 Open Access Article
Open Access ArticleInverse electron demand Diels–Alder (iEDDA) functionalisation of macroporous poly(dicyclopentadiene) foams†
Astrid-Caroline Knall*, Sebastijan Kovačič, Manuel Hollauf, David Reishofer, Robert Saf and Christian Slugovc*
Institute for Chemistry and Technology of Materials, Stremayrgasse 9/5, A-8010 Graz, Austria. E-mail: a.knall@TUGraz.at; slugovc@TUGraz.at; Fax: +43 316 873 1032284; Tel: +43 316 873 32284
First published on 15th July 2013
Abstract
Inverse electron demand Diels–Alder reactions performed on the double bonds in open cellular macroporous poly(dicyclopentadiene) monoliths yield a high degree of functionalisation (up to 2 mmol pyridazines per g or 8 mmol N per g) with grafted di(pyridyl)pyridazines in a single step.
Curing of monomers constituting the minority phase of a stabilised High Internal Phase Emulsion (HIPE) is a frequently used method to prepare macroporous polymeric foams characterised by a microcellular structure which is desirably interconnected with smaller pores.1 Such foams find manifold applications in such diverse fields as tissue engineering,2 water purification3 or as separator membranes in lithium-ion batteries.4 Common prerequisites for most applications are good mechanical properties and easy methods to post-functionalise the foams.5
A particularly promising class of HIPE templated foam is made of dicyclopentadiene (DCPD) via Ring-Opening Metathesis Polymerisation (ROMP).6 These self-crosslinked poly-(dicyclopentadiene) (pDCPD) foams combine an interconnected microcellular open porous structure with the highest mechanical resilience reported amongst HIPE templated materials.6,7 Furthermore, pDCPD bears a high degree of unsaturation (as a consequence of the metathesis polymerisation) offering ways for further functionalisation (e.g. epoxidation8 or bromination9 followed by conversion with nucleophiles or radical initiated thiol–ene chemistry10). Accordingly, pDCPD foams ideally fulfil the prerequisites for the preparation of highly functionalised porous materials.
Herein we disclose a straightforward and versatile single-step method for the post-functionalisation of pDCPD foams via the inverse electron demand Diels–Alder (iEDDA) reaction of tetrazines with the residual double bonds in the foam skeleton, which is one of the first reports on using this promising conjugation scheme in materials science.11 The iEDDA reaction of tetrazines and olefins has emerged as a high potential click chemistry scheme in life- and materials sciences in the last few years.12 In these applications mostly highly strained olefins such as trans-cyclooctenes or norbornenes and hydrolytically stable, yet less reactive, tetrazine species are used for conjugation purposes.13 However, using more reactive dienes or applying higher reaction temperatures14 allows conversion of less reactive dienophiles15 such as double bonds with a large degree of steric hindrance. For example, the iEDDA reaction has been used for the synthesis of pyridazine-based ligands16 which were used, amongst other applications in organometallic chemistry, as building blocks in metal–organic frameworks.17
A pDCPD monolith with 80% porosity was prepared according to a previously developed procedure.10 To ensure that no oligomeric DCPD units or surfactant molecules remain entrapped in the pDCPD framework, the foams were purified using sequential Soxleth extractions in dichloromethane, THF and acetone (24 h each) and dried in vacuo. For the immobilisation of pyridazines using iEDDA chemistry, pieces of this monolith were immersed in scintillation vials containing 0.5 and 0.1 equiv. (with respect to repeating units) of pyTz (3,6-di(pyridin-2-yl)-1,2,4,5-tetrazine, Fig. 1) in THF or MeOH, respectively, for 48 h. The monoliths, which were initially white, exhibited a yellow colour while the supernatant remained virtually colourless.
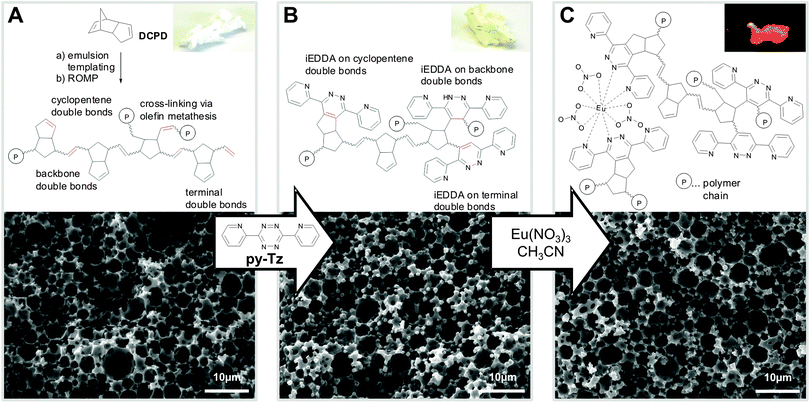 | ||
| Fig. 1 Representative chemical structures, SEM micrographs and photographs of (A) pDCPD foam, (B) after modification with 0.5 equiv. of pyTz (3,6-di(pyridin-2-yl)-1,2,4,5-tetrazine) and (C) after impregnation with Eu(NO3)3 (photograph taken under UV light (λ = 365 nm)). | ||
While the experiments in THF were completed after this time (as indicated by the discolouration of the initially pink tetrazine solution), the reactions in MeOH could not be brought to completion, even after 9 d, which is due to the more pronounced swelling of pDCPD18 in THF in comparison to MeOH. This facilitates diffusion of pyTz into the swollen pDCPD scaffold making more double bonds accessible in THF in spite of a higher reaction rate expected for MeOH.12,19
Then, the samples were purified by immersion in acetone and, after drying in vacuo, subsequently subjected to elemental analysis to determine the nitrogen content of the modified samples. Due to the high degree of unsaturation and their large surface area, pDCPD foams are known to be rapidly oxidised when stored under ambient conditions.8 Fully oxidised pDCPD has an oxygen content of about 36%.10 This was also inevitable during the purification procedure and the time allowed between sample preparation and elemental analysis. Therefore, varying oxygen contents were observed for our samples (ESI†). In Fig. 2, the pyridazine contents of reactively modified foams (calculated from elemental analysis) are compared with the respective theoretically expected ones. For samples immersed in THF, values close to the theoretical compositions (2.2 mmol grafted pyridazines per g monolith vs. 2.1 calculated) were observed which correspond to a value of 8.8 mmol g−1 nitrogen. Raising the reaction temperature to 60 °C resulted in shorter conversion times (3 h) while the same composition was obtained. In methanol, only a fraction of the applied tetrazine amount (0.37 mmol g−1vs. 2.1 calculated) was grafted.
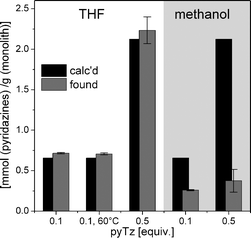 | ||
| Fig. 2 Di(pyridyl)pyridazine content of modified monoliths. | ||
About 1–2 mmol of functional units per gram of the polymeric scaffold are typical for similar, commercially available systems,20,21 such as (bipyridyl)-functionalised polystyrene, which is obtained from polystyrene in a three-step postmodification approach (with approximately every 8th or 9th repeating unit being derivatised).22 Following our post-modification approach, we were able to derivatise every second repeating unit in the pDCPD network, or, in other words, every 4th double bond in a single step under mild conditions.
The morphology of the pDCPD foam before and after functionalisation was found to be broadly similar according to evaluation of the scanning electron micrographs of representative samples (cf.Fig. 1). Mercury porosimetry and helium pycnometry revealed a somewhat higher porosity for the monolith functionalised in THF (85 ± 1%) compared to the unmodified specimens (80 ± 1%) and the sample functionalised in methanol (70 ± 1%). An increase in skeletal density from 1.20 g cm−3 (initial sample) to 1.52 g cm−3 (MeOH) and 1.75 g cm−3 (THF) reflected the increasing loading with pyridazines.
To confirm the presence of pyridazine moieties in the foams a model reaction was elaborated. Soluble low molecular weight oligo-DCPD (n = about 10) was prepared using a 1st generation Grubbs initiator at 0 °C and subsequently reacted with pyTz. The reaction was followed by 1H NMR spectroscopy (Fig. 3) which revealed complete consumption of pyTz after 24 h at room temperature. Furthermore, no characteristic dihydropyridazine signals (which should appear at 9–9.5 ppm) were found, and characteristic signals of the respective polymer-grafted pyridazine products peaking up at 7.1, 7.4, 7.5 and 8.5 ppm confirmed conversion of pyTz. Indeed, in a model experiment using pyTz and cyclopentene we also observed fast oxidation of the intermediate dihydropyridazine product without additional oxidants and found similar chemical shifts for the corresponding pyridazine (ESI†). The sharp peaks found in the NMR spectrum of fully converted oligo-DCPD (Fig. 3C) can be attributed to terminal vinyl groups transformed in an iEDDA reaction, which was confirmed by comparison with the iEDDA pyridazine product of pyTz and 1-hexene (ESI†). This “self-oxidation” of dihydropyridazines has been reported for terminal olefins,15 styrene19 and cyclopentene.23 No pronounced preference for any type of olefinic double bond (internal, strained or terminal) was observed.
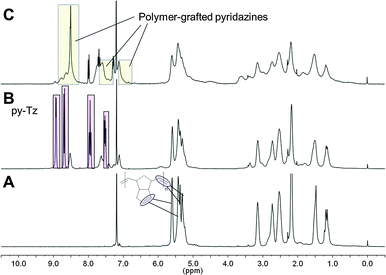 | ||
| Fig. 3 1H-NMR spectra of oligo-DCPD in CDCl3 (A) before, (B) 1 h and (C) 24 h after addition of pyTz (0.5 equiv., rt). | ||
FT-IR spectra of oligo-DCPD treated with pyTz (ESI†) showed new signals in the range of 1400 to 1600 cm−1 (ring stretching vibrations of 2-monosubstituted pyridines) while the absence of N–H stretching vibration of dihydropyridazines at about 3400 cm−1 confirmed that the grafted heterocycles were present in their oxidised (pyridazine) form.
Finally, to visualise the grafted dipyridyl(pyridazine) units and to show their principal accessibility, we selected europium(III) nitrate due to the bright red long-lived emission of Eu3+ which can be sensitised by so-called “antenna ligands”. This has also been demonstrated for pyridyl(triazine) europium(III) coordination compounds24 and similar reactions were performed on an ORMOSIL monolith bearing β-diketonates and malonamides.25 Therefore, we impregnated a piece of our pyridazinyl-bearing monolith in acetonitrile containing Eu(NO3)3(H2O)5 (1.2 equiv. with respect to grafted pyTz groups). A colour change of the monolith to a darker shade was noticed only after a few seconds and after 10 minutes, characteristic red europium emission was observed under UV light (λ = 365 nm, Fig. 1C). 3,6-Di(pyridin-2-yl)-pyridazine was reacted with Eu(NO3)3 (1 equiv.) in acetonitrile to give a red-emitting model compound which was identified as bis(3,6-di(pyridin-2-yl)pyridazine)europium(III) nitrate by MALDI-TOF-MS (ESI†) suggesting a similar stoichiometry when polymer-grafted pyridazines are used as ligands. EDX measurements of a cross-section (ESI†) proved an even distribution of Eu3+ over the surface of monoliths with an Eu content of 0.3 atom%, which can be translated into every 10th pyridazine (or every 5th pyridazine if two ligands are forming the complex) coordinating to europium while the characteristic open porous morphology is still maintained.
In conclusion, pDCPD was shown, because of its high degree of unsaturation, to have inherent reactivity in iEDDA reactions which allows facile functionalisation in a single reaction step (with nitrogen being released as the only byproduct) while involving mild reaction conditions and maintaining the characteristic open-porous morphology of emulsion-templated pDCPD foams. A high loading of up to 2 mmol g−1 of di(pyridyl)pyridazines (8 mmol g−1 of nitrogen) could be achieved. Thereby, a modular way of introducing functionalities on porous materials with a high degree of functionalisation (50% with respect to repeating units) has been disclosed and can be further exploited using other tetrazine derivatives.
Financial support from the Austrian Science Fund (FWF): [T 578-N19] is gratefully acknowledged. Karel Jerabek is thanked for mercury and helium porosimetry measurements.
Notes and references
- (a) I. Pulko and P. Krajnc, Macromol. Rapid Commun., 2012, 33, 1731 CrossRef CAS; (b) N. R. Cameron, P. Krajnc and M. S. Silverstein, Colloidal Templating, in Porous Polymers, ed. M. S. Silverstein, N. R. Cameron and M. A. Hillmyer, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2011 Search PubMed.
- (a) W. Busby, N. R. Cameron and C. A. B. Jahoda, Biomacromolecules, 2001, 2, 154 CrossRef CAS; (b) M. W. Hayman, K. H. Smith, N. R. Cameron and S. A. Przyborski, J. Biochem. Biophys. Methods, 2005, 62, 231 CrossRef CAS; (c) M. De Colli, M. Massimi, A. Barbetta, B. L. Di Rosario, S. Nardecchia, L. Conti Devirgiliis and M. Dentini, Biomed. Mater., 2012, 7, 055005 CrossRef CAS; (d) P. Viswanathan, S. Chirasatitsin, K. Ngamkham, A. J. Engler and G. Battaglia, J. Am. Chem. Soc., 2012, 134, 20103 CrossRef CAS.
- I. Pulko, M. Kolar and P. Krajnc, Sci. Total Environ., 2007, 386, 114 CrossRef CAS.
- S. Kovačič, H. Kren, P. Krajnc, S. Koller and C. Slugovc, Macromol. Rapid Commun., 2013, 34, 581 CrossRef.
- (a) L. Kircher, P. Theato and N. R. Cameron, Polymer, 2013, 54, 1755 CrossRef CAS; (b) L. Kircher, P. Theato and N. R. Cameron, Functionalisation of porous polymers from high-internal-phase emulsions and their applicationsin Functional polymers by post-polymerisation modification: concepts, practical guidelines and applications, ed. P. Theato and H.-A. Klok, Wiley-VCH, Weinheim, D, 2012 Search PubMed; (c) L. Moine, H. Deleuze and B. Maillard, Tetrahedron Lett., 2003, 44, 7813 CrossRef CAS.
- S. Kovačič, K. Jeřabek, P. Krajnc and C. Slugovc, Polym. Chem., 2012, 3, 325 RSC.
- S. Kovačič, N. B. Matsko, K. Jeřabek, P. Krajnc and C. Slugovc, J. Mater. Chem. A, 2013, 1, 487 Search PubMed.
- M. Perring, T. R. Long and N. B. Bowden, J. Mater. Chem., 2010, 20, 8679 RSC.
- M. Perring and N. B. Bowden, Langmuir, 2008, 24, 10480 CrossRef CAS.
- S. Kovačič, P. Krajnc and C. Slugovc, Chem. Commun., 2010, 46, 7504 RSC.
- (a) C. Chen, C. A. Allen and S. M. Cohen, Inorg. Chem., 2011, 50, 10534 CrossRef CAS; (b) H. Hayden, Y. K. Gun'ko and T. S. Perova, Chem. Phys. Lett., 2007, 435, 84 CrossRef CAS.
- A.-C. Knall and C. Slugovc, Chem. Soc. Rev., 2013, 42, 5131 RSC.
- N. K. Devaraj and R. Weissleder, Acc. Chem. Res., 2011, 44, 816 CrossRef CAS.
- F. Thebault, A. J. Blake, C. Wilson, N. R. Champness and M. Schröder, New J. Chem., 2006, 30, 1498 RSC.
- A. Niederwieser, A.-K. Späte, L. D. Nguyen, C. Jüngst, W. Reutter and V. Wittmann, Angew. Chem., Int. Ed., 2013, 52, 4265 CrossRef CAS.
- G. Cooke, G. M. Ó Máille, R. Quesada, L. Wang, S. Varughese and S. M. Draper, Dalton Trans., 2011, 40, 8206 RSC.
- R. Hoogenboom, G. Kickelbick and U. S. Schubert, Eur. J. Org. Chem., 2003, 4887 CrossRef CAS.
- T. R. Long, A. Gupta, A. Lee Miller, D. G. Rethwisch and N. B. Bowden, J. Mater. Chem., 2011, 21, 14265 RSC.
- J. W. Wijnen, S. Zavarise, J. B. F. N. Engberts and M. Charton, J. Org. Chem., 1996, 61, 2001 CrossRef CAS.
- J. Lu and P. H. Toy, Chem. Rev., 2009, 109, 815 CrossRef CAS.
- A. Mendonca, Metal Scavengers, in The Power of Functional Resins in Organic Synthesis, ed. J. Tulla-Puche and F. Albericio, Wiley-VCH, Weinheim, D, 2008, pp. 227–243 Search PubMed.
- R. J. Card and D. C. Neckers, Inorg. Chem., 1978, 17, 2345 CrossRef CAS.
- M. Avram, I. G. Dinulescu, E. Marica and C. D. Nenitzescu, Chem. Ber., 1962, 95, 2248 CrossRef CAS.
- G. Katsagounos, E. Stathatos, N. B. Arabatzis, A. D. Keramidas and P. Lianos, J. Lumin., 2011, 131, 1776 CrossRef CAS.
- N. Brun, B. Julián-López, P. Hesemann, G. Laurent, H. Deleuze, C. Sanchez, M.-F. Achard and R. Backov, Chem. Mater., 2008, 20, 7117 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, NMR spectra of conversion products of pyTz and norbornadiene, cyclopentene or hexene, respectively, FTIR, elemental analysis data, additional SEM micrographs, EDX, porosity and synthesis of the europium reference compound. See DOI: 10.1039/c3cc42925c |
| This journal is © The Royal Society of Chemistry 2013 |
