Turning on fluorescence by plasmonic assembly with large tunable spacing: a new observation and its biosensing application†
Shuo-Hui
Cao
,
Wei-Peng
Cai
,
Qian
Liu
,
Kai-Xin
Xie
,
Yu-Hua
Weng
and
Yao-Qun
Li
*
Department of Chemistry and the MOE Key Laboratory of Spectrochemical Analysis & Instrumentation, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005, P. R. China. E-mail: yaoqunli@xmu.edu.cn; Fax: +86-592-2185875; Tel: +86-592-2185875
First published on 7th October 2013
Abstract
Assembling nanoparticle-film metallic junctions with a spacing of up to tens of nanometers efficiently turned on fluorophores attached to the film from the quenching status to an intense surface plasmon-coupled emission. Benefiting from this new finding, a fluorescence biosensor was created based on the use of a biomolecule-linked plasmonic assembly as the trigger.
The plasmonic effects of metallic nanostructures on fluorescence have been receiving considerable attention because of their promising potential application in new methods, probes and devices for the utilization of plasmon mediated fluorescence in many important areas, such as bioanalysis, biomedical research, and clinical testing.1 Previous work has confirmed that surface plasmon-coupled emission (SPCE) could improve fluorescence detection through the propagation of surface plasmons on smooth metallic nano-films, thus showing promise as a biosensing technique.2 In addition, nanoscale junctions and interstices between metallic structures are important plasmonic geometries, which generate enormous electromagnetic enhancement, accompanied by special plasmon-mediated optical phenomena.3–7 In this paper, a fantastic process of “dequenching” SPCE is reported based on a film-coupled particle nanostructure, and for on-chip detection, it can be used to create a novel nanobiotransducer that is able to cause gold-quenched fluorescence to be luminous through the biorecognition-induced assembly of nanostructures.
It is well known that very close proximity to metal materials results in fluorescence quenching through a resonance energy transfer or “contact quenching” process.8 However, we are surprised to find here that the already quenched fluorophores located in the quenching zone of the gold nano-film are turned on by assembling silver nanoparticles (AgNPs) on the film, from the prism coupler adhering to the back of the metal film, radiating the p-polarized emission at a defined angle as SPCE. This dequenching of SPCE, as a result of integrating localized and propagating surface plasmons with fluorophores, is found to support strong coupling when the gap distances of the NPs-film are tuned from nanometers to tens of nanometers which is comparable to the size of biomolecules; thus, the assembly of nanostructures through biomolecular binding events should enable the effective measurement of the dequenched coupling signal. Dequenching-SPCE provides an “off-on” mode which is beneficial to chip-based fluorescence detection with fluorophores directly immobilized on a gold surface, thereby avoiding the complex labeling of biomolecules and an elaborate conformation design for manipulating the distance between the dyes and the gold surface.8 A convenient and general biosensing platform was established based on dequenching-SPCE, and its potential to create a new generation of plasmon-assisted fluorescence biosensing was demonstrated.
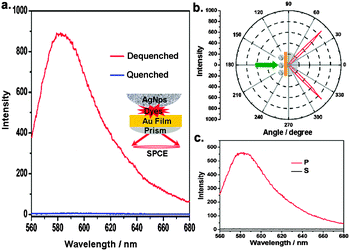
Typical results for dequenching-SPCE are shown in Fig. 1. The dyes (rhodamine B isothiocyanate) were directly modified on a smooth gold film (Fig. S1, ESI†), and complete quenching happened at a close fluorophore–metal distance (Fig. 1a, blue curve). While a strong signal with the same spectrum as that of the fluorophores was observed when the AgNPs were absorbed on the top of the dyes, meaning that the quenched fluorescence was dequenched (Fig. 1a, red curve). In comparison to the isotropic free space emission (FSE) observed for the air side of the gold film, the dequenched signal was distributed at a direction through a prism introduced beneath the film (Fig. 1b), and the emission was found to be highly p polarized (Fig. 1c). The directional and highly polarized emission proves that there was coupling between the fluorophores and plasmons, because only the defined angle and p-polarized emission can satisfy the necessary match of plasmon wavevectors.2 The blank experiments without fluorophores on the film (Fig. S2, ESI†) showed that there was just a low background signal before and after the adsorption of AgNPs on the film. These results prove that the detected signal must be from the interaction of fluorophores with the adjacent nanoparticles and nano-film: fluorophores are strongly influenced by both the localized surface plasmons on the nanoparticles and propagating surface plasmons on the nano-film;6,9 the prism coupler provides matching of the wavevector on the interface, inducing the release of energy as radiation from the prism. Although the physical details of the process are still under investigation, this strong dequenching-SPCE has displayed promising potential in sensing.
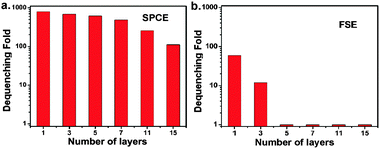
The dequenching ability of SPCE under different conditions was tested to explore its relationship to potential applications, and was evaluated by determining the dequenching fold (DF) which is defined as DFSPCE = (Fafter − Fbackground)/(Fbefore − Fbackground), where Fbefore and Fafter are the detected fluorescence signals before and after the adsorption of metal nanoparticles on the dyes-modified film, and Fbackground is the detected background intensity from the equipment. First, silver and gold nanoparticles with different diameters10 were used in the dequenching experiments. Fig. S3a (ESI†) shows that the dequenching fold increases with increasing the size of the AgNPs, and a huge dequenching ability up to 800-fold could be achieved when 100 nm AgNPs were placed. By contrast, the dequenching degree was much weaker when gold nanoparticles were used: the dequenching degree was no more than 60-fold even when 120 nm gold nanoparticles (Fig. S6, ESI†) were used. This can be explained by the fact that silver supports a much stronger surface plasmon, and the extinction cross section becomes larger with an increase in the size of the nanoparticles, inducing stronger coupling.11 Second, the quantity of dyes modified on the gold film was modulated by varying the concentration of the dyes used for covalent connection. As shown in Fig. S3b (ESI†), a continuous increase in the degree of dequenching can be observed with an increase in the concentration of the fluorophores even up to 100 μM, without the drastic self-quenching (Fig. S7, ESI†) which usually limits the numbers of labels for detection. Thus the dequenching SPCE can be improved simply by greatly increasing the number of fluorophores on the surface. Third, the concentration of AgNPs used for adsorption on the gold surface was varied. The numbers of discernable nanoparticles adsorbed on the gold surface, as shown by SEM images (Fig. S8, ESI†), increase with an increase in the added concentration, leading to the enhancement of the degree of dequenching in the tested range (Fig. S3c, ESI†). This implies that the attachment of nanoparticles is a key factor for dequenching fluorophores on the surface, with the quantity of nanoparticles positively correlated to the dequenching ability, holding the promise for utilizing this attachment to develop assays for analyzing binding events. And obviously a dequenched signal in the presence of a small number of nanoparticles (Fig. S8a, ESI†) also indicates its sensitivity in potential applications. Fourth, we tuned the distance between the AgNPs and the underlying dye-coated gold nano-film through the layer-by-layer deposition of the polyelectrolyte layers at a thickness of around 1 nm to 35 nm.6 It is worth noting that a greater than 100-fold dequenched SPCE signal was still observed with a separation of 15 layers (∼35 nm) (Fig. 2a). While there was little dequenching in FSE when the number of layers was up to 5 (∼12 nm) (Fig. 2b). This could be explained by the slower decay of propagating surface plasmons6 in SPCE on the dimension normal to the surface, compared to the short range surface plasmons in FSE, which contributes to the coupling for strong dequenching at the tested separation distances, and this demonstrates the possibility of introducing a sufficient space between the “sphere” and “surface” which is suitable at the scale of many biological molecules (such as proteins) in binding events for biorecognition. To sum up, large AgNPs and abundant fluorophores in this system are conducive for fluorescence dequenching; assembled AgNPs can be used as the triggers for dequenching-SPCE and work well at a biomolecular scale distance beyond the smooth surface.
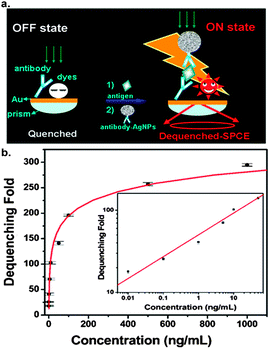
According to the above analysis, we propose a versatile dequenching SPCE biosensor based on assembling AgNPs on a smooth metal surface through the intermediacy of the binding of biomolecules, so as to achieve the revival of quenched fluorophores preloaded on the surface as the directional radiation. A sandwich immunoassay12 was established. As schematically illustrated in Fig. 3a, a large quantity of fluorophores were modified on the gold surface, but these fluorophores were not fluorescent because of quenching. In the presence of antigens, the antibody-tethered AgNPs (diameter ∼100 nm) were attached to the antibody immobilized surface through a sandwich immunoassay, and the strong coupling resulted in a dequenched SPCE signal. Fig. 3b shows that the degree of dequenching increases with an increase in the concentration of the antigens after the immunoassay, and a linear relationship is displayed between the degree of dequenching and the antigen concentration in the range of 0.01–50 ng mL−1 on bi-logarithmic coordinates (R = 0.993), with a detection limit of 7 × 10−3 ng mL−1. This design utilizes the characteristics of SPCE dequenching in an artful way, providing many advantages: first, fluorophores are directly modified on the metal surface, avoiding the intricate labeling of fluorophores to biomolecules; second, the metal surface efficiently quenches the fluorophores, thus suppressing the signal before biorecognition, which contributes to the identification of the signal from the background in applications; third, dequenching-SPCE allows strong coupling for a distance between the nanoparticles and the surface up to the scale of biomolecules, ensuring a sensitive response to the binding of nanoparticles through the agency of biomolecules, and the directional signal is convenient for collection; fourth, the homogeneous distribution of fluorophores and biomolecules on the smooth surface guarantees the reproducibility.
In summary, we have demonstrated that the fluorescence signal can be modulated by the assembly of plasmonic structures. The quenched fluorescence is turned on as a directional emission by the NPs-film assembly and over an 800-fold dequenched signal can be observed under the strong plasmonic coupling. The factors influencing the dequenching ability are described, thus providing a guide for the development of dequenching-SPCE based sensing techniques. A general sensing platform is proposed based on the assembly of AgNPs on a gold nano-film through biorecognition binding, and the sensing of the target species was therefore conveniently confirmed by the appearance of an intense fluorescence signal. In this design, propagating surface plasmons support strong coupling when the spacing between the nanoparticles and film is tuned up to accommodate large biomolecules during binding events, thus improving the feasibility and sensitivity of the biosensors. This method facilitates the introduction of fluorophores and simplifies the labeling of biomolecules; it works on an “off-on” mode with low initial background, holding great promise for effective use in a variety of biosensing applications. Here, the nanostructure doesn’t just play an assisting role for better signal expression, but the process of assembling the nanostructure itself acts as the trigger for fluorescence. This study also provides new insight into plasmonic coupling between metallic nanostructures with the aid of fluorophores.
NSFC (21127005), the 973 Program (2013CB933703), the Doctoral Fund of MOE (20110121110011) and scholarship award for excellent doctoral student granted by MOE are acknowledged.
Notes and references
- J. R. Lakowicz, Anal. Biochem., 2005, 337, 171 CrossRef CAS PubMed; I. A. Larmour and D. Graham, Analyst, 2011, 136, 3831 RSC.
- J. R. Lakowicz, Anal. Biochem., 2004, 324, 153 CrossRef CAS PubMed; K. Aslan and C. D. Geddes, Anal. Chem., 2009, 81, 6913 CrossRef PubMed; T. T. Xie, Q. Liu, W. P. Cai, Z. Chen and Y. Q. Li, Chem. Commun., 2009, 3190 RSC; S. H. Cao, T. T. Xie, W. P. Cai, Q. Liu and Y. Q. Li, J. Am. Chem. Soc., 2011, 133, 1787 CrossRef PubMed; S. H. Cao, W. P. Cai, Q. Liu and Y. Q. Li, Annu. Rev. Anal. Chem., 2012, 5, 317 CrossRef PubMed.
- F. Le, N. Z. Lwin, J. M. Steele, M. Kall, N. J. Halas and P. Nordlander, Nano Lett., 2005, 5, 2009 CrossRef CAS PubMed; V. D. Miljkovic, T. Shegai, M. Kall and P. Johansson, Opt. Express, 2011, 19, 12856 CrossRef PubMed; C. Ciracì, R. T. Hill, J. J. Mock, Y. Urzhumov, A. I. Fernández-Domínguez, S. A. Maier, J. B. Pendry, A. Chilkoti and D. R. Smith, Science, 2012, 337, 1072 CrossRef PubMed.
- K. Leong, Y. Chen, D. J. Masiello, M. T. Zin, M. Hnilova, H. Ma, C. Tamerler, M. Sarikaya, D. S. Ginger and A. K. Y. Jen, Adv. Funct. Mater., 2010, 20, 2675 CrossRef CAS; M. Schmelzeisen, Y. Zhao, M. Klapper, K. Mullen and M. Kreiter, ACS Nano, 2010, 4, 3309 CrossRef PubMed; Y. Fu, J. Zhang and J. R. Lakowicz, Langmuir, 2013, 29, 2731 CrossRef PubMed; Z. Guan, N. Gao, X. F. Jiang, P. Yuan, F. Han and Q. H. Xu, J. Am. Chem. Soc., 2013, 135, 7272 CrossRef PubMed.
- G. Braun, S. J. Lee, M. Dante, T. Q. Nguyen, M. Moskovits and N. Reich, J. Am. Chem. Soc., 2007, 129, 6378 CrossRef CAS PubMed; Y. Liu, S. P. Xu, H. B. Li, X. G. Jian and W. Q. Xu, Chem. Commun., 2011, 47, 3784 RSC; L. P. Du, D. Y. Tang, G. H. Yuan, S. Wei and X. C. Yuan, Appl. Phys. Lett., 2013, 102, 081117 CrossRef.
- J. J. Mock, R. T. Hill, A. Degiron, S. Zauscher, A. Chilkoti and D. R. Smith, Nano Lett., 2008, 8, 2245 CrossRef CAS PubMed.
- N. J. Halas, S. Lal, W. S. Chang, S. Link and P. Nordlander, Chem. Rev., 2011, 111, 3913 CrossRef CAS PubMed.
- B. Dubertret, M. Calame and A. J. Libchaber, Nat. Biotechnol., 2001, 19, 365 CrossRef CAS PubMed; H. Du, C. M. Strohsahl, J. Camera, B. L. Miller and T. D. Krauss, J. Am. Chem. Soc., 2005, 127, 7932 CrossRef PubMed.
- M. H. Chowdhury, K. Ray, C. D. Geddes and J. R. Lakowicz, Chem. Phys. Lett., 2008, 452, 162 CrossRef CAS PubMed.
- X. Y. Dong, X. H. Ji, H. L. Wu, L. L. Zhao, J. Li and W. S. Yang, J. Phys. Chem. C, 2009, 113, 6573 Search PubMed; P. P. Fang, J. F. Li, Z. L. Yang, L. M. Li, B. Ren and Z. Q. Tian, J. Raman Spectrosc., 2008, 39, 1679 CrossRef CAS.
- M. Rycenga, C. M. Cobley, J. Zeng, W. Y. Li, C. H. Moran, Q. Qin, D. Zhang and Y. N. Xia, Chem. Rev., 2011, 111, 3669 CrossRef CAS PubMed.
- J. Ling, Y. F. Li and C. Z. Huang, Anal. Chem., 2009, 81, 1707 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and supplementary figures. See DOI: 10.1039/c3cc46392c |
| This journal is © The Royal Society of Chemistry 2014 |