 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Colorimetric enantioselective recognition of chiral secondary alcohols via hydrogen bonding to a chiral metallocene containing chemosensor†
Su-Ying
Xu
a,
Bin
Hu
b,
Stephen E.
Flower
a,
Yun-Bao
Jiang
c,
John S.
Fossey
bd,
Wei-Ping
Deng
*b and
Tony D.
James
*a
aDepartment of Chemistry, University of Bath, Bath BA2 7AY, UK. E-mail: t.d.james@bath.ac.uk
bShanghai Key Laboratory of Functional Materials Chemistry & School of Pharmacy, East China University of Science and Technology, Shanghai, 20023, China
cDepartment of Chemistry, College of Chemistry and Chemical Engineering, and the MOE Key Laboratory of Analytical Sciences, Xiamen University, Xiamen 361005, China
dSchool of Chemistry, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK
First published on 6th June 2013
Abstract
An operationally simple colorimetric method for enantioselective detection of chiral secondary alcohols via hydrogen bonding interactions using a chiral ferrocene derivative is reported.
This century has seen an increasing demand for determining the concentration and purity of enantiomers due to the importance of enantiopurity in the pharmaceutical industry.1,2 Chiral molecular recognition systems have been employed, to assess enantiopurity, which exploit both covalent interactions,3–8 and non-covalent interactions.9 Among non-covalent recognition systems reported ionic interactions, hydrogen bonding,10–12 π–π interactions,13 metal coordination14–16 and hydrophobic interactions have all been shown to be effective, and these interactions have attracted great interests as they are employed for many applications such as self-assembly17–19 and molecular recognition.20–30 The hydrogen bond is an important directional inter- or intra-molecular interaction, which is crucial for controlling molecular conformation and molecular aggregation.12 In the area of molecular recognition, hydrogen bonding controls the strength of binding between ligands and receptors. For example, in biological systems, the binding between a substrate and an enzyme, as well as cell surface recognition, in great degree, depends on the hydrogen bond interactions.31
Steiner outlined the palette of hydrogen bonding patterns available including O–H⋯N and N–H⋯O/N interactions.12 These interactions have been extensively explored in the crystal engineering of supramolecular structures,32,33 catalytic reactions and molecular recognition.23,34 Ghosh designed a series of pyridine derivatives for distinguishing carboxylic acids from non-hydroxyl analogues through hydrogen bonding.23,34 Shinkai introduced chiral acids as templates to create enantiomerically pure aggregated structures using hydrogen bonding interactions, which have the potential for enantioselective sensing of chiral acids.20 However, to the best of our knowledge, no one has yet used the nitrogen (N)–hydroxyl (C–OH) hydrogen bonding interaction for enantioselective detection of chiral alcohols. Herein, we report a strategy to enantioselectively detect alcohols through hydrogen bond interactions.
Compounds 1a and 1b were previously synthesised and tested as enantioselective catalysts for the kinetic resolution of secondary alcohols (Scheme 1 and S1, ESI†).35,36 It was especially noteworthy that whilst diastereoisomer 1b functioned exquisitely as a catalyst for kinetic resolution by acylation diastereoisomer 1a was completely inactive (an open top face was reasoned to be required for the acylated catalyst to be able to effectively deliver its cargo). The imidazole nitrogens on 1a and 1b are strongly Lewis basic and can themselves form a hydrogen bond with alcohols.37–39 Therefore, we decided to investigate whether 1a and 1b were able to enantioselectively recognise chiral alcohols. From the outset it was observed that the hydrogen bond interactions between 1a and chiral alcohols are strongly dependent on the solvent, spectral changes in acetonitrile are the most pronounced (S2, ESI†). The binding between 1a and a series of chiral alcohols was investigated using UV-vis spectroscopy. With dimethyl D/L-tartrates (D-DT and L-DT) the absorption peak was red-shifted from 516 nm to 576 nm (Fig. 1). Enantioselectivity was observed as dimethyl D-tartrate produced larger spectral shifts with 1a and 1b than that of dimethyl L-tartrate (Fig. 1 and 2). The observed binding constants for dimethyl D/L-tartrates with 1a and 1b are 392.5 ± 63.2/112.5 ± 29.3 dm3 mol−1 and 298.3 ± 84.7/141.4 ± 26.1 dm3 mol−1 respectively (S3, ESI†). Meanwhile enantioselective recognition could be observed colorimetrically, since after the addition of six equivalents of dimethyl D-tartrate to a solution of 1a, a colour difference is observed as shown in (Fig. 3).
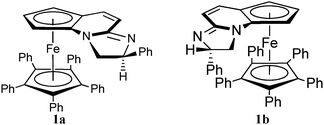 | ||
| Scheme 1 Structures of compounds 1a and 1b. | ||
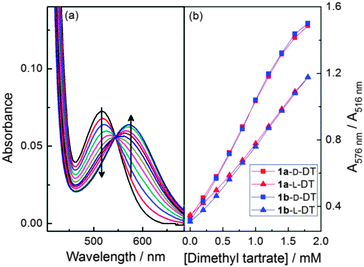 | ||
| Fig. 1 (a) UV spectra changes of 0.1 mM 1a in MeCN upon addition of dimethyl D-tartrate; (b) the ratio of absorbance at 576 nm to 516 nm versus concentration of D/L-tartrate for 1a and 1b. | ||
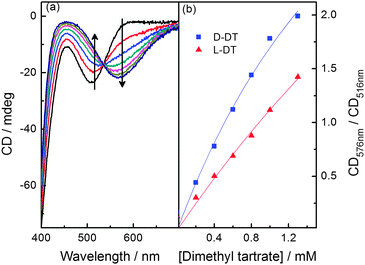 | ||
| Fig. 2 (a) CD spectra changes of 0.1 mM 1a in MeCN upon the addition of dimethyl D-tartrate; (b) the ratio of absorbance at 576 nm to 516 nm versus concentration of D/L-tartrate. | ||
 | ||
| Fig. 3 From left to right: 0.1 mM 1a, 6 eq. dimethyl L-tartrate, 6 eq. dimethyl D-tartrate in MeCN. | ||
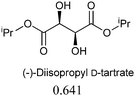
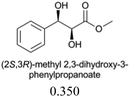
The sensing behaviours of the diastereoisomers 1a and 1b with dimethyl D/L-tartrates were identical, demonstrating a divergence between applications to alcohol recognition as opposed to acylation catalysis, i.e. the inactive catalyst, 1a, works equally well as a sensor. As such we chose to make further use of the inactive catalyst and continued our investigations with compound 1a only. A series of chiral ester containing secondary alcohols were studied and chiral alcohols with pKa ≤ 12 displayed higher chiral discrimination Δ ≥ 0.1 (Δ is the difference between (A576nm/A516nm) for each pair of enantiomers with 0.1 mM 1a and 0.6 mM of the chiral alcohol)40 (Table 1). D/L-Tartaric acids were also investigated and while pronounced spectral and colorimetric changes were observed, no enantioselectivity was detected (S4 and S5, ESI†). The observed binding constants for D/L-tartaric acid with 1a are 3131 ± 1157 dm3 mol−1 and 4636 ± 1755 dm3 mol−1 respectively (S6, ESI†). 1HNMR titrations of 1a with dimethyl D-tartrate and D-tartaric acid indicate that similar hydrogen bonding species are responsible for the observed spectral changes (S7–S9, ESI†). We believe that enantioselectivity is controlled by steric demands within the hydrogen bonding complexes formed between the guest (acid or alcohol) and compound 1a (S9, ESI†). Therefore, the increased distance between 1a and the chiral centres for the hydrogen bonding complexes formed with the tartaric acid (3 bonds) over the alcohols (2 bonds) explains the lack of enantioselectivity observed between the D/L-tartaric acids.
| Chiral alcohols (A576nm/A516nm)a | Δ | pKa41 | |
|---|---|---|---|
| a Ratio of absorption at 576 nm to 516 nm, [1a] = 0.1 mM, [chiral alcohols] = 0.6 mM. b Δ is the difference between (A576nm/A516nm) for each pair of enantiomers. | |||

|

|
0.240 | 11.44 ± 0.20 |
|

|
0.195 | 11.70 ± 0.20 |

|
|
0.016 | 12.33 ± 0.20 |

|

|
0.071 | 13.07 ± 0.20 |
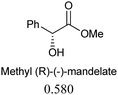
|

|
0.123 | 12.19 ± 0.20 |
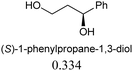
|

|
0.001 | 13.93 ± 0.20 |

|
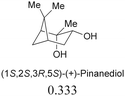
|
0.015 | 14.68 ± 0.60 |
A simple and enantioselective colorimetric sensing strategy for chiral secondary alcohols has been developed based on chiral chemosensor 1. The hydrogen bond is crucial for the enantioselectivity with more acidic alcohols exhibiting greater enantio-differentiation upon interaction with 1. Whilst the planar chirality in the sensors was not the overriding chiral recognition motif it is important to note that the metallocene fragment provides a coloured sensor that was vital in establishing a colorimetric assay and the potential to extend this work into the electrochemical arena in the future.
TDJ and SX are grateful for financial support from China Scholarship Council (CSC) and University of Bath Full Fees Scholarship. The Natural Science Foundation of China fellowship for young foreign scientists (No. 21050110426) provided support (JSF and W-PD). Thanks also go to the Catalysis and Sensing for our Environment (CASE) network for facilitating this collaboration. TDJ and JSF thank ECUST for guest professorships. TDJ thanks Xiamen University for a guest professorship.
Notes and references
- S. C. Stinson, Chem. Eng. News, 2000, 78, 55–78 Search PubMed.
- A. Thayer, Chem. Eng. News, 2005, 83, 49–53 Search PubMed.
- J. Z. Zhao, T. M. Fyles and T. D. James, Angew. Chem., Int. Ed., 2004, 43, 3461–3464 CrossRef CAS.
- J. Z. Zhao, M. G. Davidson, M. F. Mahon, G. Kociok-Kohn and T. D. James, J. Am. Chem. Soc., 2004, 126, 16179–16186 CrossRef CAS.
- X. Zhang, L. N. Chi, S. M. Ji, Y. B. Wu, P. Song, K. L. Han, H. M. Guo, T. D. James and J. Z. Zhao, J. Am. Chem. Soc., 2009, 131, 17452–17463 CrossRef CAS.
- Y. B. Wu, H. M. Guo, T. D. James and J. Z. Zhao, J. Org. Chem., 2011, 76, 5685–5695 CrossRef CAS.
- F. Han, L. N. Chi, X. F. Liang, S. M. Ji, S. S. Liu, F. K. Zhou, Y. B. Wu, K. L. Han, J. Z. Zhao and T. D. James, J. Org. Chem., 2009, 74, 1333–1336 CrossRef CAS.
- T. D. James, K. R. A. S. Sandanayake and S. Shinkai, Nature, 1995, 374, 345–347 CrossRef CAS.
- G. A. Hembury, V. V. Borovkov and Y. Inoue, Chem. Rev., 2008, 108, 1–73 CrossRef CAS.
- R. G. Desiraju, The weak hydrogen bond in structural chemistry and biology, Oxford University Press, 1999 Search PubMed.
- M. C. Etter, Acc. Chem. Res., 1990, 23, 120–126 CrossRef CAS.
- T. Steiner, Angew. Chem., Int. Ed., 2002, 41, 48–76 CrossRef CAS.
- C. G. Claessens and J. F. Stoddart, J. Phys. Org. Chem., 1997, 10, 254–272 CrossRef CAS.
- G. R. Whittell, M. D. Hager, U. S. Schubert and I. Manners, Nat. Mater., 2011, 10, 176–188 CrossRef CAS.
- J. H. Jia, P. Hubberstey, N. R. Champness and M. Schroder, in Molecular Networks, ed. M. W. Hosseini, 2009, vol. 132, pp. 135–161 Search PubMed.
- B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629–1658 CrossRef CAS.
- G. M. Whitesides and B. Grzybowski, Science, 2002, 295, 2418–2421 CrossRef CAS.
- J. Seo, J. W. Chung, E. H. Jo and S. Y. Park, Chem. Commun., 2008, 2794–2796 RSC.
- M. D. Yilmaz and J. Huskens, Soft Matter, 2012, 8, 11768–11780 RSC.
- T. Ishi-i, M. Crego-Calama, P. Timmerman, D. N. Reinhoudt and S. Shinkai, J. Am. Chem. Soc., 2002, 124, 14631–14641 CrossRef CAS.
- R. Nandhakumar, J. Ryu, H. Park, L. Tang, S. Choi and K. M. Kim, Tetrahedron, 2008, 64, 7704–7708 CrossRef CAS.
- H. Watarai, K. Mitani, N. Morooka and H. Takechi, Analyst, 2012, 137, 3238–3241 RSC.
- K. Ghosh, T. Sen and R. Frohlich, Tetrahedron Lett., 2007, 48, 2935–2938 CrossRef CAS.
- L. Pu, Acc. Chem. Res., 2012, 45, 150–163 CrossRef CAS.
- L. Cavallo, M. E. Cucciolito, A. De Martino, F. Giordano, I. Orabona and A. Vitagliano, Chem.–Eur. J., 2000, 6, 1127–1139 CAS.
- M. M. Wanderley, C. Wang, C.-D. Wu and W. Lin, J. Am. Chem. Soc., 2012, 134, 9050–9053 CrossRef CAS.
- Y. Liu, B. Li, T. Wada and Y. Inoue, Tetrahedron, 2001, 57, 7153–7161 CrossRef CAS.
- H. Y. Cun, Y. L. Wang, B. Yang, L. Zhang, S. X. Du, Y. Wang, K. H. Ernst and H. J. Gao, Langmuir, 2010, 26, 3402–3406 CrossRef CAS.
- A. Mravik, Z. Bocskei, K. Simon, F. Elekes and Z. Izsaki, Chem.–Eur. J., 1998, 4, 1621–1627 CrossRef CAS.
- A. M. Kelly, Y. Perez-Fuertes, J. S. Fossey, S. L. Yeste, S. D. Bull and T. D. James, Nat. Protoc., 2008, 3, 215–219 CrossRef CAS.
- R. E. Hubbard and M. K. Haider, in Encyclopedia of Life Sciences (ELS), John Wiley & Sons, Ltd., 2010 Search PubMed.
- E. Kolomiets, V. Berl and J. M. Lehn, Chem.–Eur. J., 2007, 13, 5466–5479 CrossRef CAS.
- V. Berl, I. Huc, R. G. Khoury, M. J. Krische and J. M. Lehn, Nature, 2000, 407, 720–723 CrossRef CAS.
- K. Ghosh and S. Adhikari, Tetrahedron Lett., 2006, 47, 3577–3581 CrossRef CAS.
- B. Hu, M. Meng, Z. Wang, W. T. Du, J. S. Fossey, X. Q. Hu and W. P. Deng, J. Am. Chem. Soc., 2010, 132, 17041–17044 CrossRef CAS.
- B. Hu, M. Meng, J. S. Fossey, W. Mo, X. Hu and W.-P. Deng, Chem. Commun., 2011, 47, 10632–10634 RSC.
- V. B. Birman, E. W. Uffman, J. Hui, X. M. Li and C. J. Kilbane, J. Am. Chem. Soc., 2004, 126, 12226–12227 CrossRef CAS.
- M. Wang, Y.-H. Shi, J.-F. Luo, W. Du, X.-X. Shi, J. S. Fossey and W.-P. Deng, Catal. Sci. Technol., 2011, 1, 100–103 CAS.
- M. Wang, Z. Wang, Y.-H. Shi, X.-X. Shi, J. S. Fossey and W.-P. Deng, Angew. Chem., Int. Ed., 2011, 50, 4897–4900 CrossRef CAS.
- K. N. Kim, K. C. Song, J. H. Noh and S.-K. Chang, Bull. Korean Chem. Soc., 2009, 30, 197 CrossRef CAS.
- Calculated using advanced chemistry development (ACD/Labs) software V11.02 ACD/Labs, 1994–2013 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Solvent screen and investigations compound 1a. See DOI: 10.1039/c3cc43083a |
| This journal is © The Royal Society of Chemistry 2013 |
