Basset, Chem. Rev., 2011, 111, 3036 CrossRef CAS.
- R. Richards, Surface and Nanomolecular Catalysis, Taylor & Francis (CRC Press), New York, USA, 2006 Search PubMed.
- V. Polshettiwar and R. S. Varma, Green Chem., 2010, 12, 743 RSC.
- S. Shylesh, V. Schünemann and W. R. Thiel, Angew. Chem., Int. Ed., 2010, 49, 3428 CrossRef CAS.
- A. Katiyar, S. Yadav, P. G. Smirniotis and N. G. Pinto, J. Chromatogr., 2006, 1122, 13 CrossRef CAS.
- B. G. Trewyn, J. A. Nieweg, Y. Zhao and V. S. Y. Lin, Chem. Eng. J., 2008, 137, 23 CrossRef CAS.
- H. Yarahmadi and H. R. Shaterian, J. Chem. Res., 2012, 36, 49 CrossRef CAS.
- H. Yarahmadi and H. R. Shaterian, J. Chem. Res., 2012, 36, 52 CrossRef CAS.
- H. R. Shaterian, M. Mohammadnia and F. Moradi, J. Mol. Liq., 2012, 172, 88 CrossRef CAS.
- H. R. Shaterian and M. Ranjbar, J. Mol. Liq., 2011, 160, 40 CrossRef CAS.
- M. Z. Kassaee, H. Masrouri and F. Movahedi, Appl. Catal., A, 2011, 395, 28 CrossRef CAS.
- X. Wang, K. S. K. Lin, J. C. C. Chan and S. Cheng, J. Phys. Chem. B, 2005, 109, 1763 CrossRef CAS.
- R. Ghahremanzadeh, T. Amanpour and A. Bazgir, Tetrahedron Lett., 2010, 51, 4202 CrossRef CAS.
- A. K. Gupta, K. Kumari, N. Singh, D. S. Raghuvanshi and K. N. Singh, Tetrahedron Lett., 2012, 53, 650 CrossRef CAS.
- A. Zonouzi, M. Biniaz, R. Mirzazadeh, M. Talebi and S. W. Ng, Heterocycles, 2010, 81, 1271 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |