A three-dimensional π-electron acceptor, tri-phenyl-o-carborane, bearing a rigid conformation with end-on phenyl units†
Abstract
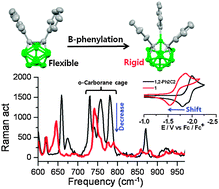
Electron accepting capability is greatly improved for B-phenylated tri-phenyl-o-carborane (1) through a favorable electronic interaction between the two adjoining phenyl-π* and cage carbon-σ* orbitals.

 Please wait while we load your content...
Please wait while we load your content...