Facile synthesis of novel Ag3PO4 tetrapods and the {110} facets-dominated photocatalytic activity†
Jun Wanga, Fei Teng*a, Mindong Chena, Jingjing Xua, Yueqin Songb and Xiaolong Zhoub
aJiangsu Key Laboratory of Atmospheric Environment Monitoring and Pollution Control, Innovative Research Laboratory of Environment and Energy, School of Environmental Sciences and Engineering, Nanjing University of Information Sciences and Engineering, Nanjing 210044, P. R. China. E-mail: tfwd@163.com; Fax: +86-25-58731090; Tel: +86-25-58731090
bPetroleum Processing Research Center, East China University of Science and Technology, Shanghai 200237, P. R. China
First published on 18th October 2012
Abstract
Highly uniform silver orthophosphate microcrystals with novel tetrapod morphology are, for the first time, synthesized via a simple hydrothermal route with the assistance of urea. The effect of active crystal facets on the photocatalytic activity is principally investigated. The silver orthophosphate tetrapods exhibit significantly higher visible light activity than the polyhedrons for the degradation of toxic organic compounds due to the highly exposed {110} facets.
Morphology control of photocatalysts has been considered to be one of the most promising avenues to improve their photocatalytic properties.1–5 For example, various TiO2 nanostructures including nanotubes,3 nanosheets,4 and nanorods5 have been designed and synthesized, all of which exhibit higher activity than P25. However, its relatively wide band gap limits the photocatalytic applications under visible light irradiation. Therefore, it is desirable and compulsory to explore efficient visible-light-driven photocatalysts. Recently, silver orthophosphate (Ag3PO4) has been demonstrated to be an active photocatalyst for the degradation of organic pollutants and the oxidation of water under visible light.6,7 Specifically, Ag3PO4 photocatalysts are reported to achieve a quantum efficiency up to 90% at wavelengths longer than 420 nm.8 The research of Ag3PO4 is thus attracting considerable interest. Up to now, many efforts have been devoted to further improving and optimizing their photoelectric and photocatalytic properties by means of semiconductor coupling8,10–12 and polymer composites.13 However, most of the as-mentioned researches mainly focus on the modification of Ag3PO4. It is well known that the morphology of materials is closely related to the exposed facets of the crystals, which directly affect the properties of the catalysts.4b,c In this aspect, Ye and co-workers have succeeded in synthesizing Ag3PO4 rhombic dodecahedrons and cubes.7 Cubic Ag3PO4 submicrocrystals with sharp corners and edges were also obtained in their further research.14 These authors have demonstrated that the Ag3PO4 rhombic dodecahedrons with only {110} facets exposed have a higher photocatalytic activity than the cubes bounded by {100} facets. To the best of our knowledge, only limited morphologies (i.e. cubes and dodecahedrons) have been reported for Ag3PO4.9,15 Thus, the fabrication of Ag3PO4 with novel morphology or microstructure still remains a big challenge. Herein we report, for the first time, the successful synthesis of uniform tetrapod-like Ag3PO4 microcrystals (designated as T-Ag3PO4). Other crystals with tetrapod-like structures, such as CdSe,16 CdTe,17 ZnO,18 CdSe–ZnS,19 and so on, are generally prepared by vapor transport, metallo-organic compound chemical vapor deposition and colloidal chemical methods, in which specialised equipment or the sophisticated operations are needed. It is desirable to explore a simple template-free approach to synthesize the crystals with a novel tetrapod morphology.
In this work, uniform silver orthophosphate tetrapods are synthesized by a simple hydrothermal method without using any template or organic surfactant, during which phosphoric acid was used as the precursor, and urea was added to tune the pH values of the reaction system. It was found that the crystal structure and morphology of the product were significantly affected by the amount of urea added, reaction time and reaction temperature. Particularly, urea was demonstrated to be the key factor to regulate the growth of crystal facets. The experimental details are given in the ESI.† Furthermore, the facet effect of T-Ag3PO4 microcrystals on photocatalytic activity was principally investigated. For comparison, irregular Ag3PO4 crystals were synthesized by a solid-state reaction method.6 The experiment and the SEM results are given in Fig. S1(A, B) of the ESI.†
The characteristic morphology of the sample is shown in Fig. 1(A, B). The sample is composed of uniform tetrapods, in which the four arms are cylindrical microrods with an average diameter of 5 μm and a length of 15–30 μm. To the best of our knowledge, this is the first report on the synthesis of tetrapod-like silver orthophosphate. Fig. 1C shows the X-ray diffraction (XRD) patterns of the as-obtained samples. All the diffraction peaks can be well indexed to the body-centered cubic structure of Ag3PO4 (JCPDS no. 06-0505). However, obvious changes in the intensity ratios for specific peaks are observed. The intensity ratios of (110)/(200) and (222)/(321) for T-Ag3PO4 are 2.9 and 1.6, which are remarkably higher than those (0.56 and 0.9) of the irregular sample, respectively. The results are similar to the report on Ag3PO4 rhombic dodecahedrons with only (110) facets exposed by Ye et al.7 These authors have ascribed the higher intensity ratio of (110)/(200) to the higher surface energy. The UV-vis diffuse absorption spectra of the samples were measured and shown in Fig. S1(C, D) of the ESI.† It was found that the absorption spectra of all the samples exhibit the same absorption edge, and the indirect band gap value is calculated to be 2.38 eV. The degradation activities of rhodamine B (RhB) dyes over the samples are shown in Fig. 1D. Compared with N-doped TiO2,20 both Ag3PO4 samples exhibit higher catalytic activities, and the T-Ag3PO4 shows the highest photocatalytic activity among them. We attribute the higher activity of T-Ag3PO4 to the higher surface energy and more active sites of (110) facet than (200) facet, which has been demonstrated by Ye et al.7
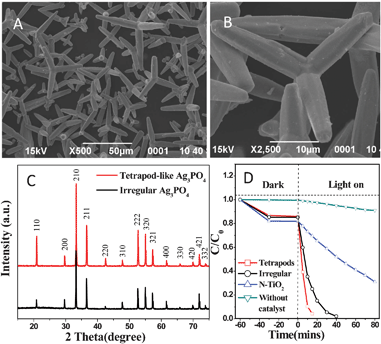 | ||
| Fig. 1 (A, B) SEM images of Ag3PO4 at different magnifications; (C) XRD patterns of tetrapod-like and irregular Ag3PO4; (D) degradation activities of rhodamine B over tetrapod-like Ag3PO4, irregular Ag3PO4 and N-doped TiO2 under visible-light irradiation (λ > 420 nm). | ||
The urea-dependent results are shown in Fig. 2. It was found that the Ag3PO4 can only be obtained in a certain concentration range of urea. When the molar ratios of urea to AgNO3 are lower than 10 or higher than 20, no product can be obtained in our experiments. On one hand, the excess phosphoric acid cannot be neutralized with a small amount of urea. On the other hand, no products can be obtained owing to the formation of Ag(NH3)2+ when much larger amount of urea is used. Herein, Fig. 2(A, B) presents the typical SEM images of the products obtained at molar ratios of urea to AgNO3 (10 and 15) in which the Ag3PO4 polyhedrons and tetrapods are obtained, respectively (low magnification SEM images are given in Fig. S2 of the ESI†). The XRD patterns are shown in Fig. 2C, and the intensity ratios of {110}/{200} and {222}/{321} are given in Fig. 2D. For Ag3PO4 polyhedrons, the peak intensity ratios of (110)/(200) and (222)/(321) are 0.9 and 2.7, respectively (Fig. 2D(a)). When the molar ratios of urea![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AgNO3 was increased to 15, the peak intensity ratio of (110)/(200) for T-Ag3PO4 increased to 2.9, but the peak intensity ratio of (222)/(321) decreased to 1.6 (Fig. 2D(b)). It is obvious that when the particle morphology changes from polyhedron to tetrapod, the (110) facet intensity increases and the (222) facet intensity decreases.
AgNO3 was increased to 15, the peak intensity ratio of (110)/(200) for T-Ag3PO4 increased to 2.9, but the peak intensity ratio of (222)/(321) decreased to 1.6 (Fig. 2D(b)). It is obvious that when the particle morphology changes from polyhedron to tetrapod, the (110) facet intensity increases and the (222) facet intensity decreases.
![SEM images of Ag3PO4 microcrystals prepared at different molar ratios of urea to AgNO3: (A) 10; (B) 15. (C) XRD patterns of Ag3PO4: (a) A, (b) B. (D) Peak intensity ratios of {110}/{200} and {222}/{321}. (E, F) Growth diagram of T-Ag3PO4 tetrapods preferentially along the [110] direction.](/image/article/2013/CE/c2ce26060c/c2ce26060c-f2.gif) | ||
| Fig. 2 SEM images of Ag3PO4 microcrystals prepared at different molar ratios of urea to AgNO3: (A) 10; (B) 15. (C) XRD patterns of Ag3PO4: (a) A, (b) B. (D) Peak intensity ratios of {110}/{200} and {222}/{321}. (E, F) Growth diagram of T-Ag3PO4 tetrapods preferentially along the [110] direction. | ||
In order to understand the formation of tetrapods, an open refluxing system, instead of the hydrothermal method, was further used to synthesize Ag3PO4, while the other conditions were kept constant. In the refluxing conditions, the NH3 and CO2 gases produced by urea can escape from solution at high temperatures. It is clear that only irregular particles are obtained under refluxing conditions (Fig. S3, ESI†). Hence, we could conjecture that the generated NH3 and CO2 molecules may play an important role in the formation of T-Ag3PO4, in which NH3 or CO2 molecules may selectively adsorb on the facets. When a small amount of urea is added, the NH3 molecules produced by urea may be used to neutralize phosphoric acid. We tentatively conjecture that the pH value and adsorption of CO2 may play an important role in the formation of Ag3PO4 polyhedrons. This needs extensive research in the future. With increasing urea, the selective adsorption of NH3 on the {111} planes may restrain the growth of {111} planes so that the peak intensity of (222) decreases correspondingly. Meanwhile, the {110} planes, which may not be absorbed easily by the small molecules, will grow faster than the former (Fig. 2E). As a result, the tetrapod-like particles form. As shown in Fig. 2F, it is clear that polyhedral crystal nucleus with the exposed {111} facets is in the center of tetrapod. While, the four arms are preferentially grow along the [110] direction. Furthermore, the photocatalytic activities of Ag3PO4 polyhedrons and tetrapods were investigated (Fig. S4, ESI†). The result shows that T-Ag3PO4 with 83.8% {110} planes exposed exhibits higher catalytic activity than the polyhedrons covered with only 32.0% {110} planes. The texture properties of the tetrapods and polyhedrons are also measured (as shown in Table S1, ESI†). The specific surface areas of the tetrapods and polyhedrons are 38.0 and 29.9 m2 g−1, respectively. It is found that the area of {110} planes in the unit mass of tetrapods is three times larger than that of polyhedrons, which is consistent to the reaction rate ratio of tetrapods to polyhedrons in the decomposition experiments. Therefore, we tentatively conjecture that the higher degradation activity of tetrapods may result from its higher percentage of {110} facets than that of polyhedrons. Ye et al. have demonstrated that the {110} facets have higher activities than the other facets in the degradation of organic contaminants.7
The time-dependent experiments were further performed in order to explore the crystal growth process (Fig. 3). The Ag3PO4 microrods were obtained at 4 h, and the pH value was determined to be 3.8–4.2 (Fig. 3(a)). It was found that a few of tetrapods formed at 6 h while many polyhedrons also formed (Fig. 3(b)). At 12 h and 24 h, the uniform tetrapods formed and the polyhedrons disappeared completely (Fig. 3(c, d)). Further prolonging the reaction time to 36 h, the tetrapods were still present (Fig. 3(e)).
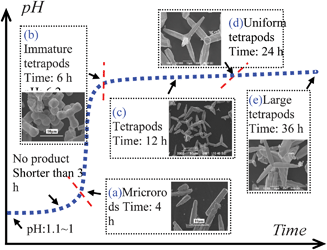 | ||
| Fig. 3 The effects of pH value and reaction time on the particle morphology. | ||
Based on the results above, it is proposed that Ag3PO4 tetrapods form via a process of precipitation–dissolution–recrystallization. The possible reactions could be described as follows:
a) Precipitation.
| CO(NH2)2 + H2O + H3PO4 → H2PO4− + CO2 | (1) |
| Ag+ + H2PO4− → Ag3PO4 (microrods) | (2) |
In the early stages of reaction (pH ≤ 3.2 at ≤ 4 h), the alkali (NH3) produced by urea was mainly used to neutralize the excess phosphoric acid. The Ag3PO4 microrods were precipitated primarily through the reaction between Ag+ and H2PO43−.
b) Dissolving–recrystallization.
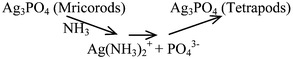 | (3) |
Further prolonging the reaction time, the Ag3PO4 microrods were dissolved by the ammonia released from urea hydrolysis, and the recrystallization process was realized through the reaction of Ag(NH3)2+ with PO43−. As result, the T-Ag3PO4 was obtained.
In summary, we have developed a facile approach to preparing uniform tetrapod-like silver orthophosphate. The crystal morphology can be easily tailored by controlling the amount of urea, and the silver orthophosphate tetrapods exhibit high degradation activity for RhB under visible light irradiation.
Acknowledgements
This work is financially supported by the Project of Six Talents Climax Foundation of Jiangsu (20100292), Science Foundation of Jiangsu Province (BK2012862), Jiangsu Province of Academic Scientific Research Industrialization Projects (JHB2012-10), Jiangsu Province Innovation Platform for Superiority Subject of Environmental Science and Engineering, the Project of “333” High Level Talents Foundation of Jiangsu (20112015), National Science Foundation of China (20943004, 21103049), and Open Project from Jiangsu Key Laboratory of Atmospheric Environment Monitoring and Pollution Control of Nanjing University of Information Science and Technology (kHK1106).References
- X. Chen, S. Shen, L. Guo and S. Mao, Chem. Rev., 2010, 110, 6503 CrossRef CAS.
- (a) L. Spanhel, H. Weller and A. Henglein, J. Am. Chem. Soc., 1987, 109, 6632 CrossRef CAS; (b) R. I. Bickley, T. Gonzalez-Carreno, J. S. Lee, L. Palmisano and R. J. D. Tilley, J. Solid State Chem., 1991, 92, 178 CrossRef CAS; (c) K. E. Karakitsou and X. E. Verykios, J. Phys. Chem., 1993, 97, 1184 CrossRef CAS; (d) J. Zhu, W. Zheng, B. He, J. Zhang and M. Anpo, J. Mol. Catal. A: Chem., 2004, 216, 35 CrossRef CAS.
- K. Shankar, G. K. Mor, H. E. Prakasam, S. Yoriya, M. Paulose, O. K. Varghese and C. A. Grimes, Nanotechnology, 2007, 18, 065707 CrossRef.
- (a) J. Li and D. Xu, Chem. Commun., 2010, 46, 2301 RSC; (b) H. G. Yang, C. Sun, S. Qiao, J. Zou, G. Liu, S. C. Smith, H. M. Cheng and G. Q. Lu, Nature, 2008, 453, 638 CrossRef CAS; (c) H. G. Yang, G. Liu, S. Qiao, C. Sun, Y. Jin, S. C. Smith, J. Zou, H. M. Cheng and G. Q. Lu, J. Am. Chem. Soc., 2009, 131, 4078 CrossRef CAS.
- Y. Li, T. Sasaki, Y. Shimizu and N. Koshizaki, J. Am. Chem. Soc., 2008, 130, 14755 CrossRef CAS.
- Z. Yi, J. Ye, N. Kikugawa, T. Kako, S. Ouyang, H. S. Williams, H. Yang, J. Cao, W. Luo, Z. Li, Y. Liu and R. L. Withers, Nat. Mater., 2010, 9, 559 CrossRef CAS.
- Y. Bi, S. Ouyang, N. Umezawa, J. Cao and J. Ye, J. Am. Chem. Soc., 2011, 133, 6490 CrossRef CAS.
- Y. Bi, S. Ouyang, J. Cao and J. Ye, Phys. Chem. Chem. Phys., 2011, 13, 10071 RSC.
- C. Dinh, T. Nguyen, F. Kleitz and T. Do, Chem. Commun., 2011, 47, 7797 RSC.
- L. Zhang, H. Zhang, H. Huang, Y. Liu and Z. Kang, New J. Chem., 2012, 10, 1039 Search PubMed.
- W. Yao, B. Zhang, C. Huang, C. Ma, X. Song and Q. Xu, J. Mater. Chem., 2012, 22, 4050 RSC.
- G. Li and L. Mao, RSC Adv., 2012, 2, 5108 RSC.
- H. Zhang, H. Huang, H. Ming, H. Li, L. Zhang, Y. Liu and Z. Kang, J. Mater. Chem., 2012, 22, 10501 RSC.
- Y. Bi, H. Hu, S. Ouyang, G. Lu, J. Cao and J. Ye, Chem. Commun., 2012, 48, 3748 RSC.
- Q. Liang, W. Ma, Y. Shi, Z. Li and X. Yang, CrystEngComm, 2012, 14, 2966 RSC.
- X. G. Peng, J. Wickham and A. P. Alivisatos, J. Am. Chem. Soc., 1998, 120(21), 5343 CrossRef CAS.
- L. Manna, D. J. Milliron, A. Meisel, W. C. Scher and A. P. Alivisatos, Nat. Mater., 2003, 2, 382 CrossRef CAS.
- W. D. Yu, X. M. Li and X. D. Gao, Cryst. Growth Des., 2005, 5, 151 CAS.
- X. Xia, Z. Liu, G. H. Du, Y. B. Li and M. Ma, J. Phys. Chem. C, 2010, 114, 13414 CAS.
- D. Mitoraj and H. Kisch, Angew. Chem., Int. Ed., 2008, 47, 9975 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ce26060c |
| This journal is © The Royal Society of Chemistry 2013 |
