 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
“Integrated” and “insulated” boronate-based fluorescent probes for the detection of hydrogen peroxide†‡
Xiaolong
Sun
a,
Su-Ying
Xu
a,
Stephen E.
Flower
a,
John S.
Fossey
b,
Xuhong
Qian
c and
Tony D.
James
*a
aDepartment of Chemistry, University of Bath, Claverton Down, Bath, BA2 7AY, UK. E-mail: t.d.james@bath.ac.uk; Fax: +44 (0)1225 386231; Tel: +44 (0)1225 383810
bSchool of Chemistry, University of Birmingham, Edgbaston, Birmingham, West Midlands B15 2TT, UK
cShanghai Key Laboratory of Chemical Biology, School of Pharmacy, East China University of Science and Technology, Shanghai, 200237, China. E-mail: xhqian@ecust.edu.cn
First published on 3rd June 2013
Abstract
Integrated and insulated boronate-based fluorescent probes have been evaluated for the detection of hydrogen peroxide in the presence of saccharides.
Hydrogen peroxide (H2O2) is the simplest peroxide (a compound with an oxygen–oxygen single bond) and it is a strong oxidizing agent, which has been widely employed as a bleach and as a cleaning reagent to reduce BOD1 and COD2 from industrial wastewater. Hydrogen peroxide, one of reactive oxygen species (ROS), plays an important role as a signalling molecule in the regulation of a variety of biological processes, such as immune response, cell signalling,3 Alzheimer's diseases4 and cancer.5 The importance of H2O2 has led to researchers seeking effective and applicable approaches for its detection.
Among the powerful tools available for H2O2 detection are synthetic fluorescent probes. Hydrogen peroxide reacts with arylboronic acids under mild alkaline conditions to generate phenols (Scheme 1).6 Over the last decade, several groups introduced different fluorescent probes for the detection of hydrogen peroxide by utilising this property. Chang and co-workers have developed a series of boronate-based derivatives for the fluorescent detection of H2O2 in living systems,7–13 and Tomapatanaget et al. used the H2O2 generated from glucose by the action of glucose oxidase to convert boronic acids to phenols in their glucose sensing system.14
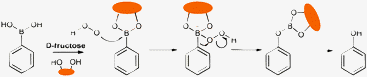 | ||
| Scheme 1 Proposed reaction mechanism between a generic aryl boronic acid/ester and H2O2. | ||
We have a long-standing interest in boronic acids for saccharide and anion detection,15–18 boronic acid derivatives rapidly and reversibly interact with saccharides in aqueous media.19,20 During the course of our investigations, we and others have employed boronic acids as sensors where the boron atom is directly attached to a fluorphore (integrated) and separated by a Lewis basic spacer (insulated). As a model integrated boronate-based fluorescent probe we chose 2-naphthylboronic acid (1). 2-Naphthylboronic acid (1) can be employed to detect saccharides via fluorescence intensity changes on addition of saccharides at constant pH resulting in an “on–off” or “off–on” fluorescence switch on saccharide binding.21 While as a model insulated boronate-based fluorescent probe we chose N-methyl-o-(aminomethyl)phenylboronic acid (2). N-Methyl-o-(aminomethyl)phenylboronic acid (2) has been previously demonstrated as an “off–on” fluorescent switch on saccharide binding, operating via a PET mechanism.22
Bearing this in mind, we decided to investigate how the reaction of H2O2 with two classes of boronic acid (integrated and insulated) fluorescent probes changes in the presence of saccharides. This is conceptually similar to the inspirational work by Jiang into the effect of saccharides on Suzuki homocoupling reactions.23 Understanding the effect of saccharides on this reaction and fluorescence may be important in developing advanced intracellular probes to accurately map H2O2 concentrations in living systems. Probes 1 and 2 (Scheme 2) and their saccharide (D-fructose was chosen as a model saccharide since it has a high binding constant) complexes were evaluated as fluorescent indicators for hydrogen peroxide both in neutral and alkaline aqueous solutions.
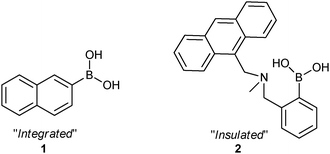 | ||
| Scheme 2 Chemical structures of probe 1 and 2. | ||
In the corresponding experiments, fluorescence titrations with H2O2 were carried out at 25 °C in pH 7.20 buffer (52.1% methanol in water with KCl, 10 mM; KH2PO4, 2.752 mM; Na2HPO4, 2.757 mM and the pH was adjusted to 7.20 using HCl)24 and pH 9.70 buffer (10.0% methanol in water with Na2CO3, 50 mM; NaHCO3, 50 mM). The saccharide-boronic acid complexes were formed by mixing free boronic acid (10 μM) with D-fructose (100 mM) in situ.25,26
The binding D-fructose to 2-naphthylboronic acid (1), in the absence of H2O2, causes a decrease and an increase in fluorescence intensity at pH 7.20 (Fig. S1, ESI‡) and pH 9.70 (Fig. S2, ESI‡) respectively.21 When H2O2 is present, 2-naphthylboronic acid is oxidatively converted to 2-naphthol (Scheme 1), and the fluorescence is reduced at 340 nm and red-shifted to 410 nm from pH 7.2 to 9.7, when excited at 290 nm (Fig. S4, ESI‡).
Fig. 1 summarises the reaction scheme and the fluorescence intensity changes FT (in the presence of H2O2)/F0 (in the absence of H2O2) for fluorescent probe 1 and the 1-D-fructose complex in pH 7.20 and 9.70 buffer. It is known that on saccharide binding and formation of a cyclic boronate ester, the pKa of the boronic acid is enhanced, or in other words the ‘ester’ is more acidic than the ‘acid’.26 Therefore, binding with D-fructose increases the electrophilicity of boron in probe 1, making it more easily oxidised by H2O2. This observation was borne out by our experiments. As shown in Fig. 1(b), the fluorescence decrease over the observed time (55 min) of probe 1 (10 μM) was ca. 0.85 (FT/F0) while there was a larger change for the 1-D-fructose complex, ca. 0.80 (FT/F0) upon expose to H2O2 (0.10 mM) over the same period in neutral buffer solution. Under alkaline conditions, the 1-D-fructose boronate ester complex reacted even faster with H2O2 (0.10 mM) (FT/F0 = ca. 0.30) than the unbound boronic acid 1 (FT/F0 = ca. 0.60). The responses of 1 and the 1-D-fructose complex towards H2O2 became more rapid at high pH since not only is the binding between boronic acid and saccharide enhanced, but also H2O2 is also more reactive at higher pH.
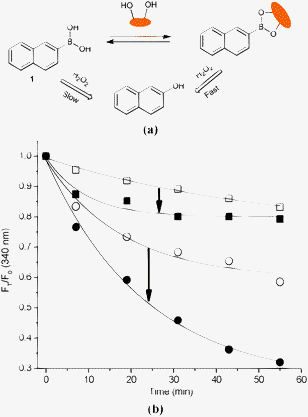 | ||
| Fig. 1 (a) Proposed strategy of probe 1 and 1-D-fructose complex for sensing of H2O2; (b) time curve of fluorescence intensity changes with probe 1 (10 μM) and D-fructose (100 mM) in aqueous H2O2 (0.10 mM) at different pH values. (Empty square – 1 and solid square – 1-D-fructose in pH 7.20; empty circle – 1 and solid circle – 1-D-fructose in pH 9.70.) The mixture was incubated in pH 7.20 PBS buffer and pH 9.70 Na2CO3/NaHCO3 buffer at 25 °C, respectively. Fluorescence intensities at 340 nm were measured with excitation at 290 nm. | ||
Fluorescence was confirmed to be due to the generation of 2-napthol by comparison with spectroscopic properties of an authentic sample. i.e. The emission maxima for 2-naphthol (10 μM, pKa = 9.51) was found at 340 nm in pH 7.20 PBS buffer while the maxima shifted to 410 nm in pH 9.70 alkaline buffer (Fig. S4, ESI‡). These results demonstrate the proton-induced fluorescence switching of 2-naphthol27 at pH 7.20 and the electron donation of the negative oxygen to the fluorophore causing the red-shift at pH 9.70.
In the case of N-methyl-o-(aminomethyl)phenylboronic acid (2), a significant “off–on” signal response has been seen on binding with D-fructose (Fig. S5, ESI‡) due to a PET mechanism.28 However, when the arylboronic acid moiety of probe 2 was transformed into a phenol upon adding H2O2, the fluorescence was further reduced due to the stronger PET from the amine in the boron free system (Fig. S5–S7, ESI‡).
The reaction scheme and fluorescence intensity changes for fluorescent probe 2 and the 2-D-fructose complex in pH 7.20 and 9.70 buffer are given in Fig. 2. From Fig. 2(b), it can be seen that the fluorescence intensity ratios with time (FT/F0) of 2-D-fructose complex slowly change to ca. 0.72, while for the saccharide free system a bigger change FT/F0 = ca. 0.55 on addition of 0.05 mM H2O2 over three hours in pH 7.20 PBS buffer (probe 2, 10 μM). The difference between 2 and the 2-D-fructose complex towards H2O2 became much larger in pH 9.70 buffer solution with FT/F0 = ca. 0.05 within 20 min upon addition of only 0.02 mM H2O2 (Fig. S8, ESI‡).29 More importantly, the signal to noise ratio output of probe 2 was enhanced through binding with saccharide allowing for colorimetric detection (bright blue to colourless) in the reaction with H2O2 (Fig. 3, Fig. S9, ESI‡).
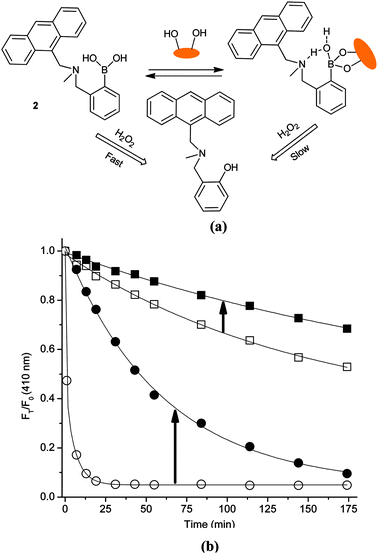 | ||
| Fig. 2 (a) Proposed strategy of probe 2 and 2-D-fructose complex for sensing of H2O2; (b) time curve of fluorescence intensity changes with probe 2 (10 μM) and D-fructose (100 mM) in aqueous H2O2 (0.05 mM for the first three samples and 0.02 mM for last sample). (Solid square – 2-D-fructose and empty square – 2 in pH 7.20; solid circle – 2-D-fructose and empty circle – 2 in pH 9.70.) The mixture was incubated in pH 7.20 PBS buffer and pH 9.70 Na2CO3/NaHCO3 buffer at 25 °C. Fluorescence intensities at 410 nm were measured with excitation at 370 nm. | ||
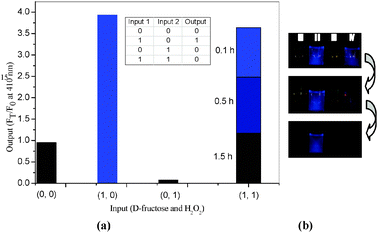 | ||
| Fig. 3 (a) Column spectral and truth table with D-fructose (100 mM) and H2O2 (0.05 mM) as inputs; (b) fluorescent colourimetric detection using naked eye with I (probe only); II (+D-fructose 100 mM); III (+ H2O2 0.10 mM); IV (+D-fructose 100 mM + H2O2 0.10 mM) after 0.1 h, 0.5 h, 1.0 h. The mixture was incubated in pH 9.70 Na2CO3/NaHCO3 buffer at 25 °C. Fluorescence intensities at 410 nm were measured with excitation at 370 nm. | ||
The fluorescence of 2 is turned on by saccharide binding, since boronic ester formation causes an enhanced interaction between the neighbouring amine and the boron atom (mediated via an inserted solvent molecule).17,30,31 This N–B interaction hinders the reaction between boron and H2O2 in the presence of saccharides resulting in a slower decrease in fluorescence (cf. saccharide free system).
In conclusion, D-fructose effects the reaction of integrated (compound 1) and insulated (compound 2) boronic acid-based fluorescent probes with hydrogen peroxide in opposite directions. Integrated boronic acid fluorophores (compound 1) display enhanced reactivity with H2O2 in the presence of D-fructose. While the PET fluorophore systems (compound 2) display reduced reactivity with H2O2 in the presence of D-fructose. The insulated PET systems are particularly interesting, (compound 2) because, in the presence of D-fructose the initial fluorescence intensity is much higher and produces a blue visible fluorescence, which implies that they could be used as temporal fluorescent probes to map both intracellular H2O2 and saccharide concentrations. The insulated systems also produce a larger fluorescence response to low concentrations of H2O2. Therefore, we believe that insulated (PET) systems are the fluorescent probes of choice for use as imaging agents and sensors for H2O2.
We are currently exploring the development of longer wavelength insulated systems for biologically relevant imaging applications.
TDJ and XS are grateful for financial support from China Scholarship Council (CSC) and University of Bath Full Fees Scholarship. The Catalysis And Sensing for our Environment (CASE) network is thanked for research exchange opportunities.
Notes and references
- Biochemical oxygen demand (BOD) is the amount of dissolved oxygen needed by aerobic biological organisms in a body of water to break down organic material present in a given water sample at certain temperature over a specific time period.
- In environmental chemistry, the chemical oxygen demand (COD) test is commonly used to indirectly measure the amount of organic compounds in water.
- J. P. Fruehauf and F. L. Meyskens, Clin. Cancer Res., 2007, 13, 789–794 CrossRef CAS.
- M. P. Mattson, Nature, 2004, 430, 631–639 CrossRef CAS.
- H. Ohshima, M. Tatemichi and T. Sawa, Arch. Biochem. Biophys., 2003, 417, 3–11 CrossRef CAS.
- H. G. Kuivila and A. G. Armour, J. Am. Chem. Soc., 1957, 79, 5659–5662 CrossRef CAS.
- M. C. Y. Chang, A. Pralle, E. Y. Isacoff and C. J. Chang, J. Am. Chem. Soc., 2004, 126, 15392–15393 CrossRef CAS.
- E. W. Miller, A. E. Albers, A. Pralle, E. Y. Isacoff and C. J. Chang, J. Am. Chem. Soc., 2005, 127, 16652–16659 CrossRef CAS.
- A. E. Albers, V. S. Okreglak and C. J. Chang, J. Am. Chem. Soc., 2006, 128, 9640–9641 CrossRef CAS.
- E. W. Miller, O. Tulyathan, E. Y. Isacoff and C. J. Chang, Nat. Chem. Biol., 2007, 3, 263–267 CrossRef CAS.
- D. Srikun, E. W. Miller, D. W. Domaille and C. J. Chang, J. Am. Chem. Soc., 2008, 130, 4596–4597 CrossRef CAS.
- B. C. Dickinson, C. Huynh and C. J. Chang, J. Am. Chem. Soc., 2010, 132, 5906–5915 CrossRef CAS.
- G. C. Van de Bittner, C. R. Bertozzi and C. J. Chang, J. Am. Chem. Soc., 2013, 135, 1783–1795 Search PubMed.
- K. Wannajuk, M. Jamkatoke, T. Tuntulani and B. Tomapatanaget, Tetrahedron, 2012, 68, 8899–8904 Search PubMed.
- T. D. James, P. Linnane and S. Shinkai, Chem. Commun., 1996, 281–288 RSC.
- R. Nishiyabu, Y. Kubo, T. D. James and J. S. Fossey, Chem. Commun., 2012, 48, 1106–1123 Search PubMed.
- S. D. Bull, M. G. Davidson, J. M. H. van den Elsen, J. S. Fossey, A. T. A. Jenkins, Y.-B. Jiang, Y. Kubo, F. Marken, K. Sakurai, J. Zhao and T. D. James, Acc. Chem. Res., 2012, 46, 312–326 Search PubMed.
- E. Galbraith and T. D. James, Chem. Soc. Rev., 2010, 39, 3831–3842 RSC.
- K. Tsukagoshi and S. Shinkai, J. Org. Chem., 1991, 56, 4089–4091 CrossRef CAS.
- T. D. James, K. R. A. S. Sandanayake and S. Shinkai, J. Chem. Soc., Chem. Commun., 1994, 477 RSC.
- H. Suenaga, M. Mikami, K. R. A. S. Sandanayake and S. Shinkai, Tetrahedron Lett., 1995, 36, 4825–4828 CrossRef CAS.
- T. D. James, K. R. A. S. Sandanayake and S. Shinkai, Nature, 1995, 374, 345–347 CrossRef CAS.
- S. Y. Xu, Y. B. Ruan, X. X. Luo, Y. F. Gao, J. S. Zhao, J. S. Shen and Y. B. Jiang, Chem. Commun., 2010, 46, 5864–5866 RSC.
- D. D. Perrin and B. Dempsey, Buffers for pH and Metal Ion Control, Chapman and Hall, London, 1974 Search PubMed.
- J. C. Norrild and H. Eggert, J. Chem. Soc., Perkin. Trans. 2, 1996, 2583–2588 RSC.
- J. Yoon and A. W. Czarnik, J. Am. Chem. Soc., 1992, 114, 5874–5875 CrossRef CAS.
- C. M. Harris and B. K. Selinger, J. Phys. Chem. B, 1980, 84, 891–898 Search PubMed.
- T. D. James, K. R. A. S. Sandanayake, R. Iguchi and S. Shinkai, J. Am. Chem. Soc., 1995, 117, 8982–8987 CrossRef CAS.
- Given that the reactions take time to reach completion we confirmed that H2O2 is stable in pH 9.70 buffer at 25 °C for two hours. (Fig. S10, ESI‡).
- L. Zhu, S. H. Shabbir, M. Gray, V. M. Lynch, S. Sorey and E. V. Anslyn, J. Am. Chem. Soc., 2006, 128, 1222–1232 CrossRef CAS.
- S. Franzen, W. Ni and B. Wang, J. Phys. Chem. B, 2003, 107, 12942–12948 CrossRef CAS.
Footnotes |
| † Joseph Priestley, the discoverer of oxygen, was born in 1733. This paper is dedicated to the 280th anniversary of his birth. |
| ‡ Electronic supplementary information (ESI) available: Detailed procedures, characterization data, and additional plots. See DOI: 10.1039/c3cc43265c |
| This journal is © The Royal Society of Chemistry 2013 |
