Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Directed assembly via selectively positioned host functionality†
Piotr P.
Cholewa
a,
Christine M.
Beavers
b,
Simon J.
Teat
b and
Scott J.
Dalgarno
*a
aInstitute of Chemical Sciences, Heriot – Watt University, Riccarton, Edinburgh, EH14 4AS, Scotland, UK. E-mail: S.J.Dalgarno@hw.ac.uk; Fax: +44 (0)131 451 3180; Tel: +44 (0)131 451 8025
bLawrence Berkeley National Laboratory, 1 Cyclotron Road, MS 6R2100, Berkeley, California 94720, USA
First published on 14th March 2013
Abstract
Introduction of distal carboxylic acid groups over two positions at the upper-rim of a di-O-alkylcalix[4]arene provides facile control over coordination polymer or discrete metal–organic capsule formation.
Attaining high levels of control over the formation of discrete and polymeric assemblies from multi-component small-molecule systems is a continuing challenge in supramolecular coordination chemistry. The calix[n]arenes are a class of host molecule that have featured extensively as supramolecular building blocks due to their ability to adopt different conformations and the relative ease with which they can be functionalised.1 Calix[4]arenes (C[4]s) have attracted significant attention in this regard as they are typically bowl-shaped (C4-symmetric) due to lower-rim H-bonding interactions (Fig. 1A); in this conformation neutral or functionalised C[4]s present a cleft that is suitable for either inorganic or organic guest binding.2 In addition the conformation of the general C[4] framework can be readily controlled through synthetic modification at the lower-rim; di-O-alkylation affords a C2-symmetric partially pinched-cone C[4] that is stabilised by two lower-rim H-bonds and that retains a cavity for guest binding, whilst tetra-O-alkylation affords the fully pinched-cone conformer due to steric bulk at the lower-rim (Fig. 1B).1b
![(A) The common C4-symmetric bowl conformation found for C[4]s. (B) Examples of common C2-symmetric partially pinched-cone and pinched-cone conformations found for C[4]s when di- or tetra-functionalised at the lower-rim respectively. (C) Metal–organic super-molecules containing sufficient tilt to promote nanotube formation in the solid state based on packing preferences.7 (D) 1-D coordination polymer chains formed from TMII2(pCO2[4])4 panels and 4,4-bipyridine linkers.8](/image/article/2013/CC/c3cc40564h/c3cc40564h-f1.gif) | ||
| Fig. 1 (A) The common C4-symmetric bowl conformation found for C[4]s. (B) Examples of common C2-symmetric partially pinched-cone and pinched-cone conformations found for C[4]s when di- or tetra-functionalised at the lower-rim respectively. (C) Metal–organic super-molecules containing sufficient tilt to promote nanotube formation in the solid state based on packing preferences.7 (D) 1-D coordination polymer chains formed from TMII2(pCO2[4])4 panels and 4,4-bipyridine linkers.8 | ||
The p-carboxylatocalix[n]arenes (general notation pCO2[n]) are a relatively unexplored family of building block. This is somewhat surprising given the importance of benzoates in the programmed formation of metal–organic frameworks and polyhedra (MOFs and MOPs respectively),3,4 and that these molecules present the opportunity to construct novel materials from cavity containing sub-units. We recently began investigating their supramolecular chemistry and reported the non-covalent assembly of a series of rare p-carboxylatocalix[4]arene nanotubes, utilising pyridine (Py) as a template in the formation of constituent hydrogen-bonded capsules.5 We have also used coordination chemistry motifs to program the formation of metal–organic supermolecules that promote the assembly of novel nanotube arrays due to solid state packing constraints (Fig. 1C),6 and 1-D coordination polymers synthesised from targeted transition metal/pCO2[4] panels linked by bipyridyl co-ligands (Fig. 1D).7 The former structures comprise pinched-cone tetra-O-alkylcalix-[4]arene tetracarboxylic acids, while the latter are formed from tetra-O-alkylcalix[4]arene monocarboxylic acids. With respect to the current contribution it is noteworthy to mention that de Mendoza and co-workers reported the formation of giant regular polyhedra through assembly of pCO2[4]s and pCO2[5]s with the uranyl ion.8 These assemblies have huge internal volumes and conform to the expected polyhedral structures based on calixarene size. More recently Burrows and co-workers reported a series of pCO2[4] MOFs formed with a tetra-O-alkylcalix[4]arene dicarboxylic acid;9 the tetra-O-alkylcalix[4]arene dicarboxylic acids in these MOFs are pinched-cone conformers, with upper-rim CO2− functionality directed away from the C[4] centre.
Here we show that controlling the position of two distal carboxylic acid groups across the upper-rim of a C2-symmetric, cavity containing C[4] has dramatic consequences over metal-directed assembly to afford either a metal–organic capsule or coordination polymer from a tri-component mixture. As a general strategy we used 1,10-phenanthroline (phen) as a co-ligand to block off coordination positions around directing Cd centres and prevent the formation of systems akin to those reported by Burrows et al.9 In the case of the coordination polymer we found that symmetry equivalent phen ligands also function as complementary guests for C[4] cavities from a neighbouring chain. Assembly of the molecular components clearly relies on the angles associated with this partially pinched-cone C[4] conformer in a manner akin to that observed for benzene dicarboxylic acids in the formation of MOPs and MOFs.3,4
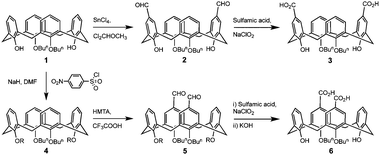
Upper-rim formylation para to the lower-rim OH groups of di-O-butylcalix[4]arene (1) to afford compound 2 is readily achieved by reaction with tin(IV) chloride and dichloromethylmethylether in DCM, and subsequent oxidation with sodium chlorite and sulfamic acid furnishes the di-carboxylic acid, 3 (Scheme 1).10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ‡ In order to formylate at the alternative upper-rim positions it is first necessary to protect the remaining lower-rim OH groups by reaction with nosyl chloride.11 Subsequent formylation with HMTA in trifluoroacetic acid, oxidation with sodium chlorite and sulfamic acid and deprotection of the lower-rim affords the alternative di-carboxylic acid, 6, in good yield (Scheme 1).5c
‡ In order to formylate at the alternative upper-rim positions it is first necessary to protect the remaining lower-rim OH groups by reaction with nosyl chloride.11 Subsequent formylation with HMTA in trifluoroacetic acid, oxidation with sodium chlorite and sulfamic acid and deprotection of the lower-rim affords the alternative di-carboxylic acid, 6, in good yield (Scheme 1).5c
In order to make structural comparison with metal complexes formed in dmf we attempted to crystallise both 3 and 6 from this solvent. Single crystals were only obtained for 3·dmf (Fig. S1, ESI†) and structural analysis showed that the calixarene adopts the expected partially pinched-cone conformation with narrow and wide cone angles of ∼88° and ∼108° respectively (Fig. S1, ESI†).§ It is unlikely that a change in upper-rim positioning (from 3 to 6) would cause significant deviation from this partially pinched-cone conformation and we therefore assume that the associated cone angles for 6·dmf would be similar.
Reaction of 3 or 6 (in equivalent stoichiometries) with cadmium nitrate tetrahydrate and phen in dmf produced single crystals that were suitable for X-ray diffraction studies (ESI†). Structural analysis of crystals formed from reaction with 3 (with two splayed upper-rim carboxylic acid groups) shows that the components form a 1-D coordination polymer (7) with formula [Cd(3-2H)(phen)(H2O)0.5(dmf)0.5]·(H2O)0.5(dmf)0.5.§ The Cd(II) centre bonds to one carboxylate of 3, the phen and one solvent molecule (found to be disordered dmf–H2O) in the asymmetric unit (Fig. 2A). Structure expansion reveals that it also bonds to a symmetry equivalent of the distal carboxylate group within 3, generating a 1-D coordination polymer chain (Fig. 2B). Expansion of the asymmetric unit around the cavity of 3 reveals that this is occupied by a symmetry equivalent phen ligand, with the two fragments knitting together through two crystallographically unique host–guest CH⋯π interactions (Fig. 2A). The result of this is that the extended structure contains interwoven pairs of 1-D chains as shown in Fig. 2B. These assemble to form bi-layer type arrays as is often observed for C[4] building blocks.12 Analysis of the cone angles of crystallographically unique 3 found in 7 shows that the molecule retains the partially pinched-cone conformation with narrow and wide cone angles of ∼88° and ∼111° respectively (Fig. S2, ESI†).
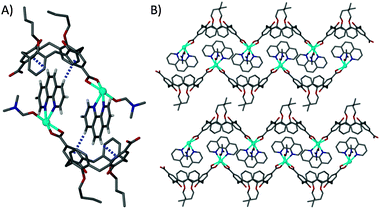 | ||
| Fig. 2 (A) Section of the extended structure in 7 showing complementary host–guest CH⋯π interactions (blue dashed lines) between neighbouring cavities of 3 and ligated phenanthrolines. (B) Extended structure in 7 showing the interdigitation of 1-D chains and the bi-layer assembly. H atoms except those involved in CH⋯π interactions are omitted for clarity. Disordered solvent ligands are shown only as dmf. | ||
Structural analysis of the crystals formed from reaction with 6 (with two proximal upper-rim carboxylic acids) shows that the components form a discrete metal–organic capsule (8) of formula [Cd2(3-2H)2(phen)2(H2O)(dmf)⊂(dmf)]·(H2O)3(MeOH).§ The asymmetric unit in 8 contains the entire molecular capsule and the two Cd(II) centres display slight, yet important differences in their coordination chemistry, a feature presumably linked to the pronounced distortion in the assembly (Fig. 3). One Cd(II) centre bonds to two upper-rim carboxylates (one from each unique molecule of 6), one phen and a dmf that is positioned within a calixarene cavity as shown in Fig. 3A. The second Cd(II) centre also bonds to two upper-rim carboxylates and a phen, but displays different coordination by ligation of a water molecule rather than dmf. Inspection of the structure (Fig. 3A) suggests that ligation of dmf would be impossible based on steric interactions, which is supported by the fact that second cavity in the capsule is occupied by a dmf guest; this occurs with a hydrogen bonding interaction from the ligated water to the dmf carbonyl with an O⋯O distance of 2.653 Å.
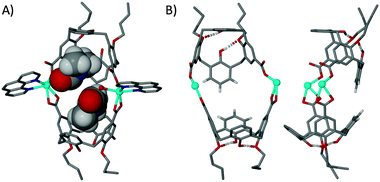 | ||
| Fig. 3 (A) The skewed molecular capsule in 8 with ligated and guest dmf molecules shown in space filling representation. (B) Two views of the metal–organic skeleton in 8 showing the offset nature of the calixarenes, the tilted nature of the capsule and the H-bonding interactions (as dashed lines) present at the lower-rims of symmetry unique molecules of 6. H atoms (except those involved in lower-rim H-bonding in (B)) are omitted for clarity. | ||
Analysis of the pairs of narrow and wide cone angles in the two unique molecules of 3 found in 8 shows that the partially pinched-cone conformation is again retained in both calixarenes; the pairs of narrow and wide cone angles have values of ∼91°/∼107° and ∼89°/∼113°. The coordination chemistry associated with the two directing Cd(II) centres also results in tilting of the two calixarenes due to coordination of the phen ligands; the tilt angle found between calixarene lower-rim centroids and one generated between the two Cd(II) centres is ∼128°. Inspection of the proximal H atoms para to the OH groups in Fig. 3B shows that these are offset. This could be due to steric reasons, or result from the coordination chemistry imposed on the Cd(II) centres by the phen ligands, or be a combination of both effects. These features clearly show that the acute angle between the acid functionalities in 6 (which is in the partially pinched-cone conformation) direct the assembly of this new discrete capsule.
We have demonstrated that selective positioning of distal carboxylic acid functionality across a C2-symmetric C[4] upper-rim affords facile control over the assembly of metal–organic capsule and coordination polymer motifs from tri-component small-molecule systems. Phenanthroline restricts the coordination sites available around the directing cadmium centres and the prevailing structure depends on the acid/lower-rim centroid/acid cone angle. An obtuse angle produces a coordination polymer, whilst a more acute building block results in discrete metal–organic capsule formation. Future work will focus on host–guest solution behaviour of this new capsule and enhanced design of new metal–organic frameworks and polyhedra possessing cavity-containing components.
The Advanced Light Source is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under contract no. DE-AC02-05CH11231. We thank the EPSRC for financial support of this work.
Notes and references
- (a) C. D. Gutsche, Calixarenes 2001, Kluwer Academic Publishers, Dordrecht, 2001, ch. 1 Search PubMed; (b) I. Thondorf, A. Shivanyuk and V. Böhmer, Calixarenes 2001, Kluwer Academic Publishers, Dordrecht, 2001, ch. 1 Search PubMed.
- p-Sulfonatocalix[4]arene has been used extensively in this regard. For a relevant review see: J. L. Atwood, L. J. Barbour, M. J. Hardie and C. L. Raston, Coord. Chem. Rev., 2001, 222, 3 CrossRef CAS.
- For recent reviews and book chapters on MOFs see: D. J. Tranchemontagne, J. L. Mendoza-Cortés, M. O'Keeffe and O. M. Yaghi, Chem. Soc. Rev., 2009, 38, 1257 RSC; K. K. Tanabe and S. M. Cohen, Chem. Soc. Rev., 2011, 40, 498 RSC; M. H. Alkordi and M. Eddaoudi, Zeolite like metal–organic frameworks (ZMOFs): design, structure and properties, in Supramolecular Chemistry: From Molecules to Nanomaterials, John Wiley & Sons, Chichester, 2012, vol. 6, p 3087 CrossRef CAS; J.-R. Li, J. Sculley and H.-C. Zhou, Chem. Rev., 2012, 112, 869 CrossRef CAS; P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Férey, R. E. Morris and C. Serre, Chem. Rev., 2012, 112, 1232 CrossRef; T. R. Cook, Y.-R. Zheng and P. J. Stang, Chem. Rev., 2013, 113, 734 CrossRef.
- For recent papers and reviews on MOPs see: D. J. Tranchemontagne, Z. Ni, M. O'Keeffe and O. M. Yaghi, Angew. Chem., Int. Ed., 2008, 47, 5136 CrossRef CAS; T. D. Hamilton, G. S. Papaefstathiou, T. Friščić, D.-K. Bučar and L. R. MacGillivray, J. Am. Chem. Soc., 2008, 130, 14366 CrossRef; J. J. Perry IV, J. A. Perman and M. J. Zaworotko, Chem. Soc. Rev., 2009, 38, 1400 RSC . Please also see papers in ref. 4 as these also cover this topic in places.
- (a) S. J. Dalgarno, J. E. Warren, J. Antesberger, T. E. Glass and J. L. Atwood, New J. Chem., 2007, 31, 1891 CAS; (b) S. Kennedy and S. J. Dalgarno, Chem. Commun., 2009, 5275 RSC; (c) S. Kennedy, P. Cholewa, R. D. McIntosh and S. J. Dalgarno, CrystEngComm, 2013, 15, 1520 RSC.
- S. Kennedy, G. Karotsis, C. M. Beavers, S. J. Teat, E. K. Brechin and S. J. Dalgarno, Angew. Chem., Int. Ed., 2010, 49, 4205 CrossRef CAS.
- P. Cholewa, C. M. Beavers, S. J. Teat, S. J. Dalgarno, submitted.
- S. Pasquale, S. Sattin, E. C. Escudero-Adan, M. Martinez-Belmonte and J. de Mendoza, Nat. Commun., 2012, 3, 785 CrossRef.
- S. P. Bew, A. D. Burrows, T. Duren, M. F. Mahon, P. Z. Moghadam, V. M. Sebestyen and S. Thurston, Chem. Commun., 2012, 48, 4824 RSC.
- A. Arduini, M. Fabbi, M. Mantovni, L. Mirone, A. Pochini, A. Secchi and R. Ungaro, J. Org. Chem., 1995, 60, 1454 CrossRef CAS.
- O. Hudecek, P. Curinova, J. Budka and P. Lhotak, Tetrahedron, 2011, 67, 5213 CrossRef CAS.
- For an example of a p-tert-butylcalix[4]arene bi-layer assembly see: J. L. Atwood, L. J. Barbour, A. Jerga and B. L. Schottel, Science, 2002, 298, 1000 CrossRef CAS.
- There was diffuse electron density associated with a badly disordered DMF of crystallisation that could not be modelled effectively. The routine SQUEEZE (ref. 14) was applied to the data in order to remove this density, the results of which matched closely with the anticipated number of electrons for one DMF per asymmetric unit.
- A. Spek, J. Appl. Crystallogr., 2003, 36, 7 CrossRef CAS; P. van der Sluis and A. L. Spek, Acta Crystallogr., Sect. A, 1990, 46, 194 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details for the synthesis of 3 and 6–8. Additional figures showing cone angles of 3 and 6 in 7 and 8 respectively. CCDC 910962–910964. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3cc40564h |
| ‡ All materials were purchased from Aldrich and used as supplied. |
§ General crystallographic details: Data for compound 3·dmf were collected on a Bruker Apex II CCD Diffractometer at 100(2) K with Mo-Kα radiation (λ = 0.71073 Å). Data for 7 and 8 were collected on a Bruker Apex II CCD Diffractometer at 100(2) K with synchrotron radiation (λ = 0.77490 Å). Given that single crystals of 7 and 8 were observed to be solvent dependent several unit cells were determined for both structures in order to confirm sample homogeneity. Crystal data for 3·dmf (CCDC 910962): C41H47NO9, M = 697.80, colourless block, 0.45 × 0.40 × 0.30 mm3, monoclinic, space group C2/c (No. 15), a = 11.4651(9) Å, b = 21.2583(18) Å, c = 15.0509(13) Å, β = 94.064(4)°, V = 3659.1(5) Å3, Z = 4, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 103 reflections collected, 3600 unique (Rint = 0.0388), final GooF = 1.039, R1 = 0.0509, wR2 = 0.1301. Crystal data for 7 (CCDC 910963)13: C56H62CdN4O11, M = 1079.50, colourless block, 0.02 × 0.02 × 0.01 mm3, triclinic, space group P 103 reflections collected, 3600 unique (Rint = 0.0388), final GooF = 1.039, R1 = 0.0509, wR2 = 0.1301. Crystal data for 7 (CCDC 910963)13: C56H62CdN4O11, M = 1079.50, colourless block, 0.02 × 0.02 × 0.01 mm3, triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (No. 2), a = 10.9671(5) Å, b = 14.2215(6) Å, c = 18.5034(8) Å, α = 110.695(2)°, β = 90.387(2)°, γ = 106.035(2)°, V = 2577.2(2) Å3, Z = 2, 44 (No. 2), a = 10.9671(5) Å, b = 14.2215(6) Å, c = 18.5034(8) Å, α = 110.695(2)°, β = 90.387(2)°, γ = 106.035(2)°, V = 2577.2(2) Å3, Z = 2, 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 494 reflections collected, 18 494 reflections collected, 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 545 unique (Rint = 0.0376), final GooF = 1.275, R1 = 0.0655, wR2 = 0.1850. Crystal data for 8 (CCDC 910964): C107H118Cd2N6O23, M = 2080.87, colourless block, 0.16 × 0.04 × 0.04 mm3, monoclinic, space group P21/n (No. 14), a = 19.2266(10) Å, b = 24.9668(14) Å, c = 20.7082(12) Å, β = 98.402(4)°, V = 9833.8(9) Å3, Z = 4, 71 545 unique (Rint = 0.0376), final GooF = 1.275, R1 = 0.0655, wR2 = 0.1850. Crystal data for 8 (CCDC 910964): C107H118Cd2N6O23, M = 2080.87, colourless block, 0.16 × 0.04 × 0.04 mm3, monoclinic, space group P21/n (No. 14), a = 19.2266(10) Å, b = 24.9668(14) Å, c = 20.7082(12) Å, β = 98.402(4)°, V = 9833.8(9) Å3, Z = 4, 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 162 reflections collected, 24 162 reflections collected, 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 unique (Rint = 0.0455), final GooF = 1.034, R1 = 0.0671, wR2 = 0.1879. 200 unique (Rint = 0.0455), final GooF = 1.034, R1 = 0.0671, wR2 = 0.1879. |
| This journal is © The Royal Society of Chemistry 2013 |