Recent progress in the development of near-infrared fluorescent probes for bioimaging applications
Zhiqian
Guo
ab,
Sookil
Park
c,
Juyoung
Yoon
*a and
Injae
Shin
*c
aDepartment of Chemistry and Nano Science, Global Top 5 Research Program, Ewha Womans University, Seoul 120-750, Korea. E-mail: jyoon@ewha.ac.kr
bKey Laboratory for Advanced Materials and Institute of Fine Chemicals, East China University of Science & Technology, Shanghai 200237, P. R. China
cDepartment of Chemistry, Yonsei University, Seoul 120-749, Korea. E-mail: injae@yonsei.ac.kr
First published on 19th September 2013
Abstract
Near-infrared (NIR) fluorescent dyes have emerged as promising modalities for monitoring the levels of various biologically relevant species in cells and organisms. The use of NIR probes enables deep photon penetration in tissue, minimizes photo-damage to biological samples, and produces low background auto-fluorescence from biomolecules present in living systems. The number of new analyte-responsive NIR fluorescent probes has increased substantially in recent years as a consequence of intense research efforts. In this tutorial review, we highlight recent advances (2010–2013) made in the development and applications of NIR fluorescent probes. The review focuses on NIR fluorescent probes that have been devised to sense various biologically important species, including ROS/RNS, metal ions, anions, enzymes and other related species, as well as intracellular pH changes. The basic principles involved in the design of functional NIR fluorescent probes and suggestions about how to expand applications of NIR imaging agents are also described.
Key learning points(1) Usefulness of NIR fluorescent probes for in vitro and in vivo studies(2) NIR sensing systems to detect biologically relevant species (3) Strategy for design of NIR fluorescent probes (4) Sensing mechanisms of NIR fluorescent probes (5) Applications of NIR fluorescent probes |
1. Introduction
Fluorescence imaging techniques have become powerful tools for noninvasive visualization of biological processes in real time with high spatial resolution.1–3 In particular, near-infrared (NIR) emission greatly facilitates in vivo imaging of molecular processes.4,5 Over the past decade, NIR fluorescent probes have become promising modalities for monitoring in vitro and in vivo levels of various biologically important species.6–8 In general, NIR fluorophores are defined as substances that emit fluorescence in the NIR region (650–900 nm).Compared to most other conventional fluorescent probes, those that rely on NIR fluorescence possess unique advantages for tracing molecular processes in vitro and in vivo.9 First of all, high tissue auto-fluorescence taking place from indigenous biomolecules in the living systems does not interfere with NIR emission. Moreover, NIR photons can penetrate relatively deeply into tissues and they cause less damage to biological samples. Undoubtedly, NIR fluorescent probes will provide chemists and biologists with many opportunities to conduct studies leading to a greater understanding of biological processes at the molecular level. Actually, NIR probes have been employed to successfully image tumors both in vitro and in vivo. In addition, some other applications in clinical practices have also been developed.
Quite a few reviews have been compiled describing NIR fluorophores.4–8 For example, in 2010, Strongin and coworkers published a nice review on “NIR dyes for bioimaging applications”,4 which covers recent progress made up to 2009 in the synthesis and evaluation of new NIR-active organic dyes. Very recently, a critical review of far-red to NIR fluorescent probes was published by Lin and coworkers.8 However, for the most part, these reviews focused on improvements made in the photophysical and photochemical properties of conventional NIR organic dyes used for potential bioimaging applications. Many interesting studies, related to the use of NIR fluorescent probes for tracing biologically important species in vitro and in vivo, have been carried out recently. In particular, advances have been made in improving in vivo performance of NIR imaging agents through encapsulation of NIR fluorescent probes in nanoparticles.
In this tutorial review, the results of recent studies of NIR fluorescent probes are described using a format that is based on sensing biological species. Attention is given to contributions appearing in the 2010–2013 time period and to NIR fluorescent probes used for sensing various important biological species, including reactive oxygen species (ROS) and reactive nitrogen species (RNS), metal ions and anions, enzymes and other related species, as well as intracellular pH changes. The primary focus is given to progress made in the application of functional NIR fluorescent probes for tracing important intracellular species. In addition, the design strategies, sensing mechanisms, and interaction modes of representative NIR fluorescent probes are also discussed. Finally, suggestions are given about the possible design and application of new NIR fluorescent probes.
2. ROS and RNS sensing
Free radicals and other intracellular reactive species serve as important signaling agents in physiological and pathological events that govern cellular functions. Currently, it is clear that ROS/RNS are important messengers that mediate various biological processes. However, a great challenge still exists in detecting ROS/RNS in vivo owing to their reactive and short-lived nature.10 Because of their advantageous properties, NIR fluorescent probes would be highly useful for tracking ROS and RNS in cells, tissues and organisms at or near sites of their production and action.2.1 Sensing ROS
Reactive oxygen species (ROS), including hydrogen peroxide (H2O2), hydroxyl radicals (HO˙), superoxide anion radicals (O2˙−) and singlet oxygen (1O2), are highly reactive molecules, which are produced as by-products of cellular metabolism. A reaction-based strategy is generally employed to design NIR fluorescent probes for various ROS targets.As a major ROS in living systems, H2O2 plays a key role as a second messenger in normal cellular signal transduction. The involvement of H2O2 in cellular signaling and disease states has motivated the development of chemical tools that can be employed to understand how cells produce and funnel H2O2 into specific signaling pathways in living systems. Fluorescent imaging with H2O2-responsive NIR fluorophores creates a unique opportunity for real-time, noninvasive detection of H2O2 in biological specimens. Currently, boronate-based fluorescent probes have been widely employed to detect intracellular H2O2. The operation of boronate-based NIR probes takes advantage of the fact that aryl boronates are selectively oxidatively transformed to the corresponding phenols by H2O2. Recently, Satchi-Fainaro, Shabat and coworkers exploited NIR cyanine-based probe 1, which contains boronate as a specific reactive moiety, for sensing H2O2 (Fig. 1a).11 The boronate-masked phenol group causes 1 to possess a reduced conjugated π-electron system. Thus, in the absence of H2O2, probe 1 displays almost no fluorescence in aqueous solutions. In contrast, H2O2-promoted transformation of the boronate group in 1 to a phenol group results in generation of an extensively conjugated π-electron system that emits a strong NIR fluorescence signal (λex = 590 nm, λem = 720 nm). This probe was demonstrated to be effective for imaging endogenous H2O2 produced in an acute inflammation mouse model (Fig. 1b).
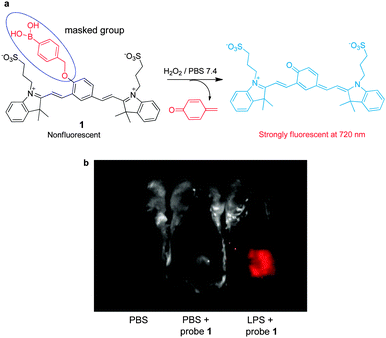 | ||
| Fig. 1 (a) A mechanism for sensing hydrogen peroxide by 1; (b) detection of endogenous hydrogen peroxide produced in a mouse during a lipopolysaccharide (LPS)-induced inflammatory response, using 1 (reprinted from ref. 11 with permission). | ||
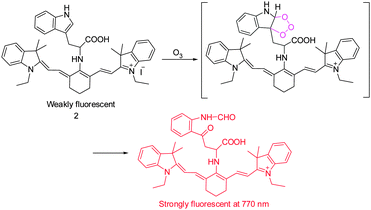
It has been suggested that ozone (O3) is produced endogenously as part of inflammatory and antibacterial responses of the immune system. However, generation of O3 in cells is difficult to prove mainly as a consequence of the lack of sensitive detection methods. In studies aimed at this goal, the tricarboncyanine 2 conjugated L-tryptophan (Trp) as an O3-indicator was developed by Tang's group to serve as a NIR ozone probe (Fig. 2).12 Probe 2 displays weak fluorescence because its excited state is quenched by a twisted intramolecular charge transfer (TICT) mechanism. Upon addition of O3, the L-tryptophan moiety is oxidized to form a ring-opened product, then leading to concurrent turn-on of a NIR fluorescence signal (λem = 770 nm). Probe 2 was found to have a low detection limit of ca. 17 nM for O3 and a high selectivity over other ROS. This NIR probe was employed to monitor the subcellular locations of endogenous O3 in macrophages stimulated by phorbol 12-myristate 13-acetate (PMA).
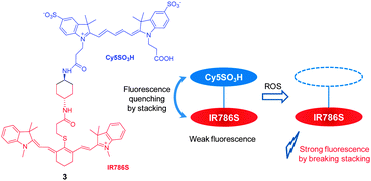
The reactivity patterns of cyanine dyes with ROS have served as the basis for design of a two cyanine-conjugated dye as a novel ROS-sensitive NIR fluorescent probe. It is known that the central polymethine chains of cyanine dyes can be oxidatively cleaved by ROS species, such as singlet oxygen or superoxide anion radical. However, the reactivity of ROS is greatly influenced by chemical structures of the cyanine dyes. In probe 3, where two different cyanine dyes (Cy5SO3H and IR786S) are conjugated to each other via a short linker (Fig. 3),13 fluorescence is quenched owing to stacking of two cyanine dyes but not by a FRET (fluorescence resonance energy transfer) mechanism. Because the Cy5SO3H moiety in 3 is more reactive with ROS than the IR786S group, reaction of the Cy5SO3H moiety in the probe with ROS disrupts the stacking. As a consequence, NIR fluorescence of the IR786S fluorophore at 813 nm is recovered. This probe was successfully used for in vivo imaging of oxidative stress in a mouse model of peritonitis.
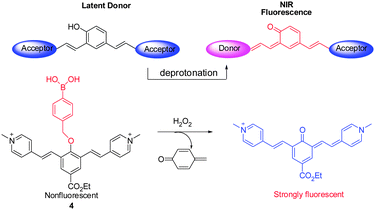
Cyanine dyes are considered to be the most efficient NIR fluorophores, but difficulties in chemical modifications of cyanine dyes serve as a limitation to their widespread application. In order to address this problem, a library of NIR fluorescent probes based on the distinctive donor-two-acceptor (D2A) rational design approach has been constructed by Shabat and coworkers.14 Latent donors and multiple acceptor molecules were incorporated into the fluorescent probes. These probes are designed to undergo intramolecular charge transfer (ICT) in their excited states via the longer π-conjugated systems. For example, the ICT from a donor (e.g., phenolate) moiety to one of the two acceptor moieties (e.g., picolinium moiety) leads to formation of a new fluorochrome that emits NIR fluorescence (700 nm). A representative example of this system is found in NIR probe 4, where a phenol group is masked in the form of a H2O2 reactive boronate (Fig. 4).14 Notably, members of this new family of NIR probes display significant Stokes shifts that lead to a greater signal-to-noise ratio. Analysis of confocal images of H2O2 in HeLa cells utilizing 4 indicates that the probe penetrates into lysosomes.
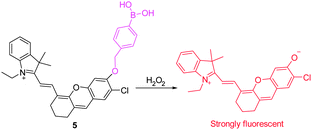
Recently, a new family of NIR fluorescent dyes has been devised for detection of H2O2 by Lin's group (Fig. 5).15 These NIR fluorescent dyes have longer NIR emission maxima (>700 nm) with high fluorescence quantum yields. Significantly, these new NIR dyes have similar properties to those containing the fluorescein/7-hydroxycoumarin platform. Specifically, they are applicable to the design of fluorescent probes based on conversion of a boronate to a phenol group. Taking advantage of their integrated tunable optical properties, the novel NIR fluorescent probe 5 was designed for H2O2 detection. This probe itself exhibits almost no fluorescence when excited at ca. 690 nm. However, following addition of H2O2, a large fluorescence turn-on response occurs with about a 180-fold enhancement in emission at 708 nm. Probe 5 was used to image endogenous H2O2 in macrophages and the peritoneal cavity of mice during LPS-induced inflammatory response.
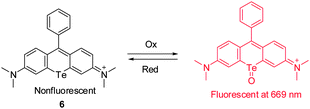
Rhodamines are widely used fluorescent probes and molecular markers because of their excellent photophysical properties. However, the absorption and emission wavelengths of most rhodamine derivatives are below 600 nm. The design of new far-red to NIR rhodamine analogues through chemical modifications of their xanthene core has attracted great interest. Advances have been achieved by replacing the oxygen atom at the 10 position of the xanthene ring system with Si (Si-rhodamine, SiR), Ge (GeR), Sn (SnR) and Te (TeR). For instance, Nagano and coworkers described the new Te-rhodamine derivative 6 and showed that it is a reversible NIR fluorescence probe for detecting ROS (Fig. 6).16 The probe was designed by using an oxidation–reduction strategy that takes advantage of the redox properties of the tellurium (Te) atom. The Te-rhodamine derivative 6 displays very weak fluorescence but its oxidized form strongly fluoresces at 690 nm owing to the reduced heavy-atom quenching effect of Te in the oxidized form. Notably, the oxide form can be readily reduced in the presence of glutathione to regenerate 6. This probe was employed to monitor endogenous production of ROS and subsequent homeostatic recovery of the intracellular reductive environment in H2O2-stimulated HL-60 cells. The redox characteristics and reversible NIR-fluorescence response of 6 suggest that the probe has the potential for continuously monitoring the dynamics of ROS production in vivo.
2.2 Sensing RNS
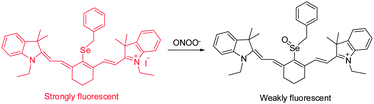
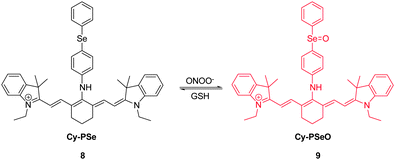
RNS, including peroxynitrite (ONOO−), nitroxyl (HNO), nitrosonium cation (NO+) and nitric oxide (NO), play crucial roles in many physiological and pathological processes. It has been suggested that overproduction of RNS can result in damage and inhibition of the normal functions of lipids, proteins and DNA. For instance, ONOO−, a highly reactive RNS species, reacts with biomolecules, causing cell damage and the onset of numerous diseases. Several NIR probes have been devised for monitoring ONOO−, which are based on the unique reactivity of the organoselenium reagents with peroxynitrite. Inspired by the specific oxidation of divalent selenium (Se2+) by ONOO−, which generates quadrivalent selenium (Se4+), NIR probe 7 was designed to incorporate a benzylselenide moiety into the tricarbocyanine skeleton (Fig. 7).17 The probe displays an intense fluorescence signal at 770 nm. However, after the oxidation of Se2+ to Se4+ oxide by ONOO−, almost no fluorescence is detectable owing to the operation of photoinduced electron transfer (PET) quenching between benzylselenide oxide and the tricarbocyanine group. In order to improve the ONOO− selectivity of 7, the probe was encapsulated into polymeric micelles to produce nanoparticles. The 7-encapsulated nanoprobe was applied for monitoring ONOO− produced in live macrophages.Redox homeostasis is critical for maintaining cellular function and metabolism. NIR fluorescent probes, which monitor ONOO− and enable imaging redox cycles in living cells, have been devised to follow the dynamic redox homeostasis. The NIR cyanine probe 8 operates by reversible ONOO−-promoted oxidation–reduction of the selenium moiety (Fig. 8).18 NIR fluorescence of this probe is quenched by PET between the signal transducer cyanine unit and the modulator 4-(phenylselenyl) aniline group. Oxidation of the selenium moiety by ONOO− inhibits PET, a phenomenon that results in an increase of a fluorescence signal at 775 nm in a “switched on” manner. Notably, as a consequence of the operation of an enzymatic catalytic cycle, the selenoxide group in the oxidized 9 is effectively and rapidly reduced by glutathione (GSH) to produce the original selenide 8, accompanied by a “switched off” decrease of the NIR fluorescence. The probe 8 was successfully used for real-time visualization of the intracellular peroxynitrite concentrations during reversible endogenous ONOO− redox cycles in living cells.
3. Thiol sensing
3.1 Sensing cysteine, homocysteine and glutathione
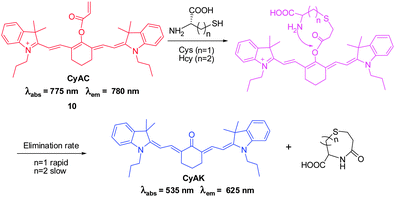
Intracellular thiol-containing molecules, such as cysteine (Cys), homocysteine (Hcy) and glutathione (GSH), play essential roles in maintaining the redox homeostasis of protein, cells and organisms. Abnormal levels of these molecules have been shown to be associated with detrimental effects, such as slow growth, liver damage and skin lesions, on human health.Recently, Yoon, Park and coworkers developed the ratiometric NIR fluorescent probe CyAC ( 10) for selective detection of Cys that operates based on Michael addition of Cys and subsequent intramolecular cyclization of a thioether adduct (Fig. 9).19 In probe 10, an acrylate group serves as the thiol specific trigger moiety. Once the acrylate group is removed from 10 by reaction with Cys, the π-conjugation system of the probe is changed. The results of studies show that probe 10 displays a colorimetric and ratiometric response to Cys. In addition, the probe has an excellent selectivity for Cys over Hcy and GSH due to the greatly different rates of the intramolecular cyclization process that produces the cyclohexanone containing product. A ratiometric fluorescent response of 10 to Cys is enabled by the significant shift in the emission wavelength from 780 to 625 nm in the presence of Cys. The probe was utilized for bioimaging Cys in breast cancer cells. Notably, the modulation of a polymethine π-electron system by conjugation and removal of a specific trigger moiety paves the way for a new strategy for the generation of ratiometric cyanine-based NIR probes.
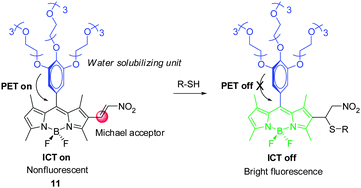
In 2013, Akkaya and coworkers created the Michael reaction-based, nitroethenyl-BODIPY conjugated NIR fluorescent probe 11 for selective detection of biological thiols in aqueous media (Fig. 10).20 Addition of Cys results in blocking the intramolecular charge transfer (ICT) process in 11 owing to disruption of the π-conjugation system. In addition, PET from the electron rich trimethoxyphenyl moiety to the electron deficient BODIPY core is also suppressed. By controlling the reaction time, NIR probe 11 displays selective and sensitive response to Cys over Hcy and GSH.
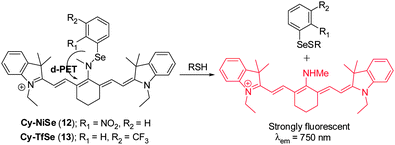
Chen's group designed two NIR fluorescent probes Cy–NiSe ( 12) and Cy–TfSe ( 13) that are based on a Se–N bond cleavage reaction by thiols to produce the selenenyl sulfide (Fig. 11).21 Two strong electron-withdrawing groups, 2-nitrophenylselane and 3-(trifluoromethyl)phenylselane, are present in 12 and 13, respectively. These groups act to modulate the NIR cyanine fluorescence signal through a donor-excited photoinduced electron transfer (d-PET) process. Upon addition of GSH to either probe, a NIR fluorescence enhancement (λem = 750 nm) occurs. Probe 12 and 13 exhibit highly sensitive and selective responses to sulfhydryl-containing molecules compared to other biologically relevant analytes. These two probes were applied to monitoring thiol levels in macrophages and to imaging thiols in rat liver tissues.
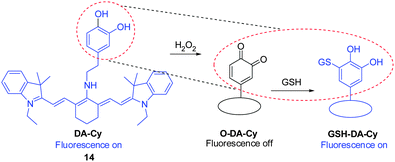
It is important to visualize states of redox stress by using a probe that can respond to changes in both oxidation and reduction events. Guided by the oxidation of the catecholaminergic neurotransmitter dopamine, the dopamine moiety was incorporated as a redox modulator into a NIR cyanine scaffold to produce DA–Cy ( 14, Fig. 12).22 This probe exhibits on–off–on fluorescence changes for detection of both H2O2 oxidative stress and GSH reducing repair processes. Specifically, reaction of 14 with H2O2 triggers formation of a non-fluorescent product O–DA–Cy (switch-off) that is accompanied by a color change from blue to purple. Subsequently, O–DA–Cy undergoes Michael addition reaction with GSH to produce GSH–DA–Cy, which displays NIR fluorescence at 755 nm (switch-on). Probe 14 was used to detect intracellular redox environments in living cells. Studies using fresh rat hippocampus tissues show that 14 is suitable for monitoring H2O2 oxidative stress and GSH reducing repair in vivo.
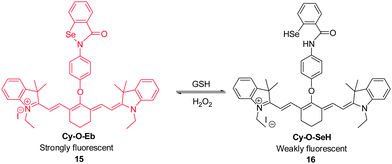
Because alterations in the redox status of substrates are closely associated with physiological and pathological processes, it is important to be able to trace these changes in living systems. For example, the redox couple composed of GSH and hydrogen peroxide in living cells plays key roles in redox homeostasis and signaling. In order to establish a real-time in vivo imaging method to monitor changes in the redox status of this couple, Tang's group developed the NIR fluorescent probe Cy–O–Eb ( 15, Fig. 13).23 This probe can be used to determine changes in GSH–H2O2 levels based on an on–off fluorescence change associated with opening of the five-membered ring promoted by Se–N bond cleavage. In the presence of GSH, the Se–N bond of 15 is cleaved to form Cy–O–SeH ( 16), in which NIR fluorescence at 768 nm is efficiently quenched by a PET process. Addition of H2O2 to 16 induces its oxidation that leads to recovery of the five-membered ring in 15 concomitant with an increase in the fluorescence intensity. Notably, this reversible redox cycle can be repeated several times. Probe 15 has been applied to real-time imaging of changes in the redox status of GSH/H2O2 during apoptosis and used for monitoring H2O2 concentration changes at the wound margin in zebrafish larvae.
3.2 Sensing H2S
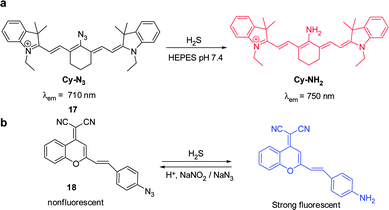
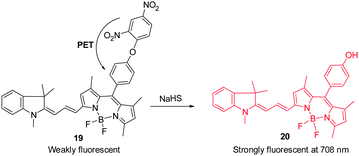
Hydrogen sulfide is produced through reduction of sulfate, sulfite and thiosulfate by anaerobic bacteria. This molecule freely diffuses into cells where it reaches toxic levels, leading to suppression of cellular respiration and proliferation. Endogenously produced H2S is involved in a number of biological processes. Advances made in studies of H2S have provided a greater understanding of the properties and functions of this substance in biological systems.24 The strong reducing or nucleophilic properties of H2S have been used advantageously in the design of fluorescent probes. For example, reduction of the fluorophore-tethered azide in the probe Cy–N3 ( 17) by H2S, leading to generation of the corresponding amine Cy–NH2, is accompanied by a change in the fluorescence signal (Fig. 14a).25 This change is a consequence of the electron-donating ability of the amine substituent present in Cy–NH2. Recently, Fan and Peng et al. described a new NIR dicyanomethylene-4H-pyran chromophore ( 18) for in vivo monitoring of H2S that relies on the same strategy (Fig. 14b).26 In the presence of H2S, the azide group of 18 is converted to an NH2 group, a process that brings about a 65-fold enhancement of the fluorescence signal at 670 nm. Probe 18 has been utilized to track H2S in living cells. Notably, the probe was also applied to H2S imaging in mice.Lin's group developed the fluorescent turn-on H2S probe ( 19), which was designed using a strategy that is based on dinitrophenyl ether chemistry (Fig. 15).27 Probe 19 displays almost no fluorescence signal owing to efficient PET quenching. However, when the dinitrobenzene moiety in 19 is removed by H2S to generate 20, a fluorescence signal appears at 708 nm (turn-on). Analysis of bioimaging data shows that probe 19 senses H2S in living cells in a dose dependent manner.
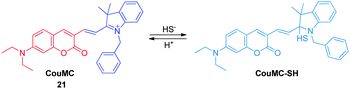
Inspired by the nucleophilic addition of a phenol anion to the indolenium C-2 atom in the spiropyran, Guo and coworkers recently designed a ratiometric H2S sensor CouMC ( 21, Fig. 16),28 which has a quick response rate to H2S and, as a result, can be utilized for real-time intracellular imaging. As a hybrid of coumarin and merocyanine fluorophores, probe 21 exhibits ratiometric sensing behavior derived from reaction with H2S at the indolenium C-2 center, which leads to suppression of merocyanine fluorescence, while retaining coumarin emission. It has been shown that probe 21 displays a higher selectivity for H2S than other biological thiols including Cys, Hcy and GSH, which are the main interfering species in intracellular H2S detection. Moreover, probe 21 has a faster response (about 30 s) than most other reported H2S probes. Thus, this probe has great benefits when account is taken of the variable nature and quick metabolism of endogenous H2S in biological systems. The results of living cell imaging experiments show that probe 21 is useful for ratiometric tracking of H2S in mitochondria.
4. Ion sensing
4.1 Sensing metal ions
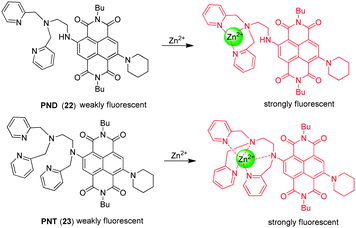
In order to detect mobile zinc ions, Tian and coworkers developed the naphthalenediimide-based NIR fluorescent probes PND ( 22) and PNT ( 23) (Fig. 17),29 in which a naphthalenediimide (NDI) fluorophore is conjugated to the zinc binding receptors, di(pyridin-2-ylmethyl)ethane-1,2-diamine (DPEA) and tri(pyridin-2-ylmethyl)ethane-1,2-diamine (TPEA), respectively. The two probes contain the strongly electron donating piperidine moiety, which causes a long wavelength shift (or bathochromic shift) of the absorption and emission maxima in comparison with the analogous bromine-substituted probe. Interestingly, probes 22 and 23 with respective DPEA and TPEA moieties display different binding behaviors toward Zn2+. PND ( 22) shows a significant increase in fluorescent intensity upon zinc ion binding as a consequence of the relief of PET quenching by the lone pair of electrons on the tertiary nitrogen atom in the DPEA moiety.
However, the fluorescence and absorption maxima of PNT ( 23) undergo distinct blue shifts with concomitant fluorescence enhancement upon binding to Zn2+. The blue shift suggests that the nitrogen attached to the NDI moiety participates in binding to Zn2+, forming a 5-coordinate complex and resulting in a decrease in its electron donating ability. The additional pyridyl nitrogen is also a ligand, forming a 5-coordinate Zn2+ complex. The synergistic chelating effect causes 23 to have a lower selectivity toward Zn2+ than 22, reflected by the fact that the response of 23 to the zinc ion is interfered with by Co2+, Ni2+ and Cd2+. Both probes have been applied to imaging Zn2+ in living cells where an increase in the fluorescence is observed in zinc ion-treated cells.
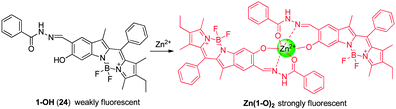
Zhu and coworkers developed the 6-hydroxyindole BODIPY-based NIR fluorescent probe 24 for Zn2+ detection (Fig. 18).30 Probe 24 contains a BODIPY scaffold with salicylaldehyde benzoyl hydrazone as a tridentate chelating moiety for Zn2+. The sensing mechanism of 24 is based on a chelation-enhanced fluorescence (CHEF) effect induced by target-triggered deprotonation. In general, dyes that contain acyclic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds are weakly fluorescent because isomerization about the C
N bonds are weakly fluorescent because isomerization about the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds efficiently deactivates the excited states. However, upon binding of Zn2+ to 24, the phenol group is deprotonated and the C
N bonds efficiently deactivates the excited states. However, upon binding of Zn2+ to 24, the phenol group is deprotonated and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond becomes incorporated in a small chelate ring system. As a consequence, the resulting complex, Zn(1-O)2, displays a long wavelength emission at 680 nm with a Zn2+ detection limit of 9.7 × 10−7 M. Importantly, probe 24 shows great selectivity for Zn2+ in aqueous solutions with little interference from Cd2+. This sensor has been used to monitor Zn2+ located in the perinuclear area of cytosol in living cells.
N bond becomes incorporated in a small chelate ring system. As a consequence, the resulting complex, Zn(1-O)2, displays a long wavelength emission at 680 nm with a Zn2+ detection limit of 9.7 × 10−7 M. Importantly, probe 24 shows great selectivity for Zn2+ in aqueous solutions with little interference from Cd2+. This sensor has been used to monitor Zn2+ located in the perinuclear area of cytosol in living cells.
We developed the cyanine-based NIR fluorescent probe 25 for detecting endogenous zinc ions in living cells and organisms (Fig. 19a).31 This probe is composed of a cyanine-based fluorophore conjugated to tris(2-pyridylmethyl)amine (TMPA) as a receptor for Zn2+. When the TMPA moiety in 25 binds to Zn2+, the amine attached to the center of the polymethine chain of tricarbocyanine is readily deprotonated to form an imine that is cross-conjugated with the less delocalized diamino-tetraene group. Reduced delocalization induces a large hypsochromic shift in both absorption and emission maxima of 25. While free 25 has an absorption maxima at 670 nm, Zn2+-bound 25 shows an absorption maxima at 510 nm and the ratio of the absorbance is proportional to Zn2+ concentrations. In addition, 25 has almost no emission at 590 nm upon excitation at 510 nm. However, the emission maximum of the probe undergoes a large hypsochromic shift from 730 nm to 590 nm upon binding to Zn2+. Moreover, probe 25 has a very strong binding affinity (Kd = 1.2 nM) and a good selectivity for Zn2+ over other metal ions. The properties of 25 enable it to serve as an excellent probe to monitor Zn2+ in the nanomolar range. Importantly, this probe was used to detect Zn2+ released during apoptosis and intact Zn2+ in the neuromasts of zebrafish (Fig. 19b), which is not observed for other zinc probes, such as NBD-TPEA and ZTRS.
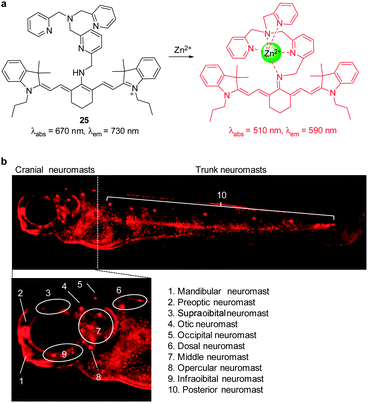 | ||
| Fig. 19 (a) A proposed mechanism for sensing Zn2+ by 25; (b) fluorescent detection of zinc ions in neuromasts of zebrafish using 25 (reprinted from ref. 31 with permission). | ||
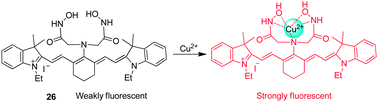
Tang and coworkers developed the NIR fluorescent probe 26, composed of tricarbocyanine as a fluorophore and 2,2′-azanediyl bis(N-hydroxyacetamide) as a Cu2+ receptor, for detection of Cu2+ (Fig. 20).32 While free 26 displays weak fluorescence because of a PET quenching mechanism, its fluorescence is strongly enhanced upon binding to Cu2+, which blocks PET quenching. Probe 26 has an absorption maximum at 750 nm and emission maximum at 800 nm. However, upon binding to Cu2+, a 30 nm blue-shift of the emission band occurs with a six-fold increase in the fluorescence quantum yield. Probe 26, which has good selectivity toward Cu2+ over other biologically relevant cations, has been employed to image exogenous Cu2+ in live cells and zebrafish. It should be noted that because probe 26 displays a more strong fluorescence in an acidic than in a neutral environment after binding to Cu2+, it can be used to image Cu2+ in the lysosome (pH ∼ 5).
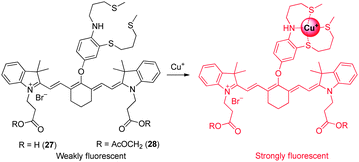
Owing to the potent redox activity of the copper ions, detection of Cu+ in biological systems is also important. Chang and coworkers developed the NIR fluorescent probe 27 for in vivo imaging of labile Cu+ (Fig. 21).33 The probe consists of a cyanine dye as a fluorophore and electron-rich 9-aza-2,6,13-trithiapentadecane as a Cu+-selective receptor. The probe does not experience a spectral shift upon binding to Cu+, but a 15-fold enhancement in fluorescence intensity at 790 nm is observed in response to Cu+ as a consequence of suppression of the PET quenching mechanism. Probe 27 has a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry and a dissociation constant (Kd) of 3.0 × 10−11 M with Cu+. For biological applications, a modified form of 27 was produced. The new probe 28 has enhanced cell-membrane permeability. Once inside cells, the acetoxymethyl group in 28 is removed by intracellular esterases to generate 27 whose carboxylates help trap the probe within cells. The result of in vitro and in vivo experiments show that probe 28 detects labile Cu+ in living cells and mice. In addition, the probe was employed to monitor the pathological development of Wilson's disease in mice by detecting copper levels.
1 binding stoichiometry and a dissociation constant (Kd) of 3.0 × 10−11 M with Cu+. For biological applications, a modified form of 27 was produced. The new probe 28 has enhanced cell-membrane permeability. Once inside cells, the acetoxymethyl group in 28 is removed by intracellular esterases to generate 27 whose carboxylates help trap the probe within cells. The result of in vitro and in vivo experiments show that probe 28 detects labile Cu+ in living cells and mice. In addition, the probe was employed to monitor the pathological development of Wilson's disease in mice by detecting copper levels.
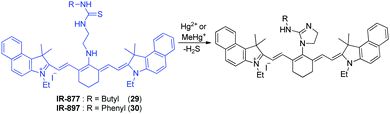
Tian and coworkers developed the tricarbocyanine-based NIR fluorescent probes 29 and 30 to monitor Hg2+ and CH3Hg+ (Fig. 22).34 The response of these dyes to mercury species is based on the strong thiophilic affinity of mercury. Binding of mercury species to the probes promotes desulfurization of the thiourea moiety and cyclization to generate the corresponding cyclic guanidines. In the cyclization process, the central bridging secondary amine of the tricarbocyanine is converted to the imidazoline tertiary amine. As a consequence, the electron-donating ability of this nitrogen is attenuated and, thus, an ICT process operating in the probe is sharply reduced. This change leads to a large red shift in absorption maxima for 29 (from 664 nm to 840 nm) and 30 (from 668 nm to 830 nm), which allows detection of the color change by the naked eye. In fluorescent properties, probe 30 displays an emission maximum at 780 nm, the intensity of which is remarkably decreased in the presence of Hg2+, upon excitation at 670 nm. However, mercury ion-induced turn-on fluorescence is observed at 830 nm upon excitation at 810 nm. A similar fluorescence response to Hg2+ is observed in 30. These probes show a great selectivity to Hg2+ over other metal ions. The results of cell experiments reveal that probes 29 and 30 can be applied to the monitoring of mercury ions in cells. Jiang and coworkers also developed a cyanine-based NIR fluorescent probe for Hg2+,35 which is composed of a tricarbocyanine fluorophore and a thymine (T) moiety that is known to associate with Hg2+ to form T–Hg2+–T. The binding stoichiometry of the probe with Hg2+ was determined to be 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, suggesting the formation of T–Hg2+–T. However, the probe was not applied for bioimaging Hg2+.
1, suggesting the formation of T–Hg2+–T. However, the probe was not applied for bioimaging Hg2+.
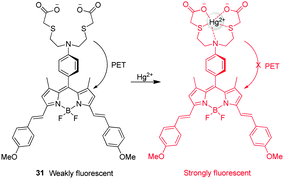
The turn-on NIR fluorescent probe 31 for Hg2+, which contains a BODIPY fluorophore, was developed by Guo and coworkers (Fig. 23).36 Despite their many advantageous features, NIR fluorescent dyes generally suffer from low fluorescence efficiencies. To overcome this problem, a new strategy was employed which relied on indirect excitation from the S0 to the S2 state of the probe. Probe 31 contains di(methoxystyryl)-BODIPY as a fluorophore and a Hg2+ binding moiety. In the absorption spectra of 31, a strong S0 → S2 transition exists at 370 nm although the typical S0 → S1 transition is also observed at 640 nm. In general, the photon number absorbed is dependent on the wavelength and it increases with a decrease in the wavelength. In probe 31, more photons with high-energy are absorbed by the indirect S0 → S2 excitation than by the direct S0 → S1 excitation, leading to a 2.5-fold emission enhancement. Upon addition of Hg2+, the long-wavelength band of 31 undergoes a slight red shift, while a remarkable 30-fold enhancement of the fluorescence signal at 655 nm takes place as a result of the suppression of PET from the aniline-containing receptor to BODIPY. Although Ag+, Ni2+ and Cd2+ cause a slight increase in fluorescence of 31 at 655 nm, the probe has a good selectivity for Hg2+ over other metal ions. This probe was successfully employed to monitor exogenous Hg2+ in living cells. Akkaya and coworkers also exploited a BODIPY-based Hg2+-selective probe,37 which consists of the dithiaazacrown ligand as a Hg2+ binder and two BODIPY fluorophores that can function as an excitation energy donor and acceptor. The excited state energy of the donor BODIPY is transferred to the receptor BODIPY. This probe has a good selectivity for Hg2+ over other metal ions, but it has not been applied for bioimaging Hg2+ due to its poor water solubility.
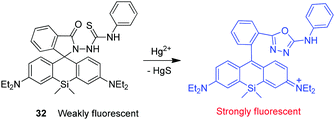
Wu and coworkers developed a silicone-incorporated rhodamine-based NIR fluorescent probe 32 for detection of Hg2+ (Fig. 24).38 Unlike typical rhodamine dyes, Si-rhodamine has absorption and emission in the NIR region because of the low-lying LUMO caused by σ* → π* conjugation. Owing to the red-shifted absorption band, Si-rhodamine has a blue color when its spirolactone or spirolactam ring is opened. Upon binding to Hg2+, 32 undergoes desulfurization of the thiourea moiety and cyclization to form the corresponding oxadiazole. During this process, the probe changes from being colorless to iconic blue. The spirolactam ring-opened form of the probe has the absorption band centered at 664 nm and a turn-on fluorescence maximum centered at 680 nm. Probe 32 shows a marked enhancement (ca. 500-fold) in fluorescence intensity in response to Hg2+. This probe has a high selectivity for Hg2+ over other ions and has been utilized to image exogenous Hg2+ in living cells.
4.2 Anions
Like their metal cation counterparts, anions often play crucial roles in biological processes. Therefore, detection of the biologically relevant anions with high selectivity and sensitivity in biological systems is an important goal. The key pathway followed by most NIR fluorescent probes, which are designed to detect anions, relies on the affinity of anions to metal cations, especially copper ions, that are incorporated into probes. This process leads to release of the NIR fluorophore (displacement approach) and concomitant restoration of its fluorescence. Several NIR fluorescent probes for detecting specific anions, which utilize this approach, have been described recently.Hydrogen cyanide (HCN), produced by Pseudomonas aeruginosa, is suggested to be involved in the pathogenesis of cystic fibrosis lung disease. Therefore, monitoring cyanide in vivo is an important component of studies aimed at gaining an understanding of the process of CN− production by bacteria and the detrimental effect of HCN on cystic fibrosis patients. Toward this end, we developed a new NIR fluorescent probe for detecting cyanide anions.39 The precursor probe ( 33) is composed of a tricarbocyanine dye and a Cu2+ receptor moiety (Fig. 25a). When 33 binds to Cu2+ to form the complex 33-Cu2+, the absorption maximum at 718 nm shifts to 743 nm and the fluorescence at 748 nm (excitation at 680 nm) is quenched. When CN− is added to a solution of 33-Cu2+, the fluorescence intensity at 748 nm increases as a result of the reaction of CN− with Cu2+ that forms the stable [Cu(CN)x]n− and it is completely recovered after addition of excess CN−. Other anions do not trigger this sensing system, indicating that 33-Cu2+ selectively responds to CN−. This probe has been successfully employed to detect biogenic CN− produced by P. aeruginosa in C. elegans (Fig. 26).
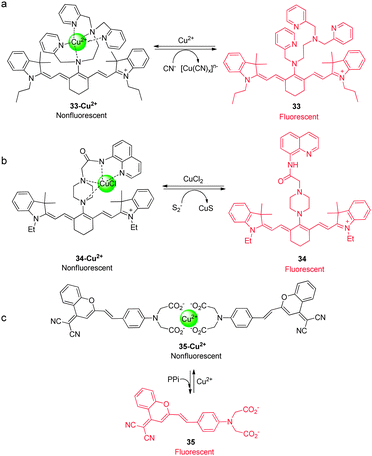 | ||
| Fig. 25 A proposed mechanism for sensing (a) CN− by 33-Cu2+, (b) sulfide anions by 34-Cu2+, and (c) PPi by 35-Cu2+. | ||
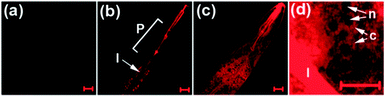 | ||
| Fig. 26 NIR imaging of CN− in C. elegans. The nematodes were exposed to various concentrations of NaCN for 4 h. (a) No cyanide; (b) 1 μM CN−; (c) 100 μM CN−; (d) the enlarged image in the vicinity of the intestine in the nematode exposed to 100 μM NaCN (P = pharynx; I = intestine; n = nucleus; c = cytosol) (reprinted from ref. 39). | ||
A similar approach was used to design probes to detect sulfide and pyrophosphate (PPi) anions. To monitor sulfide anions, Lin and coworkers developed a NIR fluorescent probe 34-Cu2+, which is composed of a tricarbocyanine dye, an 8-aminoquinoline binder and copper (Fig. 25b).40 The precursor probe ( 34) exhibits fluorescence emission at 794 nm, which is almost completely quenched by addition of Cu2+. The probe has a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding mode and the association constant of 3.0 × 105 M−1 with Cu2+. When sulfide anions are added to 34-Cu2+, turn-on fluorescence at 794 nm takes place bringing about a return of its intensity to essentially that of the free probe 34. This phenomenon is caused by coordination of Cu2+ to sulfide to form the stable CuS (Ksp = 1.27 × 10−36). Probe 34-Cu2+ has a high selectivity for sulfide over other anions, however, it has not been applied to monitoring sulfide in cells.
1 binding mode and the association constant of 3.0 × 105 M−1 with Cu2+. When sulfide anions are added to 34-Cu2+, turn-on fluorescence at 794 nm takes place bringing about a return of its intensity to essentially that of the free probe 34. This phenomenon is caused by coordination of Cu2+ to sulfide to form the stable CuS (Ksp = 1.27 × 10−36). Probe 34-Cu2+ has a high selectivity for sulfide over other anions, however, it has not been applied to monitoring sulfide in cells.
Zhu and coworkers developed a NIR fluorescent probe 35-Cu2+, which is highly selective to PPi in aqueous solutions (Fig. 25c).41 The precursor probe ( 35), which consists of dicyanomethylene-4H-chromene as a fluorophore and an iminodiacetate group as a receptor, exhibits NIR fluorescence at 675 nm that is quenched by chelation of Cu2+ to the diacetate moiety of the probe. This quenching phenomenon is observed only upon addition of Cu2+ but not other metal ions. Probe 35 forms a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with Cu2+. When PPi is added to 35-Cu2+, fluorescence at 675 nm increases, but its intensity does not completely return to that of free 35, suggesting that coordination of PPi is not strong enough to cause complete removal of Cu2+ from 35-Cu2+. This probe displays a good selectivity for PPi over the various anions, but H2PO4−, HPO42− and PO43− slightly respond to the probe. Incubation of cells with 35-Cu2+ promotes turn-on fluorescence in the perinuclear area of the cytosol.
1 complex with Cu2+. When PPi is added to 35-Cu2+, fluorescence at 675 nm increases, but its intensity does not completely return to that of free 35, suggesting that coordination of PPi is not strong enough to cause complete removal of Cu2+ from 35-Cu2+. This probe displays a good selectivity for PPi over the various anions, but H2PO4−, HPO42− and PO43− slightly respond to the probe. Incubation of cells with 35-Cu2+ promotes turn-on fluorescence in the perinuclear area of the cytosol.
5. Sensing pH changes
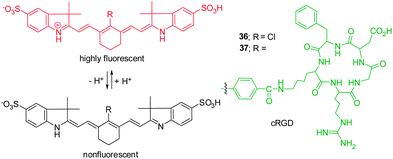
Knowledge of intracellular or extracellular pH changes provides important information about the nature of physiological and pathological processes.42 Because acidic environments are generally associated with inflammation and tumor, sensing slight decreases in pH in tumor microenvironments has become an important issue in the design of anti-tumor therapy. The requirements for imaging tumor microenvironments have driven the development of NIR pH-activatable indicators for visualization of pH changes.Cyanine-based pH probes have attracted great attention as a result of their unique NIR spectra properties. In general, two strategies have been devised for the design of pH-sensitive probes. The first relies on reversible protonation and deprotonation of the indolium ring nitrogen of pH-sensitive cyanine dyes, which leads to alternations of π electronic charge densities. For instance, Achilefu and coworkers constructed the non-N-alkylated indolium cyanine 36 as a pH indicator (Fig. 27).43 Probe 36 is almost non-fluorescent when the nitrogen atom is not protonated, but it becomes strongly fluorescent at 800 nm upon protonation in acidic environments. The characteristic alternation of π electronic charge densities in protonated 36 results in the hypsochromic shift of the absorption maxima. To improve the tumor targeting sensitivity and specificity of the probe, a cyclic arginine-glycine-aspartic acid (cRGD) group was conjugated to a pH-sensitive NIR dye to form probe 37.43 The results of in vitro and in vivo studies show that 37 is suitable for the detection of primary and metastatic breast tumors in both subcutaneous and orthotopic mouse models with high sensitivity and specificity.
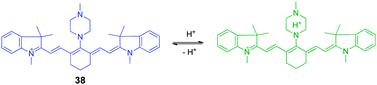
The second strategy for construction of pH-sensitive cyanine dyes relies on the suppression or enhancement of PET quenching through protonation/deprotonation of the electron donor moiety. These types of pH-sensitive dyes, exemplified by 38 (Fig. 28), consist of a cyanine fluorophore and a nitrogen-containing electron donor. For example, an ethylenediamine or piperazine moiety is conjugated to the cyanine ring system to prepare ratiometric NIR fluorescent pH probes that have various pKa values. In these probes, the excitation wavelengths of amine-substituted tricarbocyanine can be modulated by controlling the electron-donating abilities of the amine substituent. In this way, the pH-sensitive probe 38 exhibits observable shifts of its absorption maximum.44 The drastic changes in the absorption spectral maxima permit its use as a reversible ratiometric pH probe. The strategy involving tuning the pKa value of the diamine moiety provides an effective method to design and predict ratiometric measurements of pH changes over a wide range both in vitro and in vivo.
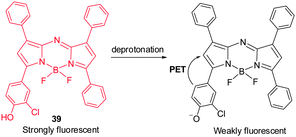
Although BODIPY dyes have good brightness and excellent photo-stability characteristics as compared to NIR cyanine dyes, they suffer from the fact that their absorption maxima lie in the visible region and, thus, they are not optimal for noninvasive in vivo imaging. As part of efforts made to improve photophysical properties of BODIPY, Borisov and coworkers explored a BF2-chelated tetraarylazadipyrromethane dye 39 as a NIR fluorescent pH probe, which is amenable to structural modifications.45 Upon deprotonation of phenol in 39, the probe exhibits large emission intensity changes in the 700 nm region (Fig. 29) as a result of efficient PET from a twisted phenolate to the aza-BODIPY subunit. This pH-sensitive probe has been employed to monitor the pH in corals with a fiber-optic pH optode.
6. Enzyme sensing
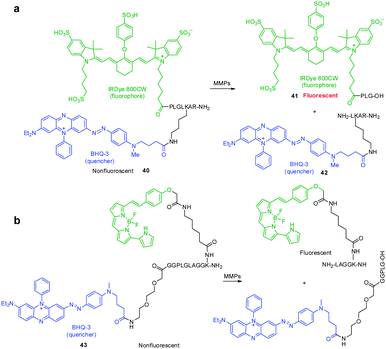
Protease-cleavable peptide probes, which contain a NIR fluorophore linked to a quencher via a peptide substrate, have been developed to detect the activity of matrix metalloproteases (MMPs), which catalyze the cleavage of the extracellular matrix and are known to be a biomarker of tumors, cardiovascular diseases and inflammation. These FRET-based probes are typically non- or very weakly fluorescent. However, when a peptide moiety in the probe is cleaved by action of a protease, the fluorophore and quencher become separated, which induces an enhancement in the fluorescence intensity.An example of this design is found in the MMP NIR probe 40 developed by Linder and coworkers, which possesses the azo-bond containing Black Hole Quencher 3 (BHQ-3) as a quencher and IRDye 800CW as a NIR fluorophore, linked via a peptide moiety (Fig. 30a).46 Although the spectral overlap between fluorescence of IRDye 800CW and absorption of BHQ-3 is not optimal for FRET applications, the fluorescence of IRDye 800CW is almost completely quenched by the presence of BHQ-3. Treatment of the probe with MMPs results in a 56-fold increase in fluorescence, caused by a process that generates the separated fragments 41 and 42 between which FRET quenching cannot occur. When probe 40 is injected into mice, the expected fragment 41 is formed together with several metabolites including a dearginated metabolite IRDye 800CW-PLGLK(BHQ-3)-A and an azo bond cleaved metabolite. This finding shows that although the IRDye 800CW/BHQ-3 pair is useful for FRET applications, instability of the azo bond in BHQ-3 needs to be considered for in vivo studies. In a later effort, Nagano and coworkers employed a similar approach in developing FRET probe 43 to detect MMP activity in vitro and in vivo (Fig. 30b).47 It should be noted that this strategy can be used to exploit FRET-based NIR fluorescent probes for detecting the activities of other enzymes.
7. Nanomaterial-based NIR fluorescent probes
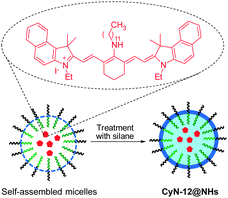
Fluorescent nanomaterials have been extensively explored in the context of biological and biomedical applications. In particular, owing to their enhanced permeability and retention effect, these materials have found great use in monitoring tumor. Zhu and coworkers prepared the NIR fluorescent nanoparticle CyN-12@NHs with a size of ca. 35 nm for in vivo bioimaging of tumors (Fig. 31).48 The nanocomposite is constructed by encapsulation of a tricarbocyanine dye into the hydrophobic core, through self-assembly of amphiphilic block copolymer PS-b-PAA (polystyrene-b-polyacrylamide), and subsequent crosslinking of the micellar assemblies with silane. CyN-12@NHs has some advantageous characteristics compared to those of the free dye, in that fluorescent dyes encapsulated in the nanoparticle display enhanced chemical and photo-stability, better resistance to ROS, and good water solubility. CyN-12@NHs has an absorption maximum at 690 nm and emits strong fluorescence at 800 nm. This nanoparticle was used to image cancer cells and tumors in xenografted mice. Li and coworkers developed biodegradable core-crosslinked polymeric micelles, which contain both NIR fluorophores and 111indium, for dual modal, NIR fluorescent and nuclear tumor imaging.49 This nanomaterial has been applied for in vivo detection of tumors in mice by γ-scintigraphy and NIR fluorescence.8. NIR chemiluminescent dyes
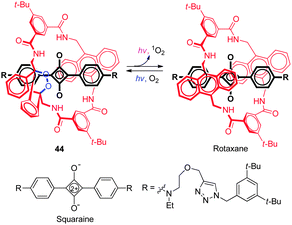
Chemiluminescent probes have some advantages over conventional fluorescent probes that arise because of their inherent high signal-to-noise ratios. However, most chemiluminescent dyes are short-lived as a consequence of their spontaneous degradation when exposed to light and, as a result, they cannot be stored for long periods, and they normally exhibit visible light emission. Recently a NIR fluorescent and chemiluminescent dye ( 44) was developed by Smith and coworkers (Fig. 32).50 This dye possesses an interlocked rotaxane skeleton, which is composed of dumb-bell-shaped squaraine encapsulated in a tetralactam macrocycle with a thermally unstable 9,10-anthracene monoendoperoxide group. Probe 44 is produced by irradiation of the parent rotaxane with red light in the presence of air and can be stored for a long period of time at −20 °C. However, upon warming 44 to 37 °C, the dye undergoes a unimolecular cycloreversion reaction to release singlet oxygen and concomitantly emit NIR light with maxima at 733 nm from the encapsulated squaraine chromophore. It was suggested that, in this process, energy transfer takes place from singlet oxygen to the encapsulated squaraine. When 44, adsorbed onto microparticles, was injected into mice, strong chemiluminescence was observed in relatively deep tissues owing to very low background emission from the animal.9. Concluding remarks
In this review, recent advances (2010–2013) made in the study of NIR fluorescent probes were described. Particular attention was given to the application of NIR fluorescent probes for bioimaging various biologically important species. The design strategies, sensing mechanisms, and interaction modes of representative NIR fluorescent probes were also discussed in the context of strategies to design and apply NIR fluorescent probes to important biological problems.Despite the great advances that have been made in this area, large challenges remain in the development of NIR fluorescent probes for in vivo biological imaging. For example, to date, the only clinically FDA approved material for this purpose is indocyanine green (ICG). The major obstacle in devising suitable fluorophores as NIR platforms is the need for these systems to have excellent chemical and photochemical properties such as high quantum yields, large Stokes shifts, greater photo-stability, and ready modification. Future efforts aimed at designing new NIR fluorescent probes will need to focus on how the NIR fluorescence signals will be integrated with more versatile functionalities. The next generation functional fluorescent probes will be required to not merely respond to the presence of reactive small biological species by NIR fluorescent signals. In addition, NIR fluorescent probes can be utilized as a component of the delivery system to carry and monitor the drug at the specific target site in an accurate manner without causing photo-damage to tissues.
Significant opportunities and great challenges remain in the development of ideal NIR fluorescence probes that can be employed as imaging and therapeutic agents in vivo. It should be noted that nanoparticle-based NIR theranostic agents, which overcome several drawbacks of small molecule cancer drugs and imaging agents, have already been designed and constructed. Clearly, devising general and effective approaches to versatile NIR fluorescent probes will be the impetus for developing exciting new methods for optical imaging in vivo.
Acknowledgements
This work was supported by the National Creative Research Initiative program (grant no. 2010-0018272 to I.S. and 2012R1A3A2048814 to J.Y.). Z. G. acknowledges NSFC/China (Grant 61177034) and the Shanghai Pujiang Program.Notes and references
- X. Chen, T. Pradhan, F. Wang, J. S. Kim and J. Yoon, Chem. Rev., 2012, 112, 1910 CrossRef CAS PubMed.
- H. N. Kim, Z. Guo, W. Zhu, J. Yoon and H. Tian, Chem. Soc. Rev., 2011, 40, 79–93 RSC.
- Y. Yang, Q. Zhao, W. Feng and F. Li, Chem. Rev., 2013, 113, 192 CrossRef CAS PubMed.
- J. O. Escobedo, O. Rusin, S. Lim and R. M. Strongin, Curr. Opin. Chem. Biol., 2010, 14, 64 CrossRef CAS PubMed.
- S. A. Hilderbrand and R. Weissleder, Curr. Opin. Chem. Biol., 2010, 14, 71 CrossRef CAS PubMed.
- K. Kiyose, H. Kojima and T. Nagano, Chem.–Asian J., 2008, 3, 506 CrossRef CAS.
- G. Qian and Z. Y. Wang, Chem.–Asian J., 2010, 5, 1006 CrossRef CAS PubMed.
- L. Yuan, W. Lin, K. Zheng, L. He and W. Huang, Chem. Soc. Rev., 2013, 42, 622 RSC.
- Near-Infrared Spectroscopy: Principles, Instruments, Applications, ed. H. W. Siesler, Y. Ozaki, S. Kawata and H. M. Heise, Wiley-VCH, 2008 Search PubMed.
- X. Chen, X. Tian, I. Shin and J. Yoon, Chem. Soc. Rev., 2011, 40, 4783 RSC.
- N. Karton-Lifshin, E. Segal, L. Omer, M. Portnoy, R. Satchi-Fainaro and D. Shabat, J. Am. Chem. Soc., 2011, 133, 10960 CrossRef CAS PubMed.
- K. Xu, L. Sun, S. P. Li, L. Li, M. Qiang and B. Tang, Chem. Commun., 2012, 48, 684 RSC.
- D. Oushiki, H. Kojima, T. Terai, M. Arita, K. Hanaoka, Y. Urano and T. Nagano, J. Am. Chem. Soc., 2010, 132, 2795 CrossRef CAS PubMed.
- N. Karton-Lifshin, L. Albertazzi, M. Bendikov, P. S. Baran and D. Shabat, J. Am. Chem. Soc., 2012, 134, 20412 CrossRef CAS PubMed.
- L. Yuan, W. Lin, S. Zhao, W. Gao, B. Chen, L. He and S. Zhu, J. Am. Chem. Soc., 2012, 134, 13510 CrossRef CAS PubMed.
- Y. Koide, M. Kawaguchi, Y. Urano, K. Hanaoka, T. Komatsu, M. Abo, T. Terai and T. Nagano, Chem. Commun., 2012, 48, 3091 RSC.
- J. Tian, H. Chen, L. Zhuo, Y. Xie, N. Li and B. Tang, Chem.–Eur. J., 2011, 17, 6626 CrossRef CAS PubMed.
- F. Yu, P. Li, G. Li, G. J. Zhao, T. S. Chu and K. L. Han, J. Am. Chem. Soc., 2011, 133, 11030 CrossRef CAS PubMed.
- Z. Guo, S. W. Nam, S. Park and J. Yoon, Chem. Sci., 2012, 3, 2760 RSC.
- M. Isik, T. Ozdemir, I. S. Turan, S. Kolemen and E. U. Akkaya, Org. Lett., 2013, 15, 216 CrossRef CAS PubMed.
- R. Wang, L. Chen, P. Liu, Q. Zhang and Y. Wang, Chem.–Eur. J., 2012, 18, 11343 CAS.
- F. Yu, P. Li, P. Song, B. Wang, J. Zhao and K. Han, Chem. Commun., 2012, 48, 4980 RSC.
- K. Xu, M. Qiang, W. Gao, R. Su, N. Li, Y. Gao, Y. Xie, F. Kong and B. Tang, Chem. Sci., 2013, 4, 1079 RSC.
- W. Xuan, C. Sheng, Y. Cao, W. He and W. Wang, Angew. Chem., Int. Ed., 2012, 51, 2282 CrossRef CAS PubMed.
- F. Yu, P. Li, P. Song, B. Wang, J. Zhao and K. Han, Chem. Commun., 2012, 48, 2852 RSC.
- W. Sun, J. Fan, C. Hu, J. Cao, H. Zhang, X. Xiong, J. Wang, S. Cui, S. Sun and X. Peng, Chem. Commun., 2013, 49, 3890 RSC.
- X. Cao, W. Lin, K. Zheng and L. He, Chem. Commun., 2012, 48, 10529 RSC.
- Y. Chen, C. Zhu, Z. Yang, J. Chen, Y. He, Y. Jiao, W. He, L. Qiu, J. Cen and Z. Guo, Angew. Chem., Int. Ed., 2013, 52, 1688 CrossRef CAS PubMed.
- X. Lu, W. Zhu, Y. Xie, X. Li, Y. Gao, F. Li and H. Tian, Chem.–Eur. J., 2010, 16, 8355 CrossRef CAS PubMed.
- J. Cao, C. Zhao, X. Wang, Y. Zhang and W. Zhu, Chem. Commun., 2012, 48, 9897 RSC.
- Z. Guo, G.-H. Kim, I. Shin and J. Yoon, Biomaterials, 2012, 33, 7818 CrossRef CAS PubMed.
- P. Li, X. Duan, Z. Chen, Y. Lin, T. Xie, L. Fang, X. Li, M. Yin and B. Tang, Chem. Commun., 2011, 47, 7755 RSC.
- T. Hirayama, G. C. Van de Bittner, L. W. Gray, S. Lutsenko and C. J. Chang, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 2228 CrossRef CAS PubMed.
- Z. Guo, W. Zhu, M. Zhu, X. Wu and H. Tian, Chem.–Eur. J., 2010, 16, 14424 CrossRef CAS PubMed.
- H. Zheng, X.-J. Zhang, X. Cai, Q.-N. Bian, M. Yan, G.-H. Wu, X.-W. Lai and Y.-B. Jiang, Org. Lett., 2012, 14, 1986 CrossRef CAS PubMed.
- Y. Zhao, X. Lv, Y. Liu, J. Liu, Y. Zhang, H. Shi and W. Guo, J. Mater. Chem., 2012, 22, 11475 RSC.
- S. Atilgan, T. Ozdemir and E. U. Akkaya, Org. Lett., 2010, 12, 4792 CrossRef CAS PubMed.
- T. Wang, Q.-J. Zhao, H.-G. Hu, S.-C. Yu, X. Liu, L. Liu and Q.-Y. Wu, Chem. Commun., 2012, 48, 8781 RSC.
- X. Chen, S.-W. Nam, G.-H. Kim, N. Song, Y. Jeong, I. Shin, S. K. Kim, J. Kim, S. Park and J. Yoon, Chem. Commun., 2010, 46, 8953 RSC.
- X. Cao, W. Lin and L. He, Org. Lett., 2011, 13, 4716 CrossRef CAS PubMed.
- W. Zhu, X. Huang, Z. Guo, X. Wu, H. Yu and H. Tian, Chem. Commun., 2012, 48, 1784 RSC.
- J. Han and K. Burgess, Chem. Rev., 2010, 110, 2709 CrossRef CAS PubMed.
- H. Lee, W. Akers, K. Bhushan, S. Bloch, G. Sudlow, R. Tang and S. Achilefu, Bioconjugate Chem., 2011, 22, 777 CrossRef CAS PubMed.
- T. Myochin, K. Kiyose, K. Hanaoka, H. Kojima, T. Terai and T. Nagano, J. Am. Chem. Soc., 2011, 133, 3401 CrossRef CAS PubMed.
- T. Jokic, S. M. Borisov, R. Saf, D. A. Nielsen, M. Kűhl and I. Klimant, Anal. Chem., 2012, 84, 6723 CrossRef CAS PubMed.
- K. E. Linder, E. Metcalfe, P. Nanjappan, T. Arunachalam, K. Ramos, T. M. Skedzielewski, E. R. Marinelli, M. F. Tweedle, A. D. Nunn and R. E. Swenson, Bioconjugate Chem., 2011, 22, 1287 CrossRef CAS PubMed.
- T. Myochin, K. Hanaoka, T. Komatsu, T. Terai and T. Nagano, J. Am. Chem. Soc., 2012, 134, 13730 CrossRef CAS PubMed.
- X. Wu, S. Chang, X. Sun, Z. Guo, Y. Li, J. Tang, Y. Shen, J. Shi, H. Tian and W. Zhu, Chem. Sci., 2013, 4, 1221 RSC.
- J. Zhao, S. Song, M. Zhong and C. Li, ACS Macro Lett., 2012, 1, 150 CrossRef CAS PubMed.
- J. M. Baumes, J. J. Gassensmith, J. Giblin, J.-J. Lee, A. G. White, W. J. Culligan, W. M. Leevy, M. Kuno and B. D. Smith, Nat. Chem., 2010, 2, 1025 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |