 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Anionic polymerization and polyhomologation: an ideal combination to synthesize polyethylene-based block copolymers†
Hefeng Zhanga,
Nazeeha Alkayala,
Yves Gnanoub and
Nikos Hadjichristidis*a
aKing Abdullah University of Science and Technology, Division of Physical Sciences & Engineering, KAUST Catalysis Center, Polymer Synthesis Laboratory, Thuwal, Kingdom of Saudi Arabia
bKing Abdullah University of Science and Technology, Division of Physical Sciences & Engineering, Thuwal, Kingdom of Saudi Arabia. E-mail: nikolaos.hadjichristidis@kaust.edu.sa
First published on 7th August 2013
Abstract
A novel one-pot methodology combining anionic polymerization and polyhomologation, through a “bridge” molecule (BF3OEt2), was developed for the synthesis of polyethylene (PE)-based block copolymers. The anionically synthesized macroanion reacts with the “bridge” molecule to afford a 3-arm star (trimacromolecular borane) which serves as an initiator for the polyhomologation.
Polyethylene (PE)-based materials are very important to modern life covering a wide spectrum of applications from commodity plastics (e.g. packaging, bottles) to precision-processed biomaterials (e.g. total joint replacement and medical devices).1 In order to better understand the behaviour and improve the performance of these materials, well-defined (controllable molecular weight, narrow molecular weight and structural distribution) PE-based polymers are needed.2 Unfortunately, anionic polymerization which gives access to well-defined polymers when chain growth occurs in a “living” fashion is not compatible with ethylene, the monomer of PE.3 The only way to prepare well-defined PEs by anionic polymerization is by hydrogenation of the corresponding anionically prepared PBd-1,4. However, the produced PE possesses butylene units originating from the hydrogenation of the unavoidable 1,2-units (minimum 5%) formed during the chain growth.4 Recently, Shea developed a novel polymerization methodology leading to perfectly linear PEs.5 The general reaction scheme involves the formation of an organoboron zwitterionic complex between a methylide (monomer) and a trialkylborane Lewis acid (initiator) which breaks down by the intramolecular 1,2-migration. As a consequence, the methylene group of methylide is randomly inserted one by one into the three branches of the trialkylborane leading to a 3-arm PE star. The resulting star is subsequently oxidized/hydrolysed to give perfectly OH-end-capped linear PEs. Molecular weights from 500 to 5.0 × 105 and the polydispersity index (PDI) typically less than 1.1 have been reported. For this type of polymerization Shea coined the name polyhomologation or C1 polymerization.5–7
By using functionalized ylides and/or organoboranes, functionalized linear and nonlinear homo/copolymers, such as α-hydroxyl-ω-allylic PE, 3-armed α-hydroxyl PE star, PE-co-PP(polypropylene) and cyclic PE have been synthesized.7 Until now, polyhomologation has been combined with controlled/living radical and ring opening polymerization reactions to afford PE-based block copolymers such as PS-b-PE, PEO-b-PE diblock and PE-b-PDMS-b-PE triblock copolymers, etc. (PS: polystyrene; PEO: polyethylene oxide; PDMS: polydimethylsiloxane).8
Here, we report a novel one-pot methodology combining anionic polymerization and polyhomologation, through a “bridge” molecule (BF3OEt2), for the synthesis of polyethylene (PE)-based block copolymers. The synthetic approach involves: (a) the synthesis of a 3-arm star polymer (trimacromolecular borane, macroinitiator) by reacting living macroanions with BF3OEt2, (b) the in situ polyhomologation of dimethylsulfoxonium methylide, with the macroinitiator to produce a 3-arm star block copolymer of PE, and (c) the oxidation/hydrolysis by trimethylamine N-oxide dihydrate (TAO) to afford the PE-based block copolymers. As examples the syntheses of PBd-b-PE (PBd: polybutadiene) and PS-b-PE are shown in Scheme 1. The details are given in the ESI.†
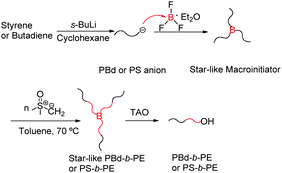 | ||
| Scheme 1 Combination of anionic polymerization and polyhomologation leading to PBd-b-PE and PS-b-PE block copolymers (BF3OEt2: “bridge” molecule). | ||
A living macroanion, PBdLi or PSLi, was first synthesized in cyclohexane at room temperature and then reacted (30% excess) with BF3OEt2 (0.7 M solution in diethyl ether) to afford a 3-arm boron-linked star. Due to the high sensitivity of boranes to air (oxygen and moisture) it was not possible to analyse the stars formed by size exclusion chromatography (SEC), since the borane species decompose during analysis (Fig. 1). The peaks corresponding to the products of coupling of PBd and PS anions with BF3OEt2 almost disappeared after 24 and 18 hours, respectively, and thus the decomposition of these macromolecular boranes (2- and 3-arm stars) was confirmed.
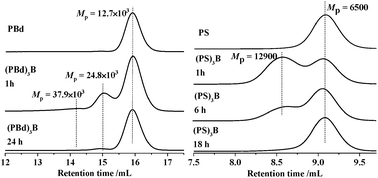 | ||
| Fig. 1 The products of coupling of PBd and PS anions with BF3OEt2 decompose during SEC measurements (THF at 35 °C). | ||
In order to avoid the decomposition, (PBd)3B and (PS)3B 3-arm stars were directly used after synthesis in situ for the polymerization of dimethylsulfoxonium methylide in toluene at 70 °C and 80 °C, respectively.
The dimethylsulfoxonium methylide (monomer) was prepared in refluxed tetrahydrofuran (THF) at 70 °C from trimethylsulfoxonium chloride in the presence of sodium hydride according to Corey's method (yield: 60–80%).9 After synthesis, THF was switched to toluene, the polymerization solvent.
The polyhomologation of dimethylsulfoxonium methylide with the star-like macroinitiators, (PBd)3B and (PS)3B, resulted in the corresponding 3-arm star block copolymers (PBd-b-PE)3B and (PS-b-PE)3B, which could not be analyzed by SEC due to reasons explained above. Oxidation/hydrolysis of the resulting starblock copolymers with trimethylamine N-oxide dihydrate (TAO) led to the linear ω-hydroxyl PBd-b-PE and PS-b-PE. Parallel experiments led to the conclusion that the methylide monomer cannot be polymerized either by the macroanion (PBdLi and PSLi) or by BF3OEt2. This indicates that only the boron-linked 3-arm star polymer has the potential for polyhomologation. The initiating efficiency of the different boron-based 3-arm stars depends on the chemical nature/steric hindrance of the arms. Macroinitiators based on polybutadiene initiate the polyhomologation much faster than PS3B. For example, polyhomologation leading to DP = 1700 could be completed in less than 10 min at 70 °C with (PBd)3B as an initiator, whereas one hour was needed when PS3B was used at 80 °C. After polyhomologation and cooling to room temperature, the toluene solution turned cloudy thus indicating successful formation of a PE block (Fig. 2).
 | ||
| Fig. 2 PS-b-PE solution in toluene after polyhomologation at 80 °C and 25 °C. | ||
The syntheses of PBd-b-PE and PS-b-PE were monitored by HT(high temperature)-SEC in trichlorobenzene (TCB) at 150 °C (Fig. 3). It is clear that the peak appearing after polyhomologation moves to the higher molecular weight region (lower elution volumes), the lower molecular weight peak corresponding to the excess precursor eliminated after fractionation. For this reason the polyhomologation products were dissolved in toluene at high temperature to give a clear and transparent solution, followed by cooling to room temperature. The solution was then centrifuged in the presence of a small amount of selective solvent for PBd (petroleum ether) and PS (acetone) to facilitate the separation. The absence of the PBd and PS precursors (Fig. 3) proves the success of the fractionation (Table 1).
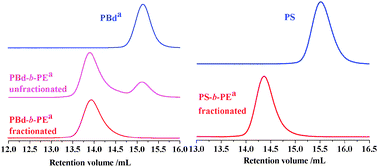 | ||
| Fig. 3 Syntheses of PBd-b-PE and PS-b-PE monitored by HT-SEC using TCB as an eluent at 150 °C. a The negative peak was shown with positive style for better comparison (dn/dc of PE and PBd are negative in TCB). | ||
| Sample | PBd or PS block | PE block | (PBd or PS)-b-PE | |||||
|---|---|---|---|---|---|---|---|---|
| Mn (× 103) | PDIHT-SEC | Mn,theor. (× 103)c | Mn,NMR (× 103) | PDIHT-SEC | Tm (°C) | Tg (°C) | UCST (°C)g | |
| a The molecular weight was determined by MALDI-ToF (Fig. S1, ESI).b The molecular weight was determined by HT-SEC using TCB as an eluent at 150 °C with a flow rate of 0.8 mL min−1 calibrated by PS standards.c The molecular weight was calculated from the ratio of the ylide to the initiator.d Mn,PE,NMR = 14 × DPPBd × (A0.8–2.4ppm − 0.6 × A6.9–7.4ppm − 2 × A4.8–5.6ppm)/A4.8–5.6ppm.e Mn,PE,NMR = 14 × DPPS × (A0.8–2.4ppm − 0.6 × A6.4–7.4ppm)/(2.5 × A6.4–6.8ppm).f The glass transition of the PS block was hidden by the melting process of the PE block.g Upper critical solution temperature (UCST) was measured in toluene (3 mg mL−1) on a UV-Vis instrument with a manual temperature controller. | ||||||||
| PBd-b-PE | 5.9a | 1.05 | 10.5 | 24.0d | 1.14 | 101 | −85 | 40–55 |
| PS-b-PE | 6.5b | 1.09 | 3.3 | 11.9e | 1.11 | 102 | —f | 40–55 |
Successful syntheses of diblock copolymers have been confirmed by Fourier-transformed infrared spectroscopy (FTIR) (Fig. 4). In the case of PBd-b-PE, the peaks at 964 and 910 cm−1 characteristic of and attributable to ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H bending vibration in PBd blocks were found.
C–H bending vibration in PBd blocks were found.
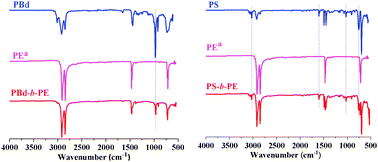 | ||
| Fig. 4 FTIR spectra of PBd-b-PE and PS-b-PE copolymers, aPE standard with a molecular weight of 2000. | ||
In the DSC curve (Fig. 5) of the PBd-b-PE diblock copolymer, a melt temperature of 101 °C attributable to the PE block was found, as well as the glass transition to the PBd block at ca. −85 °C. In the case of PS-b-PE, the glass transition of the PS block was covered by the melting process of the PE block.
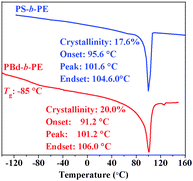 | ||
| Fig. 5 DSC curves of PBd-b-PE and PS-b-PE diblock copolymers. | ||
The PE fingerprint is clearly seen from the thermal behavior of the PE copolymers in toluene monitored by UV-Vis measurements at 450 nm. The graphs (transmittance as a function of temperature) of PBd-b-PE and PS-b-PE are shown in Fig. 6. A phase transition occurs at 40–55 °C upon heating the PBd-b-PE solution (3 mg mL−1): the transmittance then increases from ∼40% to 100%. For PS-b-PE, the phase transition is much more significant as the transmittance increases from 2% to 100% upon increasing the temperature to 40–55 °C.
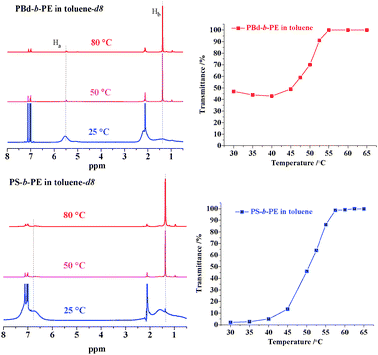 | ||
| Fig. 6 Thermal responses of PBd-b-PE and PS-b-PE copolymers revealed by 1H NMR in toluene-d8 (left) at different temperatures and UCST measurements in toluene (3 mg mL−1) (right). | ||
The PE fingerprint was also evident in the thermal responses (shielding effect) of diblock copolymers in 1H NMR spectra (Fig. 6). The single and sharp peak at 1.4 ppm, attributable to the protons in PE backbone, compared to vinyl protons in the 1,4-type double bond in the PBd block at 5.5 ppm, increases with temperature, which means that more and more PE blocks dissolved in the solvent. The area ratio of the peaks at 1.4 ppm to the one at 4.8–5.6 ppm was used to calculate the ratio of the PE block in the copolymers using: Mn,PE,NMR = 14 × DPPBd × (A0.8–2.4ppm − 0.6 × A6.9–7.4ppm − 2 × A4.8–5.6ppm)/A4.8–5.6ppm. Correspondingly, the peaks of aryl protons due to the PS blocks at 6.4–6.9 ppm were employed to calculate the respective contents of PS and PE in PS-b-PE copolymers. Surprisingly, the molecular weights calculated in this way were higher than expected. For PBd-b-PE, the molecular weight of 24.0 × 103 drawn from NMR for the PE block is 2.3 times higher than the expected value of 10.5 × 103. The deviation is more obvious for PS-b-PE (Table 1). The higher molecular weight observed indicates that the macroinitiator concentration was lower than the theoretical one, meaning that the efficiency of the linking reaction (living polymers with BF3OEt2) was about 30%. Other reasons for this disagreement in molecular weights should be excluded since the PDIs of the final block copolymers were very low (Table 1).
In conclusion, a novel strategy, based on anionic polymerization combined with polyhomologation through a “bridge” molecule (BF3OEt2), has been proposed for the synthesis of PE-based block copolymers. The examples described in this work (PBd-b-PE and PS-b-PE) are only two out of the many that can be prepared by this general methodology. A few are given below: ABE, (AB)2or3CE, (ABC)2or3DE, ABCDE, (ABCD)2or3FE, etc. (A, B, C, D, E, F: different blocks, E: polyethylene). The potential of this method is limited only by our imagination.
Notes and references
- D. B. Malpass, Introduction to Industrial Polyethylene: Properties, Catalysts, and Processes, John Wiley and Sons Ltd, 2010 CrossRef CAS; G. Chen and M. K. Patel, Chem. Rev., 2012, 112, 2082–2099 CrossRef CAS; D. Rana and T. Matsuura, Chem. Rev., 2010, 110, 2448–2471 CrossRef.
- D. Wu, F. Xu, B. Sun, R. Fu, H. He and K. Matyjaszewski, Chem. Rev., 2012, 112, 3959–4015 CrossRef CAS; J. Schmelz, A. E. Schedl, C. Steinlein, I. Manners and H. Schmalz, J. Am. Chem. Soc., 2012, 134, 14217–14225 CrossRef.
- N. Hadjichristidis, M. Pitsikalis, S. Pispas and H. Iatrou, Chem. Rev., 2001, 101, 3747–3792 CrossRef CAS; Complex Macromolecular Architectures: Synthesis, Characterization, and Self-Assembly, ed. N. Hadjichristidis, A. Hirao, Y. Tezuka and F. Du Prez, John Wiley & Sons, Singapore, 2011 Search PubMed.
- H. Rachapudy, G. G. Smith, V. R. Raju and W. W. Graessley, J. Polym. Sci., Chem. Ed., 1979, 17, 1211–1222 CrossRef CAS; N. Hadjichristidis, M. Xenidou, H. Iatrou, M. Pitsikalis, Y. Poulos, A. Avgeropoulos, S. Sioula, S. Paraskeva, G. Velis, D. J. Lohse, D. N. Schulz, L. J. Fetters, P. J. Wright, R. A. Mendelson, C. A. Garcia-Franco, T. Sun and C. J. Ruff, Macromolecules, 2000, 33, 2424–2436 CrossRef.
- J. Luo and K. J. Shea, Acc. Chem. Res., 2010, 43, 1420–1433 CrossRef CAS; K. J. Shea, B. B. Busch and M. M. Paz, Angew. Chem., Int. Ed., 1998, 37, 1391–1393 CrossRef; K. J. Shea, J. W. Walker, H. Zhu, M. M. Paz and J. Greaves, J. Am. Chem. Soc., 1997, 119, 9049–9050 CrossRef; J. Luo, F. Lu and K. J. Shea, ACS Macro Lett., 2012, 1, 560–563 CrossRef; K. J. Shea, Chem.–Eur. J., 2000, 6, 1113–1119 Search PubMed.
- E. Ihara, Adv. Polym. Sci., 2010, 231, 191–231 CrossRef CAS; E. Jellema, A. L. Jongerius, J. N. Reek and B. de Bruin, Chem. Soc. Rev., 2010, 39, 1706–1723 RSC.
- B. B. Busch, M. M. Paz, K. J. Shea, C. L. Staiger, J. M. Stoddard, J. R. Walker, X. Zhou and H. Zhu, J. Am. Chem. Soc., 2002, 124, 3636–3646 CrossRef CAS; J. M. Stoddard and K. J. Shea, Organometallics, 2003, 22, 1124–1131 CrossRef; C. E. Wagner, A. A. Rodriguez and K. J. Shea, Macromolecules, 2005, 38, 7286–7291 CrossRef; B. B. Busch, C. L. Staiger, J. M. Stoddard and K. J. Shea, Macromolecules, 2002, 35, 8330–8337 CrossRef; R. Sulc, X. Zhou and K. J. Shea, Macromolecules, 2006, 39, 4948–4952 CrossRef; J. Bai and K. J. Shea, Macromol. Rapid Commun., 2006, 27, 1223–1228 CrossRef; C. E. Wagner and K. J. Shea, Org. Lett., 2001, 3, 3063–3066 CrossRef; C. E. Wagner, J. S. Kim and K. J. Shea, J. Am. Chem. Soc., 2003, 125, 12179–12195 CrossRef; K. J. Shea, S. Y. Lee and B. B. Busch, J. Org. Chem., 1998, 63, 5746–5747 CrossRef; X. Zhou and K. J. Shea, J. Am. Chem. Soc., 2000, 122, 11515–11516 CrossRef.
- X. Zhou and K. J. Shea, Macromolecules, 2001, 34, 3111–3114 CrossRef CAS; K. J. Shea, C. L. Staiger and S. Y. Lee, Macromolecules, 1999, 32, 3157–3158 CrossRef; C. Yuan, H. Lu, Q. Li, S. Yang, Q. Zhao, J. Huang, L. Wei and Z. Ma, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 2398–2405 CrossRef; H. Lu, Y. Xue, Q. Zhao, J. Huang, S. Xu, S. Cao and Z. Ma, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 3641–3647 CrossRef; J. Chen, K. Cui, S. Zhang, P. Xie, Q. Zhao, J. Huang, L. Shi, G. Li and Z. Ma, Macromol. Rapid Commun., 2009, 30, 532–538 CrossRef; Y. Xue, H. Lu, Q. Zhao, J. Huang, S. Xu, S. Cao and Z. Ma, Polym. Chem., 2013, 4, 307–312 RSC.
- E. J. Corey and M. Chaykovsky, J. Am. Chem. Soc., 1965, 87, 1353–1364 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and the MALDI-ToF mass spectrum of PBd. See DOI: 10.1039/c3cc44928a |
| This journal is © The Royal Society of Chemistry 2013 |
