Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
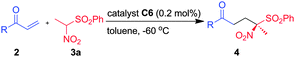
Enantioselective synthesis of α-nitro-δ-ketosulfones via a quinine–squaramide catalyzed conjugate addition of α-nitrosulfones to enones†
Kalisankar Bera and Irishi N. N. Namboothiri*
Department of Chemistry, Indian Institute of Technology Bombay, Mumbai 400 076, India. E-mail: irishi@iitb.ac.in
First published on 26th September 2013
Abstract
Conjugate addition of α-nitrosulfones to vinyl ketones in the presence of 0.2 mol% of a quinine–squaramide organocatalyst afforded α-nitro-δ-ketosulfones possessing a tetrasubstituted chiral center in excellent yield and enantioselectivity in most cases. This strategy also offers a facile and convenient entry into γ-sulfonylhydroxamates that are one carbon homologs of potent enzyme inhibitors.
Sulfonylhydroxamic acids are inhibitors of matrix metalloprotease (MMP) and are effective for the treatment of cancer and arthritis.1 For instance, β-sulfonylhydroxamate 1a, in which a sulfonyl group is attached to a chiral center, is a potent MMP and PDE (phosphodiesterase) inhibitor (Fig. 1).2,3 The enantiopure compound 1a displays superior activity as compared to the racemic one and the enantioselective synthesis of 1a involves the oxidation of an enantioenriched thioether precursor.3 Recently, sulfones attached to a tetrasubstituted chiral center4–6 received considerable attention due to their wide range of biological activities, for instance, against Alzheimer's (as γ-secretase inhibitor 1b)4 and glaucoma.6
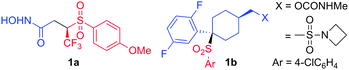 | ||
| Fig. 1 Potent inhibitors of MMP, PDE and γ-secretase. | ||
The sulfonyl group in organosulfones, viz. active methylene sulfones, vinyl sulfones and other sulfone based nucleophiles and electrophiles, takes part in a variety of synthetic transformations and is an easily removable functional group.7
Enantioselective approaches to sulfones include catalytic asymmetric Michael addition of various nucleophiles to β-unsubstituted vinyl sulfones8 and β-substituted vinyl sulfones,9 nucleophilic addition of active methylene10 and methine11,12 sulfones to various electrophiles, and other miscellaneous methods.13,14 However, to our knowledge, generation of sulfones attached to a chiral carbon remains scarcely explored under catalytic asymmetric conditions.12,14
As part of our ongoing research program to develop novel catalytic methods for the asymmetric synthesis of quaternary carbon centers, we have reported enantioselective synthesis of quaternary α-nitro/aminophosphonates.15,16 Herein we report an organocatalyzed enantioselective synthesis of quaternary α-nitro-δ-ketosulfones via Michael addition of α-nitrosulfones to enones using an alkaloid derived squaramide catalyst. Transformations of the product to quaternary γ-nitro-γ-sulfonyl hydroxamic acid and γ-nitro-γ-sulfonyl carboxylic acid are also reported here.
The reaction conditions were optimized by performing Michael addition of nitrosulfone 3a to phenyl vinyl ketone 2a using several cinchona-based bifunctional organocatalysts and solvents at different temperatures (Fig. 2 and Table 1). At the outset, the quinine–thiourea catalyst C1, recently reported from our laboratory,15 was screened (entry 1). To our delight, the Michael adduct 4a, a quaternary α-nitrosulfone, was isolated in good yield (96%) and enantioselectivity (90% ee) when 10 mol% of C1 was employed in mesitylene at rt (entry 1). Other catalysts such as quinine–thiourea C2, cinchonidine–thiourea C3 and cinchonine–thiourea C4 were also quite effective in providing the Michael adduct 4a in excellent yield and selectivity (entries 2–4). Encouraged by these results, further improvement in the enantioselectivity was explored by employing bifunctional cinchona–squaramide catalysts C5–C7 with greater H-bonding capability (entries 5–8).17 Cinchonidine–squaramide catalyst C5 provided product 4a with lower selectivity (entry 5). However, quinine–squaramide catalyst C618 furnished the Michael adduct 4a with improved selectivity (94%) and excellent yield (98%, entry 6). At this juncture, possible enhancement of enantioselectivity by screening various solvents at different temperatures in the presence of 10 mol% of C6 was investigated (entries 8–17). Thus, toluene was identified as the best solvent which at −60 °C provided Michael adduct 4a in 98% yield and 99% ee (entry 17). We were amazed to note that high yield (98%) and selectivity (99% ee) were unaffected even when the catalyst loading was gradually reduced to 5, 2 and 0.2 mol% albeit at the expense of the reaction rate (entries 18–20).
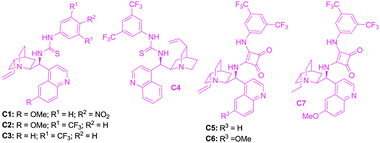 | ||
| Fig. 2 Catalysts screened. | ||
| ||||||
|---|---|---|---|---|---|---|
| Entry | Cat. | Solvent | T (°C) | Time (h) | Yieldb (%) | eec (%) |
| a The reactions were performed at the 0.2 mmol scale.b After silica gel column chromatography.c ee's determined by chiral HPLC.d Opposite enantiomer.e 5 mol% catalyst.f 2 mol% catalyst.g 0.2 mol% catalyst and 0.5 mmol of 3a. | ||||||
| 1 | C1 | Mesitylene | rt | 1 | 96 | 90 |
| 2 | C2 | Mesitylene | rt | 1 | 94 | 88 |
| 3 | C3 | Mesitylene | rt | 1 | 95 | 91 |
| 4 | C4 | Mesitylene | rt | 1 | 93 | 87d |
| 5 | C5 | Mesitylene | rt | 1 | 95 | 80 |
| 6 | C6 | Mesitylene | rt | 1 | 98 | 94 |
| 7 | C7 | Mesitylene | rt | 1 | 98 | 94 |
| 8 | C6 | Xylene | rt | 1 | 97 | 93 |
| 9 | C6 | Toluene | rt | 1 | 97 | 94 |
| 10 | C6 | PhCF3 | rt | 1 | 95 | 88 |
| 11 | C6 | CH2Cl2 | rt | 1 | 86 | 86 |
| 12 | C6 | (CH2)2Cl2 | rt | 1 | 97 | 85 |
| 13 | C6 | THF | rt | 1 | 91 | 92 |
| 14 | C6 | Diethyl ether | rt | 1 | 92 | 86 |
| 15 | C6 | MeCN | rt | 1 | 92 | 70 |
| 16 | C6 | Toluene | −20 | 2 | 98 | 96.4 |
| 17 | C6 | Toluene | −60 | 4 | 98 | 99 |
| 18e | C6 | Toluene | −60 | 5 | 98 | 98.5 |
| 19f | C6 | Toluene | −60 | 8 | 98 | 98.6 |
| 20g | C6 | Toluene | −60 | 11 | 98 | 99 |
The scope of the above reaction was subsequently investigated by treating α-nitrosulfone 3a with various enones 2b–o under the above optimized conditions, i.e. 0.2 mol% catalyst C6, in toluene at −60 °C (Table 2). It is noteworthy that regardless of the steric and electronic properties and the position of substituents on the aromatic ring of enones, the Michael adducts 4a–i were isolated in excellent yields (97–99%) and selectivities (96–99% ee) over a period of 2–20 h (entries 1–9). However, a faster reaction rate was observed in the case of enones possessing electron withdrawing substituents such as NO2, CN, Br and Cl at unhindered positions (entries 5–7 and 9) when compared to enones possessing electron donating substituents such as Me and OMe (entries 2–4). Polycyclic aromatic enones 2j–k, heteroaromatic enones 2l–m and an aliphatic enone 2n were also well tolerated in terms of the chemical and optical yields of the products (entries 10–14), except that 1-naphthyl vinyl ketone 2k furnished the Michael adduct 4k with lower selectivity (72% ee, entry 11). However, since the reaction rate dramatically decreased when using aliphatic enone 2n the reaction had to be conducted at rt with 10 mol% of the catalyst C6 (entry 14). A dienone 2o also furnished the Michael adduct 4o in 98% yield and 97% ee (entry 15). Notably, the reaction is highly regioselective in that α-nitrosulfone 3a selectively reacted with the β-unsubstituted olefin moiety in the presence of a β-substituted olefin moiety in enone 2o (entry 15).
| |||||
|---|---|---|---|---|---|
| Entry | R | Time (h) | 4 | Yieldb (%) | eec (%) |
| a The reactions were performed at the 0.5 mmol scale.b After silica gel column chromatography.c ee determined by chiral HPLC.d This reaction was also performed on a larger scale (vide infra).e Reaction performed at rt. | |||||
| 1 | C6H5 | 11 | 4a | 98 | 99 |
| 2 | 4-MeC6H4 | 10 | 4b | 99 | 96 |
| 3 | 4-OMeC6H4 | 16 | 4c | 98 | 99 |
| 4 | 3,4-(OMe)2C6H3 | 20 | 4d | 99 | 96 |
| 5 | 4-ClC6H4 | 5 | 4e | 98 | >99 |
| 6 | 4-CNC6H4 | 3 | 4f | 99 | >99 |
| 7 | 4-NO2C6H4 | 2 | 4g | 99 | 99 |
| 8 | 2-ClC6H4 | 14 | 4h | 97 | 98 |
| 9 | 3-BrC6H4 | 5 | 4i | 99 | >99d |
| 10 | 2-Naphthyl | 14 | 4j | 98 | 99 |
| 11 | 1-Naphthyl | 15 | 4k | 99 | 72 |
| 12 | 2-Furyl | 12 | 4l | 96 | 99 |
| 13 | 2-Thienyl | 7 | 4m | 97 | 97 |
| 14e | c-C6H11 | 10 | 4n | 86 | 91 |
| 15 | PhCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH CH | 15 | 4o | 98 | 97 |
Encouraged by the excellent results obtained from the reaction of nitrosulfone 3a with a variety of enones 2 (Table 2), the scope of the reaction was investigated further with other sterically and electronically different nitrosulfones 3b–g (Table 3). Thus, various alkyl, allyl and benzyl substituted nitrosulfones 3b–g were treated with a representative enone 2i under the optimal reaction conditions (Table 3). In general, the Michael adducts 5a–f were obtained in excellent yields (90–99%) and enantioselectivities (entries 1–6). The enantioselectivity dropped marginally (to 85% ee) in the case of 5a (entry 1) and substantially (to 50% ee) when a nitrosulfone 3f possessing a long alkyl chain was employed (entry 5). This is attributable to the interference of the long, linear and flexible alkyl chain in 3f in the Michael addition step (see Fig. 3, vide infra). Since the rate of the reaction was very slow at −60 °C in the case of benzyl substituted nitrosulfone 3g, the reaction was performed at rt (entry 6).
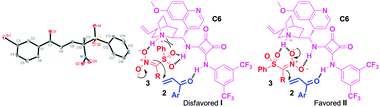 | ||
| Fig. 3 X-ray structure of 4i and proposed mechanistic model. | ||
The absolute configuration of the Michael adducts 4–5 was unambiguously assigned as R by single crystal X-ray structure analysis of a representative compound 4i (Fig. 3, see also the ESI†). The proposed mechanism based on model studies involves deprotonation of nitrosulfone 3 by the quinuclidine moiety of catalyst C6 and activation of enone 2 by the squaramide moiety (Fig. 3). In the favored approach II, the squaramide moiety also co-ordinates with the nitro group, and the quinuclidine moiety with the nitro and the sulfonyl groups, to enable Re-face approach of the enone 2 towards the nitrosulfone anion affording (R)-nitrosulfone 4 or 5. The alternative approach I appears to be disfavored due to severe steric interactions between the phenyl group of the sulfone and the quinuclidine moiety of the catalyst.
Our reaction conditions are suitable for the synthesis of nitrosulfones 4 and 5 on a multi-gram scale without any appreciable drop in the yield or selectivity. This was demonstrated by the synthesis of 2.9 g of 4i (97%) with 99% ee via Michael addition of 1.5 g of nitrosulfone 3a to 2.2 g of vinyl ketone 2i (Table 2, entry 9).
Nitrosulfonylketones 4 and 5 in which the carbonyl group is at the δ-position of the nitro and the sulfonyl group are excellent precursors for the enantioselective synthesis of carboxylic acid 7 and hydroxamic acid 8 (Scheme 1). Thus a representative nitrosulfonylketone 4b was subjected to Baeyer–Villiger oxidation using mCPBA-TFA to afford nitrosulfonyl ester 6 in 93% yield. Lithium hydroxide mediated hydrolysis of nitrosulfonyl ester 6 afforded quaternary γ-nitro-γ-sulfonyl carboxylic acid 7 in 84% yield. More importantly, the ester 6 was successfully transformed to hydroxamic acid 8 in 74% yield by treating with hydroxylamine hydrochloride.
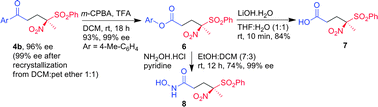 | ||
| Scheme 1 Enantioselective synthesis of hydroxamic acid. | ||
In conclusion, conjugate addition of α-nitrosulfones to vinyl ketones afforded α-nitro-δ-ketosulfones in excellent yield and enantioselectivity in the presence of as low as 0.2 mol% quinine–squaramide organocatalyst. The feasibility of scale up of the enantioselective conjugate addition as well as practical application of the products in the enantioselective synthesis of carboxylic acids and hydroxamic acids have been successfully demonstrated.
INNN thanks DAE India for financial assistance and KSB thanks CSIR India for a senior research fellowship.
Notes and references
- B. Lovejoy, A. R. Welch, S. Carr, C. Luong, C. Broka, R. T. Hendricks, J. A. Campbell, K. A. M. Walker, R. Martin, H. Van Wart and M. F. Browner, Nat. Struct. Biol., 1999, 6, 217 CrossRef CAS PubMed.
- For an article: J. M. Salvino, R. Mathew, T. Kiesow, R. Narensingh, H. J. Mason, A. Dodd, R. Groneberg, C. J. Burns, G. McGeehan, J. Kline, E. Orton, S.-Y. Tang, M. Morrisette and R. Labaudininiere, Bioorg. Med. Chem. Lett., 2000, 10, 1637 CrossRef CAS.
- (a) X.-Q. Dong, X. Fang and C.-J. Wang, Org. Lett., 2011, 13, 4426 CrossRef CAS PubMed; (b) M. Sani, G. Candiani, F. Pecker, L. Malpezzia and M. Zandaa, Tetrahedron Lett., 2005, 46, 2393 CrossRef CAS PubMed.
- I. Churcher, D. Beher, J. D. Best, J. L. Castro, E. E. Clarke, A. Gentry, T. Harrison, L. Hitzel, E. Kay, S. Kerrad, H. D. Lewis, P. Morentin-Gutierrez, R. Mortishire-Smith, P. J. Oakley, M. Reilly, D. E. Shaw, M. S. Shearman, M. R. Teall, S. Williams and J. D. J. Wrigley, Bioorg. Med. Chem. Lett., 2006, 16, 280 CrossRef CAS PubMed.
- For a recent example, see: M. Fernández and J. Caballero, Bioorg. Med. Chem., 2007, 15, 6298 CrossRef PubMed.
- M. F. Surgrue, A. Harris and I. Adamsoms, Drugs Today, 1997, 33, 283 Search PubMed.
- Selected books/reviews: (a) T. G. Back, K. N. Clary and D. Gao, Chem. Rev., 2010, 110, 4498 CrossRef CAS PubMed; (b) A. El-Awa, M. N. Noshi, X. M. D. Jourdin and P. L. Fuchs, Chem. Rev., 2009, 109, 2315 CrossRef CAS PubMed; (c) N. S. Simpkins, Sulfones in Organic Synthesis, Pergamon, Oxford, 1993 Search PubMed; (d) D. A. Alonso and C. Najera, Org. React., 2008, 72, 367 CAS; (e) E. N. Prilezhaeva, Russ. Chem. Rev., 2000, 69, 367 CrossRef CAS PubMed.
- Selected recent reviews: (a) A. R. Alba, X. Companyo and R. Rios, Chem. Soc. Rev., 2010, 39, 2018 RSC; (b) M. Nielsen, C. B. Jacobsen, N. Holub, M. W. Paixao and K. A. Jørgensen, Angew. Chem., Int. Ed., 2010, 49, 2668 CrossRef CAS PubMed.
- (a) S. Rajkumar, K. Shankland, J. M. Goodman and A. J. A. Cobb, Org. Lett., 2013, 15, 1386 CrossRef CAS PubMed; (b) P. H. Bos, A. J. Minnaard and B. L. Feringa, Org. Lett., 2008, 10, 4219 CrossRef CAS PubMed; (c) P. Mauleón, I. Alonso, M. R. Rivero and J. C. Carretero, J. Org. Chem., 2007, 72, 9924 CrossRef PubMed; (d) J.-N. Desrosiers, W. S. Bechara and A. B. Charette, Org. Lett., 2008, 10, 2315 CrossRef CAS PubMed.
- (a) J. L. García-Ruano, V. Marcos and J. Alemán, Chem. Commun., 2009, 4435 RSC; (b) A.-N. Alba, X. Companyo', A. Moyano and R. Rios, Chem.–Eur. J., 2009, 15, 11095 CrossRef CAS PubMed; (c) A. Landa, A. Puente, J. I. Santos, S. Vera, M. Oiarbide and C. Palomo, Chem.–Eur. J., 2009, 15, 11954 CrossRef CAS PubMed; (d) N. Nielsen, C. B. Jacobsen, M. W. Pixao, N. Holub and K. A. Jørgensen, J. Am. Chem. Soc., 2009, 131, 10581 CrossRef PubMed; (e) G. K. Surya Prakash, F. Wang, Z. Zhang, C. Ni, R. Haiges and G. A. Olah, Org. Lett., 2012, 14, 3260 CrossRef PubMed; (f) C. B. Jacobsen, K. L. Jensen, J. Udmark and K. A. Jørgensen, Org. Lett., 2011, 13, 4790 CrossRef CAS PubMed.
- (a) S. Mizuta, N. Shibata, Y. Goto, T. Furukawa, S. Nakamura and T. Toru, J. Am. Chem. Soc., 2007, 129, 6394 CrossRef CAS PubMed; (b) T. Furukawa, N. Shibata, S. Mizutana, S. Nakamura, T. Toru and M. Shiro, Angew. Chem., Int. Ed., 2008, 47, 8051 CrossRef CAS PubMed; (c) C. Ni, F. Wang and J. Hu, Beilstein J. Org. Chem., 2008, 4 Search PubMed; (d) A.-N. Alba, X. Companyo', A. Moyano and R. Rios, Chem.–Eur. J., 2009, 15, 7035 CrossRef CAS PubMed; (e) S. Zhang, Y. Zhang, Y. Ji, H. Li and W. Wang, Chem. Commun., 2009, 4886 RSC; (f) F. Ullah, G.-L. Zhao, L. Deiana, M. Zhu, P. Dziedzic, I. Ibrahem, P. Hammar, J. Sun and A. Córdova, Chem.–Eur. J., 2009, 15, 10013 CrossRef CAS PubMed; (g) C. B. Jacobsen, M. Nielsen, D. Worgull, T. Zweifel, E. Fisker and K. A. Jørgensen, J. Am. Chem. Soc., 2011, 133, 7398 CrossRef CAS PubMed.
- (a) M. B. Cid, J. López-Cantero, S. Duce and J. L. Garcia Ruano, J. Org. Chem., 2009, 74, 431 CrossRef CAS PubMed; (b) G. K. Surya Prakash, F. Wang, T. Stewart, T. Mathew and G. A. Olah, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 4090 CrossRef PubMed; (c) M. Kamlar, N. Bravo, A. R. Alba, S. Hybelbauerová, I. Císařová, J. Veselý, A. Moyano and R. Rios, Eur. J. Org. Chem., 2010, 5464 CrossRef CAS.
- (a) Catalytic hydrogenation of unsaturated sulfones: T. Zhou, B. Peters, M. F. Maldonado, T. Govender and P. G. Andersson, J. Am. Chem. Soc., 2012, 134, 13592 CrossRef CAS PubMed; (b) Reduction of β-ketosulfones: X. Wan, Q. Meng, H. Zhang, Y. Sun, W. Fan and Z. Zhang, Org. Lett., 2007, 9, 5613 CrossRef CAS PubMed.
- (a) Allylation of sodium sulfinate: M. Ueda and J. F. Hartwig, Org. Lett., 2010, 12, 92 CrossRef CAS PubMed; (b) Stereospecific decarboxylative allylation of sulfones: J. D. Weaver, B. J. Ka, D. K. Morris, W. Thompson and J. A. Tunge, J. Am. Chem. Soc., 2010, 132, 12179 CrossRef CAS PubMed; (c) Intramolecular cyclopropanation of α-diazo-β-ketosulfones: M. Honma, T. Sawada, Y. Fujisawa, M. Utsugi, H. Watanabe, A. Umino, T. Matsumura, T. Hagihara, M. Takano and M. Nakada, J. Am. Chem. Soc., 2003, 125, 2860 CrossRef CAS PubMed[3+2] Cycloaddition of azomethine ylides with vinyl sulfones: (d) T. Llamas, R. G. Arrayás and J. C. Carretero, Org. Lett., 2006, 8, 1795 CrossRef CAS PubMed; (e) S.-I. Fukuzawa and H. Oki, Org. Lett., 2008, 10, 1747 CrossRef CAS PubMed.
- K. Bera and I. N. N. Namboothiri, Org. Lett., 2012, 14, 980 CrossRef CAS PubMed.
- K. Bera and I. N. N. Namboothiri, Adv. Synth. Catal., 2013, 355, 1265 CrossRef CAS.
- (a) J. P. Malerich, K. Hagihara and V. H. Rawal, J. Am. Chem. Soc., 2008, 130, 14416 CrossRef CAS PubMed ; review: ; (b) J. Aleman, A. Parra, H. Jiang and K. A. Jørgensen, Chem.–Eur. J., 2011, 17, 6890 CrossRef CAS PubMed.
- W. Yang and D.-M. Du, Org. Lett., 2010, 12, 5450 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 946574. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3cc45985c |
| This journal is © The Royal Society of Chemistry 2013 |