Abasic site-binding ligands conjugated with cyanine dyes for “off–on” fluorescence sensing of orphan nucleobases in DNA duplexes and DNA–RNA hybrids†
Yusuke
Sato
,
Megumi
Kudo
,
Yu
Toriyabe
,
Shota
Kuchitsu
,
Chun-xia
Wang
,
Seiichi
Nishizawa
and
Norio
Teramae
*
Department of Chemistry, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan. E-mail: teramae@m.tohoku.ac.jp; Fax: +81-22-795-6552; Tel: +81-22-795-6552
First published on 31st October 2013
Abstract
A series of abasic site-binding ligands conjugated with cyanine dyes have been developed for “off–on” fluorescence sensing of an orphan nucleobase in DNA duplexes and DNA–RNA hybrids.
Many efforts have been recently devoted to develop fluorescent ligands that can bind to a specific site in DNA duplexes.1 This class of fluorescent ligands has been utilized as useful probes for detecting the abasic (apyrimidinic or apurinic; AP) sites,2 bulges3 and mismatched sites,4 by which biological functions of such lesion sites have been explored. In addition, these fluorescent ligands have significant potential for applicability to gene analysis, especially for typing single nucleotide polymorphisms (SNPs). The binding-induced fluorescence signaling of ligands is expected to allow easy, simple, and cost-effective analysis compared to the conventional approach based on the fluorophore-labelled oligonucleotide probes.5 These ligands also function as fluorescent reporters in label-free DNA-based assays for detecting target analytes.6
In this context, we have developed a series of AP site-binding fluorescent ligands for the analysis of single-base mutation in DNAs2a–e and RNAs.7a,b These ligands can form a pseudo-base pair with an orphan nucleobase opposite the AP site giving fluorescence signals. In DNA genotyping assay, an AP site-containing DNA oligonucleotide probe is hybridized with a target DNA so that the AP site is placed toward a target nucleobase, and subsequently, the ligands can discriminate between the target nucleobases in the preformed AP site-containing DNA duplexes. We recently developed AP site-binding ligands (APLs) conjugated with a benzofurazan derivative, 4-(N,N-dimethylaminosulfonyl)-1,2,3-benzoxadiazole (DBD), and these conjugates showed the fluorescence emission response for the target nucleobases.8 This type of response is clearly advantageous for the practical analysis of SNPs, compared to the fluorescence quenching response of most of the simple APLs.2a–e However, the emission response of a DBD moiety in the conjugates is relatively moderate (ca. 7-fold for 100 nM DNA). In addition, this conjugation does not result in the increase in the binding affinity, since the DBD moiety does not have the ability to bind to DNAs.8
In this work, APL conjugates are improved to form “off–on” fluorescent probes for sensing the target nucleobases in AP site-containing DNA duplexes. Here, DNA-binding cyanine dyes are employed due to their fluorescence enhancement properties by intercalating into DNA duplexes.9,10 Related to the present APL-intercalator conjugates, several purine nucleobase conjugates with acridine derivatives were previously developed by Lhomme's group,11 in which these conjugates were aimed to function as cleavage agents for the AP site-containing DNA duplexes to mimic the AP nucleases. In contrast, our work focused on the development of the fluorescent probes suitable for the analysis of single-base mutation in DNAs. Synthetic APLs capable of recognizing the target pyrimidine nucleobases2b or G2c were conjugated with fluorescence emitting cyanine dyes. Then, these conjugates allow the discrimination of the target nucleobases opposite the AP site by monitoring cyanine fluorescence.
2-Amino-5,6,7-trimethyl-1,8-naphthyridine (ATMND) was chosen as the APL moiety in the conjugate, due to its strong binding with cytosine (C) and thymine (T) opposite the AP site in the DNA duplexes.2b As for the cyanine dyes, we utilized thiazole orange (TO).12 Aminoethyl groups were incorporated into ATMND according to our previous study,8 and the resulting ATMND derivative was coupled with the carboxylate-terminated decanyl (C10) spacer-containing TO derivative13 (ESI†), to afford the ATMND–TO conjugate (Fig. 1).
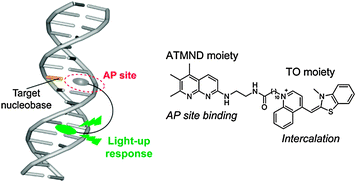 | ||
| Fig. 1 Schematic illustration of the binding of an APL–cyanine conjugate for an AP site-containing DNA duplex. Chemical structure of ATMND–TO is also shown. | ||
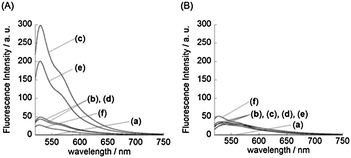
First, we examined the fluorescence response of ATMND–TO to 21-mer AP site-containing DNA duplexes (5′-d(ATT TGG GTG A![[X with combining low line]](https://www.rsc.org/images/entities/char_0058_0332.gif) A TTG CTC ACA)-3′/3′-d(TAA ACC CAC T
A TTG CTC ACA)-3′/3′-d(TAA ACC CAC T![[N with combining low line]](https://www.rsc.org/images/entities/char_004e_0332.gif) T AAC GAG TGT)-5′,
T AAC GAG TGT)-5′, ![[X with combining low line]](https://www.rsc.org/images/entities/char_0058_0332.gif) = AP site (dSpacer),
= AP site (dSpacer), ![[N with combining low line]](https://www.rsc.org/images/entities/char_004e_0332.gif) = target nucleobase; G, C, A, or T). The DNA sequences were chosen because of their relationship with the antiviral treatment response of the combination therapy of the pegylated interferon-α and ribavirin for chronic hepatitis C.14Fig. 2A shows the fluorescence spectra of the TO moiety of ATMND–TO (100 nM) in the absence and presence of DNA duplexes (100 nM). The fluorescence of the TO moiety is found to be negligible (ϕfl = 0.0043) in the absence of DNA duplexes (curve a), resulting from the nonradiative energy loss by free rotation of the benzothiazole and quinoline rings of the TO moiety.12a In contrast, the addition of DNA duplexes causes a significant increase in the fluorescence intensity of the TO moiety (λem = 530 nm). The responses are much pronounced for AP site-containing DNA duplexes (curves b–e) compared to the fully-matched DNA duplex containing no AP sites (curve f; 5′-d(ATT TGG GTG AAA TTG CTC ACA)-3′/3′-d(TAA ACC CAC TTT AAC GAG TGT)-5′), which suggests a favorable binding of ATMND–TO to the AP site-containing DNA duplexes. The fluorescence response of the TO moiety strongly depends on the target nucleobase opposite the AP site in DNA duplexes. ATMND–TO exhibits the largest response for the target C (curve c; ϕfl = 0.34), in which the fluorescence intensity increases more than 1000-fold. This response is three orders of magnitude larger than that of ATMND–DBD conjugates,8 showing the significance of the “off–on” fluorescence property of the TO moiety. ATMND–TO also shows a large response for the target T (curve e), whereas the responses are small for purine nucleobases (curves b and d). The observed responses of ATMND–TO for the pyrimidine nucleobases are rationalized by the binding selectivity of the ATMND moiety at the AP site,2b which is also supported by the observation of the fluorescence quenching responses of the ATMND moiety in ATMND–TO (Fig. S1, ESI†).
= target nucleobase; G, C, A, or T). The DNA sequences were chosen because of their relationship with the antiviral treatment response of the combination therapy of the pegylated interferon-α and ribavirin for chronic hepatitis C.14Fig. 2A shows the fluorescence spectra of the TO moiety of ATMND–TO (100 nM) in the absence and presence of DNA duplexes (100 nM). The fluorescence of the TO moiety is found to be negligible (ϕfl = 0.0043) in the absence of DNA duplexes (curve a), resulting from the nonradiative energy loss by free rotation of the benzothiazole and quinoline rings of the TO moiety.12a In contrast, the addition of DNA duplexes causes a significant increase in the fluorescence intensity of the TO moiety (λem = 530 nm). The responses are much pronounced for AP site-containing DNA duplexes (curves b–e) compared to the fully-matched DNA duplex containing no AP sites (curve f; 5′-d(ATT TGG GTG AAA TTG CTC ACA)-3′/3′-d(TAA ACC CAC TTT AAC GAG TGT)-5′), which suggests a favorable binding of ATMND–TO to the AP site-containing DNA duplexes. The fluorescence response of the TO moiety strongly depends on the target nucleobase opposite the AP site in DNA duplexes. ATMND–TO exhibits the largest response for the target C (curve c; ϕfl = 0.34), in which the fluorescence intensity increases more than 1000-fold. This response is three orders of magnitude larger than that of ATMND–DBD conjugates,8 showing the significance of the “off–on” fluorescence property of the TO moiety. ATMND–TO also shows a large response for the target T (curve e), whereas the responses are small for purine nucleobases (curves b and d). The observed responses of ATMND–TO for the pyrimidine nucleobases are rationalized by the binding selectivity of the ATMND moiety at the AP site,2b which is also supported by the observation of the fluorescence quenching responses of the ATMND moiety in ATMND–TO (Fig. S1, ESI†).
A control ligand, TO that lacks the ATMND moiety, also exhibits fluorescence enhancement upon binding to DNA duplexes (Fig. 2B); however, the responses are moderate compared to ATMND–TO (cf.Fig. 2A). In addition, TO shows little selectivity for the target nucleobases opposite the AP site in the DNA duplexes. Accordingly, the ATMND moiety in the conjugate is responsible for the significant fluorescence enhancement in the pyrimidine nucleobases.
Fluorescence titration experiments were performed to assess the binding affinity of ATMND–TO to the target nucleobase opposite the AP site in the DNA duplexes (Fig. S2, ESI†). The resulting titration curve was analyzed based on a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model, giving the binding affinity with the dissociation constant (Kd). The Kd value for the target C is thus determined to be 4.7 ± 0.74 nM (n = 3), which is 3.8-fold smaller than that for the target T (Kd = 18 ± 5.1 nM). On the other hand, ATMND–TO shows a weak binding affinity for the purine nucleobases (Kd > 300 nM). These results show the strong binding of ATMND–TO to the pyrimidine nucleobases over the purine ones. It is noteworthy that the affinity of ATMND–TO for pyrimidine nucleobases is one order of magnitude superior to that of the parent ATMND (Kd/nM; C, 44 ± 7.5; T, 193 ± 29) for the same DNA duplex as examined for ATMND–TO. Apparently, the conjugation of the APL with the TO moiety leads to the increased binding affinity in contrast to the conjugation with DBD.8 This can be ascribed to the TO binding in addition to the AP site binding of the ATMND moiety. Indeed, circular dichroism (CD) spectra have revealed the intercalation of the TO moiety into DNA duplexes, where the negative CD band appeared at approximately 506 nm, which coincides with the absorption band of the TO moiety when bound to the target C (Fig. S3, ESI†).15 We note that the spacer length of the conjugate influences the binding and fluorescence sensing properties of the target nucleobases in the AP site-containing DNA duplexes (Fig. S4, ESI†).
1 binding model, giving the binding affinity with the dissociation constant (Kd). The Kd value for the target C is thus determined to be 4.7 ± 0.74 nM (n = 3), which is 3.8-fold smaller than that for the target T (Kd = 18 ± 5.1 nM). On the other hand, ATMND–TO shows a weak binding affinity for the purine nucleobases (Kd > 300 nM). These results show the strong binding of ATMND–TO to the pyrimidine nucleobases over the purine ones. It is noteworthy that the affinity of ATMND–TO for pyrimidine nucleobases is one order of magnitude superior to that of the parent ATMND (Kd/nM; C, 44 ± 7.5; T, 193 ± 29) for the same DNA duplex as examined for ATMND–TO. Apparently, the conjugation of the APL with the TO moiety leads to the increased binding affinity in contrast to the conjugation with DBD.8 This can be ascribed to the TO binding in addition to the AP site binding of the ATMND moiety. Indeed, circular dichroism (CD) spectra have revealed the intercalation of the TO moiety into DNA duplexes, where the negative CD band appeared at approximately 506 nm, which coincides with the absorption band of the TO moiety when bound to the target C (Fig. S3, ESI†).15 We note that the spacer length of the conjugate influences the binding and fluorescence sensing properties of the target nucleobases in the AP site-containing DNA duplexes (Fig. S4, ESI†).
In our conjugates, the binding selectivity of the target nucleobases and the fluorescence emission wavelength can be tuned by adopting the suitable APLs2a–e and DNA-binding cyanine dyes,9,13 respectively. Here, we synthesized a conjugate of a guanine-selective dimethylpterine (DMP) derivative2c with benzothiazole orange (BO)16 and examined its sensing properties (Fig. 3). The DMP–BO conjugate shows fluorescence enhancement response upon binding to DNA duplexes, probably due to the intercalation of the BO moiety (λem = 483 nm).16 The most significant response is observed for the target G opposite an AP site in the DNA duplexes (Kd = 24 ± 1.8 nM), reflecting the binding selectivity of the DMP moiety in the conjugate. DMP–BO shows G-selectivity with a light-up response at 483 nm (blue), while ATMND–TO shows C- and T-selective light-up responses at 530 nm (green). Accordingly, the multiplex analysis of the target nucleobases in the DNA duplexes is facilitated by the simultaneous use of these conjugates in a single solution, where the different target nucleobases can be detected by the selective “off–on” fluorescence response of each conjugate with a specific fluorescence color (Fig. S5, ESI†).
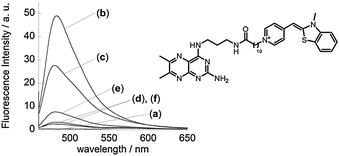 | ||
Fig. 3 Fluorescence spectra of DMP–BO (100 nM) in the (a) absence and presence of 21-mer AP site-containing DNA duplexes (100 nM; 5′-ATT TGG GTG A![[X with combining low line]](https://www.rsc.org/images/entities/char_0058_0332.gif) A TTG CTC ACA-3′/3′-TAA ACC CAC T A TTG CTC ACA-3′/3′-TAA ACC CAC T![[N with combining low line]](https://www.rsc.org/images/entities/char_004e_0332.gif) T AAC GAG TGT-5′, T AAC GAG TGT-5′, ![[X with combining low line]](https://www.rsc.org/images/entities/char_0058_0332.gif) = AP site (dSpacer), = AP site (dSpacer), ![[N with combining low line]](https://www.rsc.org/images/entities/char_004e_0332.gif) = (b) G, (c) C, (d) A, or (e) T) or (f) a fully-matched DNA duplex. Other solution conditions were the same as those given in Fig. 2. Excitation: 448.5 nm. Temperature, 20 °C. Chemical structure of DMP–BO is also shown. = (b) G, (c) C, (d) A, or (e) T) or (f) a fully-matched DNA duplex. Other solution conditions were the same as those given in Fig. 2. Excitation: 448.5 nm. Temperature, 20 °C. Chemical structure of DMP–BO is also shown. | ||
APL–cyanine conjugates exhibit the nucleobase-selective “off–on” fluorescence response for AP site-containing DNA–RNA hybrids, like DNA duplexes (cf.Fig. 2A and 3). From the examination of the hybrids composed of AP site-containing DNA probes and target RNA strands, the most significant fluorescence responses of ATMND–TO and DMP–BO are obtained for the targets C and G opposite the AP site, respectively (Fig. S6, ESI†). Thus, these conjugates are applicable to the analysis of single-base mutation in RNAs while the degree of the response is influenced by the type of the duplex, DNA duplexes or DNA–RNA hybrids. Therefore, it is highly likely that such properties of APL–cyanine conjugates can be useful for the discrimination of microRNA members in their family, as shown based on the fluorescence signaling of APLs.7a,b
In summary, a series of APL–cyanine conjugates has been developed for fluorescence sensing of the orphan nucleobases opposite an AP site in DNA duplexes and DNA–RNA hybrids. The conjugation of APLs with cyanines facilitates “off–on” fluorescence sensing as well as improvement of the binding affinity for the target nucleobases compared to the parent APLs due to the favorable contribution of the intercalation of the cyanine moiety. The present approach can be quite effective for designing fluorescent probes with the applicability to the analysis of single-base mutation in DNAs and RNAs, while their use for the quantitative analysis is limited. These conjugates exhibit the tunable properties of nucleobase-selectivity and fluorescence emission wavelength by adopting suitable APLs and cyanine dyes, respectively. Thus, the preparation of G, C, A, or T (U)-selective conjugates having different fluorescence emission wavelengths are expected to be applicable to the multicolor analysis of DNAs and RNAs. In addition, these conjugates are expected to function as affinity labelling agents for the AP site in various nucleic acid-based assays such as aptamers and molecular beacons.6 We are now undertaking further studies in these directions.
The present work was supported by Scientific Research (S) (No. 22225003) and Scientific Research (B) (No. 24350033) from the Ministry of Education, Culture, Sports, Science and Technology, Japan. Y.S. acknowledges support by Grant-in-Aid for Young Scientists (Start-up; No. 22850001).
Notes and references
- (a) Y. H. Du, J. Huang, X. C. Weng and X. Zhou, Curr. Med. Chem., 2010, 17, 173–189 CrossRef CAS; (b) G. Song and J. Ren, Chem. Commun., 2010, 46, 7283–7294 RSC; (c) B. M. Zeglis, J. A. Boland and J. K. Barton, J. Am. Chem. Soc., 2008, 130, 7530–7531 CrossRef CAS PubMed.
- (a) K. Yoshimoto, S. Nishizawa, M. Minagawa and N. Teramae, J. Am. Chem. Soc., 2003, 125, 8982–8983 CrossRef CAS PubMed; (b) Y. Sato, S. Nishizawa, K. Yoshimoto, T. Seino, T. Ichihashi, K. Morita and N. Teramae, Nucleic Acids Res., 2009, 37, 1411–1422 CrossRef CAS PubMed; (c) Q. Dai, C.-Y. Xu, Y. Satos, K. Yoshimoto, S. Nishizawa and N. Teramae, Anal. Sci., 2006, 22, 201–203 CrossRef CAS; (d) Z. Ye, B. Rajendar, Q. Dai, S. Nishizawa and N. Teramae, Chem. Commun., 2008, 6588–6590 RSC; (e) N. B. Sankaran, S. Nishizawa, T. Seino, K. Yoshimoto and N. Teramae, Angew. Chem., Int. Ed., 2006, 45, 1563–1568 CrossRef CAS PubMed; (f) A. Fakhari M and S. E. Rokita, Chem. Commun., 2011, 47, 4222–4224 RSC; (g) Y. Abe, O. Nakagawa, R. Yamaguchi and S. Sasaki, Bioorg. Med. Chem., 2012, 20, 3470–3479 CrossRef CAS PubMed.
- (a) K. Nakatani, S. Sando and I. Saito, J. Am. Chem. Soc., 2000, 122, 2172–2177 CrossRef CAS; (b) H. Suda, A. Kobori, J. Zhang, G. Hayashi and K. Nakatani, Bioorg. Med. Chem., 2005, 13, 4507–4512 CrossRef CAS PubMed; (c) Z. Xi, R. Y. Zhang, Z. H. Yu, D. Ouyang and R. Q. Huang, Bioorg. Med. Chem. Lett., 2005, 15, 2673–2677 CrossRef CAS PubMed; (d) H. C. Ong, J. F. Arambula, S. R. Ramisetty, A. M. Baranger and S. C. Zimmerman, Chem. Commun., 2009, 668–670 RSC.
- (a) A. Granzhan and M.-P. Teulade-Fichou, Chem.–Eur. J., 2009, 15, 1314–1318 CrossRef CAS PubMed; (b) K. Nakatani, S. Sando, K. Yoshida and I. Saito, Nat. Biotechnol., 2001, 19, 51–55 CrossRef CAS PubMed; (c) B. A. Jackson and J. K. Barton, J. Am. Chem. Soc., 1997, 119, 12986–12987 CrossRef CAS.
- R. T. Ranasinghe and T. Brown, Chem. Commun., 2005, 5487–5502 RSC.
- (a) Y. Xiang, A. Tong and Y. Lu, J. Am. Chem. Soc., 2009, 131, 15352–15357 CrossRef CAS PubMed; (b) Z. Xu, K. Morita, Y. Sato, Q. Dai, S. Nishizawa and N. Teramae, Chem. Commun., 2009, 6445–6447 RSC; (c) Y. Sato, Y. Zhang, S. Nishizawa, T. Seino, K. Nakamura, M. Li and N. Teramae, Chem.–Eur. J., 2012, 18, 12719–12724 CrossRef CAS PubMed.
- (a) Y. Sato, T. Ichihashi, S. Nishizawa and N. Teramae, Angew. Chem., Int. Ed., 2012, 51, 6369–6372 CrossRef CAS PubMed; (b) Y. Sato, Y. Toriyabe, S. Nishizawa and N. Teramae, Chem. Commun., 2013, 49, 9983–9985 RSC.
- C.-X. Wang, Y. Sato, M. Kudo, S. Nishizawa and N. Teramae, Chem.–Eur. J., 2012, 18, 9481–9484 CrossRef CAS PubMed.
- (a) B. A. Armitage, Top. Curr. Chem., 2005, 253, 55–76 CAS; (b) B. A. Armitage, Top. Heterocycl. Chem., 2008, 14, 11–29 CrossRef CAS; (c) A. S. Tatikolov, J. Photochem. Photobiol., C, 2012, 13, 55–90 CrossRef CAS PubMed.
- (a) E. J. Fechter, B. Olenyuk and P. B. Dervan, J. Am. Chem. Soc., 2005, 127, 16685–16691 CrossRef CAS PubMed; (b) J. R. Carreon, K. P. Mahon Jr. and S. O. Kelly, Org. Lett., 2004, 6, 517–519 CrossRef CAS PubMed; (c) M. Thompson and N. W. Woodbury, Biochemistry, 2000, 39, 4327–4338 CrossRef CAS PubMed; (d) M. Thompson, Bioconjugate Chem., 2006, 17, 507–513 CrossRef CAS PubMed.
- (a) N. Berthet, A. Boudali, J. F. Constant, J. L. Decout, M. Demeunynck, A. Fkyerat, J. Garcia, A. Laayoun, P. Michon and J. J. Lhomme, J. Mol. Recognit., 1994, 7, 99–107 CrossRef CAS PubMed; (b) A. Fkyerat, M. Demeunynck, J. F. Constant and J. J. Lhomme, Tetrahedron, 1993, 49, 11237–11252 CrossRef CAS; (c) A. Flyerat, M. Demeunynck, J. F. Constant, P. Michon and J. J. Lhomme, J. Am. Chem. Soc., 1993, 115, 9952–9959 CrossRef.
- (a) J. Nygren, N. Svanvik and M. Kubista, Biopolymers, 1998, 46, 39–51 CrossRef CAS; (b) L. G. Lee, C.-H. Chen and L. A. Chiu, Cytometry, 1986, 7, 508–517 CrossRef CAS PubMed; (c) E. Privat and U. Asseline, Bioconjugate Chem., 2001, 12, 757–769 CrossRef CAS PubMed.
- J. R. Carreon, K. M. Stewart, K. P. Mahon Jr, S. Shin and S. O. Kelly, Bioorg. Med. Chem. Lett., 2007, 17, 5182–5185 CrossRef CAS PubMed.
- Y. Tanaka, N. Nishida and M. Sugiyama, et al. , Nat. Genet., 2009, 41, 1105–1111 CrossRef CAS PubMed.
- (a) A. Larsson, C. Carlsson, M. Johnsson and N. Albinsson, J. Am. Chem. Soc., 1994, 116, 8459–8465 CrossRef CAS; (b) J. T. Petty, J. A. Bordelon and M. E. Robertson, J. Phys. Chem. B, 2000, 104, 7221–7227 CrossRef CAS; (c) C. Cosa, K. S. Focsaneanu, J. R. N. McLean, J. P. McNamee and J. C. Scaiano, Photochem. Photobiol., 2001, 73, 585–599 CrossRef.
- (a) J. Isacsson and G. Westman, Tetrahedron Lett., 2001, 42, 3207–3210 CrossRef CAS; (b) H. J. Karlsson, P. Lincoln and G. Westman, Bioorg. Med. Chem., 2003, 11, 1035–1040 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, synthesis of the conjugates, fluorescence spectra of the ATMND moiety, fluorescence titration experiments, changes in CD spectra of the conjugate upon binding, fluorescence response of different spacer-containing conjugates, multiplex analysis of the target nucleobases, and application to RNA analysis. See DOI: 10.1039/c3cc47717g |
| This journal is © The Royal Society of Chemistry 2014 |