 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Control of aggregation-induced emission by DNA hybridization†
Shaoguang
Li
,
Simon M.
Langenegger
and
Robert
Häner
*
Department of Chemistry and Biochemistry, University of Bern, Freiestrasse 3, CH-3012 Bern, Switzerland. E-mail: robert.haener@ioc.unibe.ch; Tel: +41 31 631 4382
First published on 13th May 2013
Abstract
Aggregation-induced emission (AIE) was studied by hybridization of dialkynyl-tetraphenylethylene (DATPE) modified DNA strands. Molecular aggregation and fluorescence of DATPEs are controlled by duplex formation.
DNA is a useful and versatile scaffold for the assembly of functional groups with important applications in the fields of diagnostics, electronics, and materials science.1–13 In particular, the introduction of a wide range of chromophores into DNA has drawn considerable attention.14–23 Distinct photophysical effects are observed in such DNA-organized architectures including the formation of exciplexes,24–28 H- and/or J-aggregates29–31 or distinct helical arrangements with unique optical or chiral properties.32–39 In recent years, tetraphenylethylene (TPE) and related molecules have attracted interest because of their unusual fluorescence properties. These chromophores are weakly or non-emissive as unassociated monomers but they become strongly fluorescent upon aggregation. Aggregation-induced emission (AIE) is a result of restricted intramolecular rotation in the aggregated state (Fig. 1a).40,41 AIE features are of interest in diverse areas including fluorescence sensors42 and OLEDs.40 However, reports describing the interaction of AIE molecules (AIEs) with DNA,43 proteins44,45 or other bio-structures46 are limited and only one recent publication has reported the incorporation of an AIE-active silole derivative into DNA strands using an enzymatic method.47 Herein, we describe the synthesis and incorporation of the two stereoisomers of a dialkynyl-tetraphenylethylene (DATPE) into DNA and the AIE properties of the obtained conjugates (Fig. 1b).
 | ||
| Fig. 1 AIE properties of tetraphenylethylene (a) as a monomer and (b) as a building block in single and double stranded DNA. | ||
The synthetic strategy of the two building blocks (ME and MZ) is shown in Scheme 1. Compound 1 was obtained according to the reported procedure as a mixture of E-/Z-isomers.43 The butynyl chains were introduced through Sonogashira coupling using 3-butyn-1-ol. The E- and Z-isomers (2 and 3) were separated by chromatography and obtained in nearly equal amounts. The products were recrystallized and their configurations established by X-ray crystallography (ESI†). Compounds 2 and 3 exhibited typical AIE characteristics. They are non-emissive in well-solubilizing solvents, such as THF. In THF–water mixtures, the fluorescence intensity strongly increases once the water fraction rises above 60%. In a 95/5 (v/v) water–THF solution the quantum yields (ΦF, ESI†) are 0.40 and 0.39 for 2 and 3, which compare well with similar compounds.40
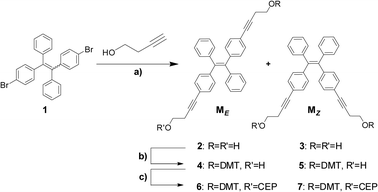 | ||
| Scheme 1 Preparation of phosphoramidites 6 and 7; (a) CuI, Pd(PPh3)2Cl2, NEt3, THF; 38%; (b) DMTCl, THF, pyridine; 29% for 4; 23% for 5; (c) CEPCl, Hünig's base, DCM; DMT = 4,4′-dimethoxytrityl; CEP = 2-cyanoethyl-N,N-diisopropyl-phosphoramidite; 61% for 6; 71% for 7. | ||
The two diols were transformed into the corresponding mono-DMT protected alcohols 4 and 5, which were subsequently converted into phosphoramidites 6 and 7. These building blocks were used in the automated synthesis of oligomers ON1-8 (Table 1) containing one or two DATPEs. All oligomers were purified by reversed-phase HPLC and characterized by ESI-MS (see ESI†).
| Sequence | T m (°C) | ΔTmb (°C) | ||
|---|---|---|---|---|
| a Conditions: 1.0 μM oligonucleotide (each strand), 10 mM sodium phosphate buffer (pH 7.4) and 100 mM NaCl, recorded in 2,2,2-trifluorethanol–water (70/30 v/v). b Compared to unmodified duplex DR. | ||||
| DR | R1 | 5′-AGC TCG GTC ATC GAG AGT GCA | 45.0 | — |
| R2 | 3′-TCG AGC CAG TAG CTC TCA CGT | |||
| D1 | ON1 | 5′-AGC TCG GTC AMEC GAG AGT GCA | 47.0 | 2.0 |
| ON2 | 3′-TCG AGC CAG TMEG CTC TCA CGT | |||
| D2 | ON3 | 5′-AGC TCG GTC AMZC GAG AGT GCA | 47.0 | 2.0 |
| ON4 | 3′-TCG AGC CAG TMZG CTC TCA CGT | |||
| D3 | ON5 | 5′-AGC TCG GTC MEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEMEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEC GAG AGT GCA | 49.5 | 4.5 |
| ON6 | 3′-TCG AGC CAG MEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEMEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEG CTC TCA CGT | |||
| D4 | ON7 | 5′-AGC TCG GTC MZZZZZZZZZZZZZZZMZZZZZZZZZZZZZZZC GAG AGT GCA | 50.0 | 5.0 |
| ON8 | 3′-TCG AGC CAG MZZZZZZZZZZZZZZZMZZZZZZZZZZZZZZZG CTC TCA CGT | |||
A mixed solvent system composed of a buffered system with 2,2,2-trifluoroethanol (TFE, 70%, v/v) and water (30%, v/v, ESI†)48 was used to analyze the spectroscopic properties of the DATPE-modified oligonucleotides.49 Measurements performed in buffered water alone or smaller fractions of TFE provided qualitatively similar results but the effects were less pronounced. Generally, single strands showed considerably weaker fluorescence in TFE–water mixtures than in water alone. We ascribe this to reduced molecular interactions between DATPE and the nucleotides and, consequently, to increased internal rotation in DATPEs42 in the presence of the less polar TFE.
The melting temperature (Tm) values of the different hybrids obtained by thermal denaturation are summarized in Table 1. The DATPE units have a positive effect on the stability of the duplex with ΔTm values ranging from +2 to +5 °C, for hybrids containing two or four DATPE units, respectively. This stabilization can be attributed to favorable stacking interactions between the propeller-twisted DATPE molecules in the duplex.
The UV-vis and fluorescence spectra recorded for hybrid D1 are shown in Fig. 2 along with the spectra of the single strands. For the single strand ON1, the absorption band of the E-DATPE molecule locates around 327 nm. The maximum of this band is shifted to 335 nm during formation of the duplex D1. Aggregation of the two DATPE molecules by hybridization of the two single strands results in a considerable enhancement of the fluorescence intensity (Fig. 2, right). The fluorescence intensity of D1 at 490 nm is about 9 times higher than that of ON1 and roughly 3 times higher than that of ON2. These differences were also reflected in the fluorescence quantum yields (ΦF) given in Table 2. ΦF rises to 0.22 upon hybridization compared to 0.05 and 0.10 for ON1 and ON2, respectively. Similar effects are observed for D2 containing two Z-isomers. Fig. 3 displays the titration of ON3 with ON4, in which ΦF gradually increases to 0.19 in the duplex D2. Formation of the duplex results in a gradual shift in the emission maximum from 476 nm (ON3) to 490 nm for D2. Fluorescence intensity is significantly increased by annealing of the two strands (an increase by a factor of 5.6 compared to ON3, Fig. 3), indicating a restriction of internal rotation by aggregation of the two DATPEs.41,42 Thus, the AIE character of the building blocks is controlled by the hybridization process. Emission maxima and quantum yields are summarized in Table 2.
 | ||
| Fig. 2 Left: absorption spectra of single strands ON1 and D1; right: fluorescence spectra of ON1, ON2, calculated sum of spectra of ON1 and ON2, and D1 (20 °C; λex: 335 nm, ex. slit: 5 nm; em. slit: 5 nm; detector: 600 V; other conditions as in Table 1; for more details see ESI†). | ||
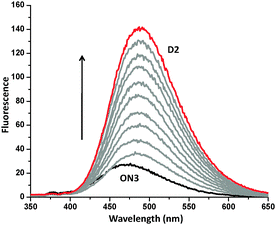 | ||
| Fig. 3 Change of fluorescence by addition of ON4 (individual steps = 0.1 μM; conditions as in Fig 2) to ON3; arrow: increasing ON4 conc. | ||
Fig. 4 illustrates the effect of molecular aggregation of DATPEs by DNA hybridization. The comparison between ON7 and D2 shows that the emission by two DATPE molecules is considerably higher in the duplex (ΦF = 0.19) than in the single strand (ΦF = 0.07). This is expected since the molecular aggregation should be positively influenced by the well-organized duplex structure compared to the single stranded random coil. A further extension of the DATPE stack, as in D4, results in a further significant increase in the fluorescence intensity (ΦF = 0.32). The ΦF values of D3 and D4 are three times higher than those of the corresponding single strands and close to the monomeric building blocks 2 and 3 in their aggregated states (ΦF ∼ 0.40). These findings reflect a steady growth of the emission with an increasing degree of molecular aggregation, i.e. the compact arrangement of DATPEs effectively suppresses the intramolecular rotation.42 The effect of AIE is best illustrated in the example shown in Fig. 5, which represents the change in fluorescence between single strand ON7 and duplex D4. Duplex formation results in a 10-fold increased emission.
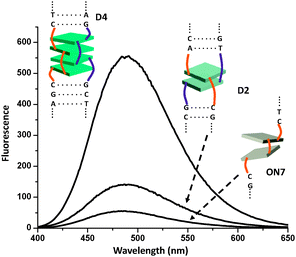 | ||
| Fig. 4 Fluorescence spectra of single strand ON7 and hybrids D2 and D4; conditions as in Fig 2. | ||
 | ||
| Fig. 5 Titration of ON7 (starting conc. 1.0 μM) by addition of ON8 (individual steps = 0.25 μM); arrow indicates increasing ON8 conc.; photo: AIE of D4 (right) compared to single strand ON7; conditions as in Fig. 2. | ||
In conclusion, we have demonstrated that AIE can be controlled by DNA hybridization. Two AIE-active, DATPE building blocks were incorporated into oligonucleotides. Hybridization of complementary strands leads to molecular aggregation of the DATPE units. Quantum yields in hybrids reach values close to those of the monomers in the aggregated state. Considering the ease of their synthesis and their unique fluorescence properties, DATPEs are promising candidates for diagnostic probes or DNA-based nanostructures with special optical properties.
This work was supported by the Swiss National Foundation (Grant 200020-132581).
Notes and references
- N. C. Seeman, Nature, 2003, 421, 427–431 CrossRef.
- J. Wengel, Org. Biomol. Chem., 2004, 2, 277–280 CAS.
- K. V. Gothelf and T. H. Labean, Org. Biomol. Chem., 2005, 3, 4023–4037 CAS.
- N. L. Rosi and C. A. Mirkin, Chem. Rev., 2005, 105, 1547–1562 CrossRef CAS.
- J. M. Kinsella and A. Ivanisevic, Langmuir, 2007, 23, 3886–3890 CrossRef CAS.
- F. A. Aldaye, A. L. Palmer and H. F. Sleiman, Science, 2008, 321, 1795–1799 CrossRef CAS.
- M. Endo and H. Sugiyama, ChemBioChem, 2009, 10, 2420–2443 CrossRef CAS.
- B. Sacca and C. M. Niemeyer, Angew. Chem., Int. Ed., 2012, 51, 58–66 CrossRef CAS.
- F. Diezmann and O. Seitz, Chem. Soc. Rev., 2011, 40, 5789–5801 RSC.
- Y. N. Teo and E. T. Kool, Chem. Rev., 2012, 112, 4221–4245 CrossRef CAS.
- M. Kwak and A. Herrmann, Chem. Soc. Rev., 2011, 40, 5745–5755 RSC.
- A. Heckel and M. Famulok, Biochimie, 2008, 90, 1096–1107 CrossRef CAS.
- M. Meng, C. Ahlborn, M. Bauer, O. Plietzsch, S. A. Soomro, A. Singh, T. Müller, W. Wenzel, S. Brase and C. Richert, ChemBioChem, 2009, 10, 1335–1339 CrossRef CAS.
- S. H. Weisbrod and A. Marx, Chem. Commun., 2008, 5675–5685 RSC.
- R. Varghese and H. A. Wagenknecht, Chem. Commun., 2009, 2615–2624 RSC.
- V. V. Filichev and E. B. Pedersen, in Wiley Encycl. Chem. Biol., ed. T. P. Begley, Wiley, Hoboken, 2009, 1, pp. 493–524 Search PubMed.
- H. Kashida, X. Liang and H. Asanuma, Curr. Org. Chem., 2009, 13, 1065–1084 CrossRef CAS.
- V. L. Malinovskii, D. Wenger and R. Häner, Chem. Soc. Rev., 2010, 39, 410–422 RSC.
- F. Seela and S. A. Ingale, J. Org. Chem., 2010, 75, 284–295 CrossRef CAS.
- O. Khakshoor and E. T. Kool, Chem. Commun., 2011, 47, 7018–7024 RSC.
- E. Stulz, Chem.–Eur. J., 2012, 18, 4456–4469 CrossRef CAS.
- E. Socher, A. Knoll and O. Seitz, Org. Biomol. Chem., 2012, 10, 7363–7371 CAS.
- J. Krim, M. Taourirte, C. Gruenewald, I. Krstic and J. W. Engels, Synthesis, 2013, 396–405 CAS.
- F. D. Lewis, Y. F. Zhang and R. L. Letsinger, J. Am. Chem. Soc., 1997, 119, 5451–5452 CrossRef CAS.
- E. V. Bichenkova, A. R. Sardarian, A. N. Wilton, P. Bonnet, R. A. Bryce and K. T. Douglas, Org. Biomol. Chem., 2006, 4, 367–378 CAS.
- I. Trkulja and R. Häner, Bioconjugate Chem., 2007, 18, 289–292 CrossRef CAS.
- I. Trkulja and R. Häner, J. Am. Chem. Soc., 2007, 129, 7982–7989 CrossRef CAS.
- S. Uno, C. Dohno, H. Bittermann, V. L. Malinovskii, R. Häner and K. Nakatani, Angew. Chem., Int. Ed., 2009, 48, 7362–7365 CrossRef CAS.
- K. C. Hannah and B. A. Armitage, Acc. Chem. Res., 2004, 37, 845–853 CrossRef CAS.
- H. Kashida, H. Asanuma and M. Komiyama, Angew. Chem., Int. Ed., 2004, 43, 6522–6525 CrossRef CAS.
- L. I. Markova, V. L. Malinovskii, L. D. Patsenker and R. Häner, Org. Biomol. Chem., 2012, 10, 8944–8947 CAS.
- M. A. Abdalla, J. Bayer, J. O. Radler and K. Müllen, Angew. Chem., Int. Ed., 2004, 43, 3967–3970 CrossRef CAS.
- M. Balaz, A. E. Holmes, M. Benedetti, P. C. Rodriguez, N. Berova, K. Nakanishi and G. Proni, J. Am. Chem. Soc., 2005, 127, 4172–4173 CrossRef CAS.
- F. D. Lewis, L. G. Zhang, X. Y. Liu, X. B. Zuo, D. M. Tiede, H. Long and G. C. Schatz, J. Am. Chem. Soc., 2005, 127, 14445–14453 CrossRef CAS.
- R. Häner, F. Samain and V. L. Malinovskii, Chem.–Eur. J., 2009, 15, 5701–5708 CrossRef.
- D. Wenger, V. L. Malinovskii and R. Häner, Chem. Commun., 2011, 47, 3168–3170 RSC.
- F. Garo and R. Häner, Angew. Chem., Int. Ed., 2012, 51, 916–919 CrossRef CAS.
- J. G. Woller, J. K. Hannestad and B. Albinsson, J. Am. Chem. Soc., 2013, 135, 2759–2768 CrossRef CAS.
- O. N. Sancho, W. R. Browne and G. Roelfes, Chem.–Eur. J., 2013, 19, 2457–2461 CrossRef.
- Z. Zhao, J. W. Lam and B. Z. Tang, J. Mater. Chem., 2012, 22, 23726–23740 RSC.
- N. B. Shustova, T. C. Ong, A. F. Cozzolino, V. K. Michaelis, R. G. Griffin and M. Dinca, J. Am. Chem. Soc., 2012, 134, 15061–15070 CrossRef CAS.
- Y. Hong, J. W. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361–5388 RSC.
- Y. Hong, H. Xiong, J. W. Y. Lam, M. Haeussler, J. Liu, Y. Yu, Y. Zhong, H. H. Sung, I. D. Williams, K. S. Wong and B. Z. Tang, Chem.–Eur. J., 2010, 16, 1232–1245 CrossRef CAS.
- H. Tong, Y. Hong, Y. Dong, M. Haeussler, Z. Li, J. W. Lam, Y. Dong, H. H. Sung, I. D. Williams and B. Z. Tang, J. Phys. Chem. B, 2007, 111, 11817–11823 CrossRef CAS.
- M. Wang, D. Zhang, G. Zhang and D. Zhu, Chem. Commun., 2008, 4469–4471 RSC.
- C. W. T. Leung, Y. Hong, S. Chen, E. Zhao, J. W. Y. Lam and B. Z. Tang, J. Am. Chem. Soc., 2013, 135, 62–65 CrossRef CAS.
- Y. Yu, J. Liu, Z. Zhao, K. M. Ng, K. Q. Luo and B. Z. Tang, Chem. Commun., 2012, 48, 6360–6362 RSC.
- J. Kypr, I. Kejnovska, D. Renciuk and M. Vorlickova, Nucleic Acids Res., 2009, 37, 1713–1725 CrossRef CAS.
- Judging from circular dichroism spectroscopy, the presence of 70% TFE does not significantly affect the B-form conformation of the present hybrids (ESI†).
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic and analytical details; additional spectroscopic data; X-ray structures. See DOI: 10.1039/c3cc42706d |
| This journal is © The Royal Society of Chemistry 2013 |
